Abstract
Current-use pesticides (CUPs) and banned organochlorine compounds (OCCs) were measured in precipitation (snowpack and rain) and lake sediments from two national parks in the Western United States to determine their occurrence and distribution in high-elevation environments. CUPs frequently detected in snow were endosulfan, dacthal, and chlorothalonil in concentrations ranging from 0.07 to 2.4 ng/L. Of the OCCs, chlordane, hexachlorobenzene, and two polychlorinated biphenyl congeners were detected in only one snow sample each. Pesticides most frequently detected in rain were atrazine, carbaryl, and dacthal in concentrations from 3.0 to 95 ng/L. Estimated annual deposition rates in one of the parks were 8.4 μg/m2 for atrazine, 9.9 μg/m2 for carbaryl, and 2.6 μg/m2 for dacthal, of which >85% occurred during summer. p,p’-DDE and p,p’-DDD were the most frequently detected OCCs in surface sediments from lakes. However, concentrations were low (0.12 to 4.7 μg/kg) and below levels at which harmful effects for benthic organisms are likely to be observed. DDD and DDE concentrations in an age-dated sediment core suggest that atmospheric deposition of DDT and its degradates, and possibly other banned OCCs, to high-elevation areas have been decreasing since the 1970s. Dacthal and endosulfan sulfate were present in low concentrations (0.11 to 1.2 μg/kg) and were the only CUPs detected in surface sediments. Both pesticides were frequently detected in snow, confirming that some CUPs entering high-elevation aquatic environments through atmospheric deposition are accumulating in lake sediments and potentially in aquatic biota as well.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
A number of studies have documented the presence of organochlorine compounds (OCCs) in remote polar ecosystems. These compounds are accumulating at high latitudes because of long-range atmospheric transport and the effects of cold condensation, a process that causes semivolatile organic compounds used at warmer temperate latitudes to accumulate in colder high-latitude regions (Simonich & Hites 1995; Wania & Mackay 1996; Blais 2005). OCCs frequently detected in these environments include DDT and its degradates, α- and γ-hexachlorocyclohexane (HCH), hexachlorobenzene (HCB), and polychlorinated biphenyls (PCBs). These compounds are of environmental concern because they tend to bioconcentrate in wildlife and, for some, are known or suspected endocrine disrupters (Tyler et al. 1998).
Several recent studies also have documented the accumulation of OCCs in high-elevation areas at temperate latitudes (Blais et al. 1998; Carrera et al. 2001; Grimalt et al. 2001). Blais et al. (1998) detected OCCs in seasonal snowpacks in Canada and found that concentrations of the more volatile compounds tended to increase with increasing elevation. Grimalt et al. (2001) reported similar patterns in concentrations of OCCs in lake sediment and fish tissue in mountainous areas of Europe. These investigators hypothesized that low annual air temperatures were causing selective accumulation of OCCs at high elevations similar to what is observed at high latitudes. Based on these results, temperate mountains in the Western United States, such as the Rocky Mountains and Sierra Nevada, also may be susceptible to accumulation of organic contaminants because of low air temperatures, high rates of precipitation, and close proximity to agricultural and urban source areas.
Although the widespread occurrence of OCCs in the environment has been documented, much less is known about the long-range atmospheric transport of current-use pesticides (CUPs) and their persistence in aquatic environments, particularly in mountainous areas. CUPs have been detected in precipitation collected in remote areas of the United States, indicating that these compounds can be transported by air currents for substantial distances. Thurman and Cromwell (2000) detected triazine herbicides in rainfall on Isle Royale in Lake Superior. Analysis of predominant wind direction indicated that the herbicides originated from the upper Midwest and were transported hundreds of kilometers before being deposited by precipitation. Organophosphate insecticides were detected in rain and snow samples collected at high elevations in the Sierra Nevada of California (McConnell et al. 1998). Atmospheric transport of pesticides from California’s Central Valley, one of the heaviest pesticide-use areas in the United States, was considered the likely source of the pesticides. These compounds also were detected in surface-water samples from two areas in the Sierra Nevada but not in frog tissue (Fellers et al. 2004) suggesting that organophosphate insecticides may not be as easily accumulated in aquatic ecosystems as OCCs.
National parks in mountainous areas of the Western United States have a large percentage of lakes and streams at high elevations that may be at risk from long-range transport and deposition of organic contaminants (National Park Service 2005). To date, only a few measurements have been made in Western parks (Heit et al. 1984; Gubala et al. 1995; Tate & Heiny 1996; Stephens & Deacon 1997; McConnell et al. 1998; Hageman et al. 2006), so relatively little is known about the degree of contamination in these remote ecosystems. The objective of this study was to examine the occurrence and distribution of selected CUPs and OCCs in precipitation and lake sediment from two national parks in the Rocky Mountain region. Snowpack and rain samples were collected to determine which pesticides are currently entering high-elevation areas by way of atmospheric deposition and at what rates. Surface sediments from lakes were collected to investigate occurrence of these compounds in park lakes and to determine if current levels in sediment might pose a risk to aquatic organisms.
Description of Study Area
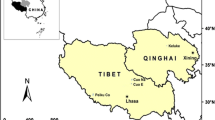
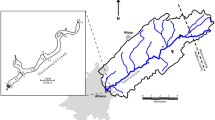
The study was conducted in Rocky Mountain National Park (ROMO) in north-central Colorado (Fig. 1) and Glacier National Park (GLAC) in northwestern Montana (Fig. 2). Both parks are characterized by high-elevation, mountainous terrain with cold, long winters and a short growing season. More than half of the precipitation occurs as snow that accumulates in a seasonal snowpack between November and April (Finklin 1986; Baron 1992). In ROMO, precipitation is associated with synoptic weather systems, with westerly airflow in winter and convective air masses originating from the south and east in spring and summer (Baron 1992). In GLAC, precipitation on the west side is associated with Pacific air masses. whereas the east side is dominated by colder Continental air masses from the north and west (Finklin 1986). Land in both parks is designated as wilderness, and human activities are limited to tourism and recreation. ROMO is situated <40 km to the west of the Front Range urban corridor, which contains the most concentrated population density in the Rocky Mountain region. The park also is situated directly west of large expanses of cropland and pasture on the plains of eastern Colorado where agricultural pesticide use exceeds 1.3 million kg/yr (Kimbrough & Litke 1997). Land-use activities in areas adjacent to GLAC include timber harvesting, low-density residential development, road networks, and ranching. The heaviest pesticide use in the region is in agricultural areas west of the park in central Washington and southern Idaho (http://www.aboutwater.usgs.gov/pnsp/) and to the northeast in Alberta, Canada (Tuduri et al. 2006).
Map of ROMO showing locations of precipitation and sediment sampling sites listed in Table 1
Map of GLAC showing locations of precipitation and sediment sampling sites listed in Table 1
Materials and Methods
Sample Collection
Snow samples were collected during spring 2001, 2002, and 2003 at sites along elevational gradients and on both sides of the Continental Divide (Table 1, Figs. 1 and 2). At each site, a single full-depth snowpack sample was collected near maximum accumulation, thus both wet and dry deposition for the entire winter period was captured. Snow samples were collected using a polycarbonate shovel from the freshly exposed face of a snow pit into 20-L, solvent-rinsed Teflon bags according to methods described by Ingersoll et al. (2002). Snow samples were transported on dry ice to the laboratory where they were stored at –15°C before processing and analysis.
A bulk-deposition collector was operated from May to August of 2002 near Bear Lake in ROMO (L1 in Fig. 1) to measure pesticides in precipitation during snow-free months (Mast et al. 2003). The collector consisted of a 40-L aluminum vessel suspended 2 m above ground. Rain samples were collected into 1-L glass bottles immediately after each storm to minimize sample evaporation and contamination. Because the sampler was continuously open to the atmosphere for as long as 1 week, the rain samples included pesticides from both wet and dry deposition. Samples were stored unfiltered at 5°C and shipped to the laboratory within 1 week of collection. The sampler was cleaned with pesticide-grade deionized water and methanol between storms or every Monday if no storms occurred during the previous week.
Surface sediments were collected at 10 lakes in ROMO and 10 lakes in GLAC during the summer 2002 and 2003 (Table 1, Figs. 1 and 2). Sediment cores were collected near the deepest point in each lake with a gravity corer. The surface sediment (top 3 cm) of each core was extruded in the field and transferred into a baked glass jar by using a metal spatula. Sediments from three to five cores from each lake were combined to acquire sufficient sample mass for pesticide analyses. Based on 210Pb age dating, the top 3 cm of the cores represented sediments deposited during the past 10 to 15 years (M. A. Mast, United States Geological Survey [USGS], 2004 unpublished data). The samples were frozen until analysis.
Analytical Methods
All samples were processed and analyzed at the USGS National Water Quality Laboratory in Denver, Colorado. Additional method details for rain and snow samples are given in Mast et al. (2003, 2006). Methods applied to snow samples were modified during the study period as the primary objective shifted from examining OCCs to examining CUPs. Snow samples were melted in the sealed Teflon collection bags, typically yielding 5 to 15 L snowmelt. The samples were shake extracted using hexane for the 2001 samples and dichloromethane for the 2002 and 2003 samples. The melted snow was not filtered, so both dissolved and particulate phases were extracted. Extracts from the 2001 and 2002 snow samples were cleaned and fractionated by alumina–silica chromatography, and extracts from the 2003 snow samples were cleaned using graphitized carbon solid-phase extraction (SPE) columns. Extracts for 2001 and 2002 snow samples were analyzed for 17 OCCs and 27 PCB congeners by gas chromatography (GC) with electron-capture detection (ECD) using conditions comparable with Noriega et al. (2004). The 2002 and 2003 snow extracts were analyzed for 62 CUPs by GC with electron-impact mass spectrometry (EIMS) operated in selected-ion mode using conditions comparable with Sandstrom et al. (2001). A subset of the 2002 to 2003 snow extracts were reanalyzed by GC with electron-capture negative ion mass spectrometry (ECNIMS) in full scan mode using conditions described in Mast et al. (2006). This third instrumental analysis was done to verify compounds detected by GC/ECD or GC/EIMS and to investigate the presence of additional organohalogen compounds.
A separate 6-L aliquot of the 2001 snow samples and the 1-L rain samples were filtered to remove suspended particulate matter and then pumped through C18 SPE columns to extract CUPs. The pesticides were eluted from the columns with ethyl acetate and analyzed for 47 CUPs (plus 3 endosulfans for snow samples) by GC/EIMS as described in Zaugg et al. (1995). No CUPs were detected in extracts of the complementary filters (particle phase) for the 2001 snow samples. Filters were not analyzed for rain samples.
Lake sediments were processed and analyzed for OCCs according to the method of Noriega et al. (2004). The sediments were extracted with dichloromethane in a Soxhlet apparatus. Extracts were cleaned by gel permeation chromatography (GPC) and fractionated by alumina–silica chromatography with Florisil cleanup for fraction 2. Both fractions were analyzed for 17 OCCs plus 27 PCB congeners by dual column GC/ECD. Sediments collected in 2003 also were analyzed for CUPs according to the method described in Mast et al. (2006). Sediments were extracted with 25% acetone in dichloromethane under pressure at 100°C; extracts were cleaned using graphitized carbon SPE and GPC and analyzed for 64 CUPs by GC/EIMS. Similar to snow samples, a subset of the 2002 to 2003 sediment extracts was reanalyzed by GC/ECNIMS.
For all mass spectrometric analyses, analyte concentrations lower than the method reporting level (MRL) or lowest calibration standard (whichever was higher) are reported as estimated. In these cases, the presence of the pesticide has been verified, but the concentration is estimated because it falls below the calibration range. Concentrations of carbaryl, chlorothalonil, and 2-chloro-4-isoproopylamino-6-amino-s-triazine (CIAT) always are reported as estimated because of recognized performance limitations. Estimated concentrations are treated as normal detected concentrations in the subsequent discussion. Reporting levels for the many undetected analytes not reported herein are given in Mast et al. (2003) and in Mast et al. (2006).
Quality Assurance
Laboratory quality-control samples included reagent blanks, reagent spikes, laboratory replicates, and, for OCCs in sediments, standard reference materials. In addition, all sample types were fortified with surrogate compounds before extraction to monitor performance of the sample preparation process. Concentrations reported for the environmental samples were not adjusted based on recoveries of surrogates in samples or analytes in reagent spikes. Laboratory quality-assurance procedures and results are given in Mast et al. (2006).
Field quality-control samples included field blanks and sample replicates. Field blanks for snow were collected by pouring pesticide residue–grade water over the sampling scoop and shovel into the Teflon bag. A field blank for the bulk collector was obtained by pouring 1 L pesticide residue–grade water over the sides of the aluminum vessel. Analyte concentrations in field blanks were at or below laboratory MRLs, indicating that the potential for contamination during sample collection and processing was minimal. Four field replicates were collected with snow samples and two with rain samples. The same compounds were detected in all the replicate pairs, indicating good reliability in identifying the analytes. The relative percent differences in concentrations for the replicates averaged 45% in snow and 20% in rain. The larger difference in snow replicates reflects concentrations that are much closer to the MRLs compared with those in rain.
Results and Discussion
Concentrations in Snow
Sixteen compounds were detected in snowpack samples at concentrations <3.2 ng/L (Table 2). Not all target compounds were analyzed in every sample because of extract losses before ECNIMS or methodologic limitations as discussed in Mast et al. (2006). CUPs detected in snow included acetochlor; atrazine; carbaryl; chlorpyrifos and its oxon degradate; chlorothalonil; dacthal; endosulfans I and II and their sulfate degradate; γ-HCH (lindane); metolachlor; and triallate (Table 2). Concentrations of CUPs ranged from 0.07 to 3.1 ng/L and were not related to site elevation or location relative to the Continental Divide. Lack of a correlation with elevation may suggest that cold condensation was not a major influence on pesticide distributions among our sites (Hageman et al. 2006). Of the banned OCCs, dieldrin, HCB, and trans-nonachlor were detected but only in one sample each. Hageman et al. (2006) and Blais et al. (1998) reported more frequent detections of some OCCs (e.g., chlordane, α-HCH, and dieldrin) in snowpack, likely because of lower MRLs obtained by using larger sample volumes. Lack of detection of DDT and chlordane family compounds in snow samples also may reflect decreases in atmospheric concentrations in North America (Sun et al. 2006).
Compounds most frequently detected in snow were the endosulfans, dacthal, and chlorothalonil, all chlorinated pesticides currently registered for use in the United States. Technical endosulfan, a 7:3 mixture of endosulfan I and II isomers, is the only chlorinated cyclodiene insecticide still used in the United States, where it is applied on cotton and tobacco and on a wide variety of fruits and vegetables, particularly potatoes and apples. Endosulfan is highly toxic to fish and aquatic invertebrates (Johnson & Finley 1980) and the freshwater aquatic-life criteria is 56 ng/L (http://www.epa.gov/waterscience/criteria/wqcriteria.html). Endosulfan I was detected in 14 of 15 measured samples in concentrations ranging from 0.20 to 2.5 ng/L, and endosulfan II was detected in 9 of 14 samples at 0.07 to 1.18 ng/L. Endosulfan I concentrations were as much as 4 times higher than endosulfan II, which might reflect the composition of the technical mixture applied in agricultural areas as well as differences in physical properties. Endosulfan I is more volatile than endosulfan II, and therefore typically has higher atmospheric concentrations. However, endosulfan II has a lower Henry’s law constant, which favors its removal from the atmosphere by wet deposition and air–water exchange (Rice et al. 1997). Isomer conversion from endosulfan II to I also can occur (Schmidt et al. 2001). Endosulfan sulfate, a primary degradation product of endosulfan I and II, was detected in 7 of 12 measured samples in concentrations ranging from 0.15 to 0.21 ng/L.
Dacthal (or DCPA) is a pre-emergent herbicide used primarily on onions and broccoli as well as on sod farms, golf courses, and residential lawns. Dacthal is only slightly toxic to aquatic organisms (Johnson & Finley 1980), and currently no established water-quality guidelines exist for this compound. Dacthal was detected in all but one of the snow samples analyzed in concentrations ranging from 0.27 to 2.2 ng/L. Concentrations in snow from ROMO were slightly higher than in snow from GLAC, which might reflect closer proximity of ROMO to agricultural areas.
Chlorothalonil is a broad-spectrum organochlorine fungicide used on a variety of fruit and vegetable crops but most commonly on potatoes and peanuts. It also has nonagricultural uses on golf courses and in nurseries and as a fungicide in paints, grouts, and other building products. Chlorothalonil is highly toxic to aquatic organisms in laboratory studies; however, it does not have the high degree of persistence in the environment that is typical of many other chlorinated pesticides (http://www.epa.gov/espp/effects/chlorothalonil). Chlorothalonil was detected in seven of nine snow samples analyzed in concentrations ranging from 0.51 to 2.4 ng/L. Concentrations of chlorothalonil in snow were 1 to 2 orders of magnitude lower than the freshwater aquatic-life criterion of 180 ng/L (Environment Canada 1999).
Because the sampling sites are remote, and none of the detected pesticides were applied in the parks during the study period, the most reasonable source for these contaminants is regional to long-range atmospheric transport and subsequent wet and dry deposition to the snowpack. Based on air concentrations as measured by passive air samplers, Shen et al. (2005) estimated that endosulfan I has a characteristic travel distance (or “half distance,” analogous to “half-life”) of approximately >650 km in air. Hageman et al. (2006) attributed the majority of endosulfan in ROMO snowpacks to regional transport from source areas within 300 km. This suggests that agricultural areas adjacent to ROMO and GLAC are the most likely source of endosulfans in snow. This is consistent with endosulfan use data, with heavy use in central Washington for fruit and moderate use in northeastern and south-central Colorado for potatoes. Endosulfan is also used for agriculture in Canadian Prairie Provinces to the northeast (Tuduri et al. 2006). Observations of endosulfans in Arctic air (Chernyak et al. 1996; Shen et al. 2005) indicate that some endosulfan deposition might come from long-range transport to the parks.
Dacthal also has properties that give it the potential for long-range atmospheric transport (Muir et al. 2004). It has been detected in snow samples from Alaska (Garbarino et al. 2002; Hageman et al. 2006); lake water in the Canadian high Arctic (Muir et al. 2004); air and rain samples collected at sites along the Great Lakes (James & Hites 1999); and in air samples from Cheeka Peak Observatory in Oregon (Killin et al. 2004). On the basis of empirical models, Muir et al. (2004) determined that dacthal had a significantly greater potential for long-range transport than other commonly used CUPs. Similar to endosulfan, the most likely source of dacthal in ROMO is in eastern Colorado, where it is among the 10 most commonly used agricultural herbicides on irrigated land primarily for the cultivation of onions (Kimbrough & Litke 1997). Use around GLAC is heaviest in agricultural areas of the Idaho panhandle and in southeastern Washington where it also is used predominantly on onions (http://www.ncfap.org/database/default.htm).
The long-range transport characteristics of chlorothalonil are less well understood, in part because its analytical determination is challenging and its physical properties less well characterized (Mackay et al. 1997). Chernyak et al. (1996) reported concentrations in fog and seawater samples collected in the Bering and Chukchi Seas, suggesting long-range atmospheric transport. McConnell et al. (1998) detected chlorothalonil in snow and rain samples collected in Sequoia National Park and the Lake Tahoe basin. It also has been detected in air samples from sites on the Great Lakes (James & Hites 1999); at Cheeka Peak, Oregon (Killin et al. 2004); and on the Chesapeake Bay (Harman-Fetcho et al. 2000). The presence of this compound in ROMO snow indicates that it is transported at least regionally in the atmosphere. Chlorothalonil was detected in all the ROMO snow samples for which it was analyzed and probably was transported from agricultural areas in eastern Colorado, likely from use during potato cultivation where loss to the atmosphere can be substantial (White et al. 2006). Chlorothalonil was not detected in the two snow samples from GLAC despite the fact that there is reported use of the pesticide in areas adjacent to the park.
Concentrations in Rain
Only a few CUPs were detected in the nine rain samples collected at the Bear Lake site during summer 2002, although at least one compound was detected in each sample (Table 3). The pesticides most frequently detected in rain were atrazine, carbaryl, and dacthal. Atrazine, a triazine herbicide, was detected in seven samples at concentrations ranging from 15 to 60 ng/L. These concentrations were 1 to 2 orders of magnitude lower than the freshwater aquatic–life criteria of 1,800 ng/L (Environment Canada 1999). The detection of atrazine was not unexpected given that it has been detected in precipitation collected throughout the United States (Goolsby et al. 1997; Majewski et al. 2000) and that it is the most heavily used pesticide on irrigated lands in eastern Colorado (Kimbrough & Litke 1997). Despite its predominance in rain, atrazine was only detected in two snowpack samples, which may reflect seasonal patterns in its application, which usually starts in late April in Colorado (Goolsby et al. 1997; Majewski & Capel 1995). Interestingly, the two snowpack samples with detections were collected in late April (Upper Andrews Tarn), which is 2 to 3 weeks later than collection of the other snow samples and more closely coincides with the period of application. Higher atrazine concentrations in rain than in snow also may be related to more efficient scavenging by rain. Based on equilibrium partition modeling, Lei and Wania (2004) showed that rain was more efficient at scavenging the vapors of small and more polar organic molecules from the atmosphere compared with snow. Snow was predicted to be a more efficient scavenger of the vapors of larger nonpolar compounds, including higher–molecular weight PCBs. However, at temperature <0°C, these compounds are predicted to be predominantly associated with particles or the snow flake surface, and particle versus gas-phase scavenging dominates removal by snow. Lei and Wania (2004) noted that the relative efficiency of the snow and rain removal process was not readily predicted under particle scavenging conditions. No similar modeling was attempted for the CUPs because of this consideration and the lack of required physical property information.
Carbaryl, which is an insecticide, was detected in eight rain samples in concentrations ranging from 7.9 to 95 ng/L, considerably higher than in the one snow sample but below the freshwater aquatic–life criterion of 200 ng/L (Environment Canada 1999). Carbaryl was measured in rain in the Mississippi River Valley and was detected more frequently and often at higher concentrations at urban sites than at agricultural sites because it is primarily used in urban environments (Majewski et al. 2000). In the South Platte River Basin, carbaryl was the most frequently detected pesticide in surface-water samples and the dominant pesticide in storm-runoff samples collected at urban sites in Denver, CO (Kimbrough & Litke 1997). Similar to atrazine, the paucity of carbaryl in snow may reflect seasonal patterns in urban use, where it is primarily used for lawn care (Majewski & Capel 1995), or more efficient scavenging by rain.
Dacthal was detected in all rain samples in concentrations slightly higher than in snow (3.0 to 9.3 ng/L). Compared with atrazine and carbaryl, dacthal was detected in every snow sample analyzed, which may reflect the seasonal patterns in application to a variety of crops and long-range transport and persistence characteristics of this compound. Dacthal is a pre-emergent herbicide, so it may be applied early enough in the spring to be deposited in snowpack. Dacthal also has a much longer atmospheric half-distance than atrazine, primarily from its estimated 2-orders-of-magnitude lower reaction rate with atmospheric hydroxyl radical compared with atrazine, indicating that long-range sources may be more important for dacthal (Muir et al. 2004). Chlorothalonil and endosulfan, the other two pesticides commonly detected in snow, were not measured in rain, so comparison with snow is not possible.
Comparison With Other Studies
For perspective, concentrations in this study are compared with other reported concentrations in snow and rain from temperate mountainous areas (Table 4). The most comparable data are for the Rocky Mountains where chlorpyrifos, dacthal, endosulfan, and γ-HCH (lindane) have been reported in snow samples collected in ROMO and GLAC (Hageman et al. 2006). The main difference with the results of this study was more frequent detections of chlorpyrifos by Hageman et al. (2006), likely because of differences in laboratory MRLs. Chlorpyrifos, dacthal, endosulfan, and γ-HCH also have been reported in snow and rain samples collected in the Sierra Nevada (McConnell et al. 1998; Hageman et al. 2006). The relatively high concentrations of organophosphorus insecticides (chlorpyrifos, malathion, diazinon) in these samples reflect heavy use of these pesticides in California’s Central Valley, which lies directly west of the Sierra Nevada. Endosulfan and γ-HCH also have been detected in snow from the Canadian Rockies and mountainous regions of Europe in concentrations similar to those reported for ROMO and the Sierra Nevada.
Of the banned OCCs, chlordane, HCB, and α-HCH have been commonly detected in snow samples from ROMO and the Sierra Nevada by Hageman et al. (2006). By contrast, HCB and trans-nonachlor were detected in only one snow sample in our study, again probably because of MRL differences. HCB also has been reported in snow from mountainous areas in Europe in concentrations (0.007 to 0.048 ng/L) similar to those measured in snow (0.007 to 0.072 ng/L) from ROMO and the Sierra Nevada. α-HCH was detected in precipitation from the Western United States, Canada, and Europe in concentrations ranging from 0.06 to 7.5 ng/L. Interestingly, concentrations were 5 to 10 times higher in precipitation from Sequoia National Park (McConnell et al. 1998) compared with other locations, perhaps reflecting the proximity of this park to Central Valley sources. PCBs have been detected in similar concentrations in snow collected in the Canadian Rockies (0.30 to 2.17 ng/L) and in Europe (0.2 to 2.2 ng/L). Other OCCs reported by these studies include dieldrin, heptachlor epoxide, and DDT.
Atmospheric Deposition Rates
Deposition rates for selected CUPs in ROMO were estimated by combining results from summer rain and winter snowpack. Volume-weighted mean concentrations of atrazine, carbaryl, and dacthal in summer rain at Bear Lake were 22, 27, and 6 ng/L, respectively. Average concentrations of atrazine, carbaryl, and dacthal in snow from ROMO were 1.0, 0.4, and 0.9 ng/L, respectively (nondetected concentrations were set to one half the MRL to compute an upper estimate of the average in snow). Using the long-term (1981 to 2005) annual precipitation amount at Bear Lake (http://www.wcc.nrcs.usda.gov/snow/), 49 cm in winter (November to April) and 36 cm in summer (May to October), annual deposition rates of atrazine, carbaryl, and dacthal at this site were estimated as 8.4, 9.9, and 2.6 μg/m2, respectively. Deposition during summer was 7.9 μg/m2 for atrazine, 9.7 μg/m2 for carbaryl, and 2.2 μg/m2 for dacthal and during winter was 0.5 μg/m2 for atrazine, 0.2 μg/m2 for carbaryl, and 0.4 μg/m2 for dacthal. Hageman et al. (2006) estimated a winter flux of 1.0 μg/m2 for dacthal at Mills Lake (elevation 3056 m) near Bear Lake in 2003. These results reveal that 85% to 98% of pesticide deposition at Bear Lake occurred during the summer months, primarily because of high concentrations in summer rain. Pesticide concentrations in air and precipitation generally are highest in spring and summer, coinciding with pesticide application times and warmer temperatures, which increase revolatilization from soil and plant surfaces (Majewski & Capel 1995). These results highlight the importance of year-round monitoring to improve estimates of pesticide deposition rates to high-elevation environments.
Concentrations in Lake Sediment
Surface sediments (top 3 cm) from all 10 lakes sampled in ROMO had detectable concentrations of DDE, ranging from 0.62 to 4.7 μg/kg (Table 5). Eight lakes also had detectable DDD, but only Black Lake had detectable DDT. In GLAC, 9 of 10 lakes had detectable concentrations of DDE; 2 lakes had detectable DDD; and none had detectable DDT. Sediment concentrations of DDE and DDD were not related to lake elevation, surface area or depth, or lake location relative to the Continental Divide. The only notable spatial pattern was higher DDE and DDD concentrations in ROMO compared with GLAC (Fig. 3), which might reflect closer proximity of ROMO to urban and agricultural source compared with GLAC. DDE and DDD concentrations in surface sediments of all lakes were below the probable effect concentrations of 31.3 μg/kg for DDE and 28.0 μg/kg for DDD, which are the concentrations above which harmful effects for benthic organisms are likely to be observed (MacDonald et al. 2000). DDE in five lakes were near or above its threshold effect concentration (TEC) of 3.16 μg/kg, but DDD exceeded its TEC of 4.88 μg/kg in only one lake. The TEC is a level below which harmful effects for benthic organisms are unlikely to be observed (MacDonald et al. 2000). Other OCCs detected in lake sediments included chlordane components trans-chlordane and cis- and trans-nonachlor, which ranged from 0.10 to 1.0 μg/kg. The TEC for total chlordane is 3.24 μg/kg (MacDonald et al. 2000). Based on published chlordane compositional information (Dearth & Hites 1991), we estimated a TEC of 0.91 μg/kg for the sum of the three detected components compared with the 2.6 μg/kg summed concentration at Ypsilon Lake in ROMO. HCB and two PCB congeners (180 and 187) also were detected but only in one sample each.
DDE and DDD are degradation products of DDT, which is a well-documented, persistent organochlorine insecticide that has been banned from use in the United States since 1972. DDT and its degradates are of concern because they are endocrine-disrupting compounds that accumulate in the food chain and persist in the environment for long periods of time (Tyler et al. 1998). Because the lakes in ROMO and GLAC are remote and there is no reported use of DDT in either park, the presence of DDE and DDD in recent lake sediment is likely the result of long-range atmospheric transport and subsequent wet and dry deposition to the lake and watershed. Recent air-quality studies in North America suggest that DDT in the atmosphere is from an aged source such as volatilization from agricultural or forest soils that were contaminated in the past (Shen et al. 2005). Another possibility is long-range transport from distant sources in Central America or Asia where some banned compounds are still in use (Shen et al. 2005; Alegria et al. 2006). DDE is also an impurity in dicofol, a current-use pesticide applied in Colorado and Montana.
Although DDD and DDE were detected in most surface sediments, the concentrations were very low and probably pose little threat to aquatic organisms. It is possible, however, that concentrations of these compounds may increase with sediment depth (and age) because of greater use in the past. Van Metre and Mahler (2005) found significant decreases in sediment DDT, DDE, and PCB concentrations between 1970 and 2001 in sediment cores collected from lakes across the United States, reflecting the discontinued use of these compounds in North America during the past 3 decades. As part of a separate USGS study in 1999, a sediment core was collected from Mills Lake (located 2 km downstream from Black Lake in ROMO), which was age dated and analyzed for OCCs (P. C. Van Metre, USGS, 2000, unpublished data). DDD and DDE concentrations in the Mills Lake sediment core show distinct increases with depth (Fig. 4), with the highest concentrations measured in sediments deposited during the 1970s (4.0 μg/kg DDE and 9.7 μg/kg DDD), which correlates with its ban from use in North America in 1972. The results at Mills Lake suggest that concentrations of some compounds measured in this study likely are higher at greater depths in undisturbed bottom sediments of park lakes. These results also suggest that atmospheric deposition of DDT and possibly other banned OCCs to high-elevation areas in the Rocky Mountains has been decreasing since the 1970s.
Of the CUP method compounds, only endosulfan sulfate and dacthal were detected in lake sediments. Endosulfan sulfate was detected in six of the eight sediments analyzed, with highest concentrations measured in Black Lake (0.44 μg/kg) and Lake Ypsilon (1.2 μg/kg), located on the east side of ROMO. Concentrations in the GLAC lakes were lower, ranging from 0.12 to 0.15 μg/kg. Endosulfan in bottom sediment has been listed as toxic to aquatic invertebrates (Leonard et al. 2001) but currently there are no established sediment guidelines for aquatic life. Nearly all the snow samples also had detectable concentrations of endosulfan, indicating that the pesticide is entering park lakes by way of atmospheric deposition. Compared with snow, only endosulfan sulfate was detected in sediments, suggesting that the parent endosulfan isomers are metabolized in the lake sediments. Endosulfan sulfate typically is more persistent in aqueous environments than either parent isomer because of its longer half-life in water and soil (Peterson & Batley 1993). Dacthal was detected in half of the sediments analyzed in concentrations ranging from 0.11 to 0.26 μg/kg (Table 5). Dacthal is considered a general hazard to fish although there are no established guidelines for sediment concentrations. Compared with endosulfan and dacthal, other CUPs commonly detected in precipitation (atrazine, carbaryl, and chlorothalonil) were not detected in lake sediments. This pattern is reasonable because endosulfan and dacthal are more hydrophobic and persistent than many other CUPs and therefore have a higher potential to accumulate in sediment and aquatic biota (Nowell et al. 1999).
To date, few studies have been conducted of pesticides and other organic contaminants in lake or stream sediment in mountainous areas in North America against which to compare the results of this study. Streambed sediment and whole-body fish samples from two locations in ROMO were collected as part of the USGS National Water-Quality Assessment Program (Stephens & Deacon, 1997; Tate and Heiny 1996). None of the 33 OCCs and PCBs analyzed for were detected in sediment, and only DDE was detected in fish tissue. Heit et al. (1984) detected PCB concentrations ranging from 21 to 540 μg/kg in surface sediments (0 to 2 cm) from four lakes in ROMO, including Lake Haiyaha. The PCB concentration of 160 μg/kg for Lake Haiyaha reported by Heit et al. (1984) was much higher than the detection level value (<15 μg/kg) reported in this study, which may reflect a 20-year difference in the age of the surface sediments analyzed. Several OCCs were measured in a sediment core from Wonder Lake in Denali National Park in Alaska (Gubala et al. 1995). DDE and DDT were detected at concentrations of 0.32 and 0.15 μg/kg in lake sediments deposited after 1950 (Gubala et al. 1995), which is in the range of concentrations measured in ROMO and GLAC. Other OCCs detected in sediments from Wonder Lake included chlordane, α-HCH, HCB, and several PCB congeners.
Summary
This study reports concentrations of CUPs and banned OCCs in precipitation (snow and rain) and lake sediments from two national parks in the Rocky Mountain region. The most frequently detected CUPs in snow were dacthal, endosulfan, and chlorothalonil. Of the OCCs, HCB, trans-chlordane, and two PCB congeners were detected but only in one snow sample each. The concentrations of the detected pesticides in snow were not related to site elevation or location relative to the Continental Divide, which may suggest that cold condensation is not a major influence on pesticide distributions among our sites. Atrazine, carbaryl, and dacthal were frequently detected in rain at concentrations substantially higher than in snow. The most reasonable source of organic contaminants in precipitation is regional to long-range atmospheric transport from surrounding agricultural and urban areas. Estimated annual deposition rates of atrazine, carbaryl, and dacthal in ROMO were 8.4, 9.9, and 2.6 μg/m2, respectively. Deposition during summer months was substantially greater than during winter, which emphasizes the importance of year-round monitoring to improve estimates of pesticide deposition to high-elevation environments.
Lake sediments were analyzed for CUPs and OCCs because they are recognized as a primary reservoir of organic contaminants to fish. DDE and DDD were the most frequently detected OCCs in lake surface sediments. Concentrations (0.12 to 4.7 μg/kg) were below levels at which harmful effects for benthic organisms are likely to be observed. Slightly higher sediment concentrations were observed in ROMO compared with GLAC, which may reflect closer proximity of ROMO to urban and agricultural source areas. DDD and DDE concentrations in a dated sediment core from Mills Lake in ROMO showed increases with depth with the highest concentrations measured in sediments deposited during the 1970s, which correlates with DDT’s ban from use in 1972. This result suggests that atmospheric deposition of DDT and possibly other banned OCCs compounds to high-elevation parks has been in decline since their agricultural and industrial uses were discontinued. Dacthal and endosulfan sulfate were the only CUP method compounds detected in lake sediments in concentrations ranging from 0.11 to 1.2 μg/kg. Both compounds also were detected in precipitation, confirming that some CUPs entering aquatic ecosystems through atmospheric deposition are persistent enough to be accumulating in lake sediments and potentially in aquatic biota as well.
References
Alegria H, Bidleman TF, Figueroa MS (2006) Organochlorine pesticides in the ambient air of Chiapas, Mexico. Environ Pollut 140:483–491
Baron J (1992) Biogeochemistry of a subalpine ecosystem—Loch Vale Watershed. Springer Verlag, New York, NY, 247 pp
Blais JM (2005) Biogeochemistry of persistent bioaccumulative toxicants—Processes affecting the transport of contaminants to remote areas. Can J Fish Aquat Sci 62:236–243
Blais JM, Schindler DW, Muir DCG, Kimpe LE, Donald DB, Rosenberg B (1998) Accumulation of persistent organochlorine compounds in mountains of western Canada. Nature 395:585–588
Carrera G, Fernández P, Grimalt JO, Ventura M, Camarero L, Catalan J (2002) Atmospheric deposition of organochlorine compounds to remote high mountain lakes of Europe. Environ Sci Technol 36:2581–2588
Carrera G, Fernandez P, Vilanova RM, Grimalt JO (2001) Persistent organic pollutants in snow from European high mountain areas. Atmos Environ 35:245–254
Chernyak SM, Rice CP, McConnell LL (1996) Evidence of currently-used pesticides in air, ice, fog, seawater and surface microlayer in the Bering and Chukchi seas. Marine Pollut Bull 32:410–419
Dearth MA, Hites RA (1991) Complete analysis of technical chlordane using negative ionization mass spectrometry. Environ Sci Technol 25:245–254
Environment Canada (1999) Canadian water quality guidelines for the protection of aquatic life, summary tables. Available at: http://www.ec.gc.ca/ceqg-rcqe/water.htm. Accessed: 2005
Fellers GM, Mcconnell LL, Pratt D, Datta S (2004) Pesticides in mountain yellow-legged frogs (Rana muscosa) from the Sierra Nevada Mountains of California, USA. Environ Toxicol Chem 23:2170–2177
Finklin AI (1986) A climatic handbook for Glacier National Park with data for Waterton Lakes National Park. United States Forest Service General Technical Report INT–204, 124 pp
Garbarino JR, Snyder-Conn E, Leiker TJ, Hoffman GL (2002) Contaminants in Arctic snow collected over northwest Alaskan sea ice. Water Air Soil Pollut 139:183–214
Goolsby DA, Thurman EM, Pomes ML, Meyer MT, Battaglin WA (1997) Herbicides and their metabolites in rainfall: Origin, transport, and deposition patterns across the midwestern and northeastern United States. Environ Sci Technol 31:1325–1333
Grimalt JO, Fernandez P, Berdie L, Vilanova RM, Catalan J, Psenner R, et al. (2001) Selective trapping of organochlorine compounds in mountain lakes of temperate areas. Environ Sci Technol 35:2690–2697
Gubala C, Landers DH, Monetti M, Heit M, Lasora B, Allen-Gil S (1995) The rates of accumulation and chronologies of atmospherically derived pollutants in Arctic Alaska, USA. Sci Total Environ 160/161:347–361
Hageman KJ, Simonich SL, Campbell DH, Wilson GR, Landers DH (2006) Atmospheric deposition of current-use and historic-use pesticides in snow at national parks in the Western United States. Environ Sci Technol 40:3174–3180
Harman-Fetcho JA, McConnell LL, Rice CP, Baker JE (2000) Wet deposition and air-water gas exchange of currently used pesticides to a subestuary of the Chesapeake Bay. Environ Sci Technol 34:1462–1468
Heit M, Klusek C, Baron J (1984) Evidence of deposition of anthropogenic pollutants in remote Rocky Mountain lakes. Water Air Soil Pollut 22:403–416
Ingersoll GP, Turk JT, Mast MA, Clow DW, Campbell DH, Bailey ZC (2002) Rocky Mountain Snowpack Chemistry Network—History, methods, and the importance of monitoring mountain ecosystems. United States Geological Survey Open-File Report 01–466
James RR, Hites RA (1999) Chlorothalonil and dacthal in Great Lakes air and precipitation samples. J Great Lakes Res 25:406–411
Johnson W, Finley MT (1980) Handbook of acute toxicity of chemicals to fish and aquatic invertebrates. Resource Publication 137. United States Department of Interior, Fish and Wildlife Service, Washington, DC
Killin RK, Simonich SL, Jaffe DA, De Forest CL, Wilson GR (2004) Trans-Pacific and regional atmospheric transport of anthropogenic semi-volatile organic compounds to Cheeka Peak Observatory during the spring of 2002. J Geophys Res 109:D23-S15 doi:10.1023/2003JD004388
Kimbrough RA, Litke DW (1997) Pesticides in surface water in agricultural and urban areas of the South Platte River Basin, from Denver, Colorado, to North Platte, Nebraska, 1993–94, 98. U.S. Geological Survey Water-Resources Investigations Report 97–4230
Lei YD, Wania F (2004) Is rain or snow a more efficient scavenger of organic chemicals? Atmos Environ 38: 3557–3571
Leonard AW, Hyne RV, Lim RP, Leigh KA, Le J, Beckett R (2001) Fate and toxicity of endosulfan in Namoi River water and bottom sediment. J Environ Qual 30:750–759
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 3:20–31
Mackay D, Shiu WY, Ma KC (1997) Illustrated handbook of physical-chemical properties and environmental fate for organic chemicals—Pesticide chemicals. Lewis Publishers, Boca Raton, FL
Majewski MS, Capel PD (1995) Pesticides in the atmosphere—Pesticides in the hydrologic system. Ann Arbor Press, Chelsea, MI
Majewski MS, Foreman WT, Goolsby DA (2000) Pesticides in the atmosphere of the Mississippi River Valley, part I—Rain. Sci Total Environ 248:201–212
Mast MA, Campbell DH, Ingersoll GP, Foreman WT, Krabbenhoft DP (2003) Atmospheric deposition of nutrients, pesticides, and mercury in Rocky Mountain National Park, Colorado. United States Geological Survey Water-Resources Investigations Report 2003–4241
Mast MA, Foreman WT, Skaates S (2006) Organochlorine compounds and current-use pesticides in snow and lake sediment from Rocky Mountain National Park, Colorado and Glacier National Park, Montana, 2002–03. United States Geological Survey Scientific Investigations Report 2006–5991
McConnell LL, LeNoir JS, Datta S, Seiber JN (1998) Wet deposition of current-use pesticides in the Sierra Nevada Mountain Range, California, USA. Environ Toxicol Chem 17:1908–1916
Muir DCG, Teixeira C, Wania F (2004) Empirical and modeling evidence of regional atmospheric transport of current-use pesticides. Environ Toxicol Chem 23:2421–2432
National Park Service (2005) Western Airborne Contaminant Assessment Project. Air Resources Division, Fact Sheet. Available at: http://www2.nature.nps.gov/air/studies/air_toxics/wacap.cfm. Accessed: 2005
Noriega MC, Wydoski DS, Foreman WT (2004) Methods of analysis by the Unites States Geological Survey National Water Quality Laboratory—Determination of organochlorine pesticides and polychlorinated biphenyls in bottom and suspended sediment by gas chromatography with electron-capture detection. United States Geological Survey Water-Resources Investigations Report 2003–4293
Nowell LH, Capel PD, Dileanis PD (1999) Pesticides in surface waters—Distribution, trends, and governing factors. Lewis, Boca Raton, FL
Peterson SM, Batley GE (1993) The fate of endosulfan in aquatic systems. Environ Pollut 82:143–152
Rice CP, Chernyak SM, Hapeman CJ, Bilboulian S (1997) Air-water distribution of endosulfan. J Environ Qual 26:1101–1106
Sandstrom MW, Stroppel ME, Foreman WT, Schroeder MP (2001) Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of moderate-use pesticides and selected degradates in water by C-18 solid-phase extraction and gas chromatography/mass spectrometry. United States Geological Survey Water-Resources Investigations Report 2001–4098
Schmidt WF, Bilboulian S, Rice CP, Fettinger JC, McConnell LL, Hapeman CJ (2001) Thermodynamic, spectroscopic, and computational evidence for the irreversible conversion of beta- to alpha-endosulfan. J Agric Food Chem 49:5372–5376
Shen L, Wania F, Lei YD, Teixeira C, Muir DCG, Bidleman TF (2005) Atmospheric distribution and long-range transport behavior of organochlorine pesticides in North America. Environ Sci Technol 39:409–420
Simonich SL, Hites RA (1995) Global distribution of persistent organochlorine compounds. Science 269:1851–1854
Stephens VC, Deacon JR (1997) Distribution and concentrations of selected organochlorine pesticides and PCB’s in streambed sediment and whole-body fish in the Upper Colorado River, 1995–96. United States Geological Survey Fact Sheet 167–97
Sun P, Backus S, Blanchard P, Hites RA (2006) Temporal and spatial trends of organochlorine pesticides in Great Lakes precipitation. Environ Sci Technol 40:2135–2141
Tate CM, Heiny JS (1996) Occurrence and distribution of pesticides in bed sediment and fish tissue in the Upper Colorado River Basin, Colorado, 1995–96. Arch Environ Contam Toxicol 30:62–78
Thurman EM, Cromwell AE (2000) Atmospheric transport, deposition, and fate of triazine herbicides and their metabolites at Isle Royale National Park. Environ Sci Technol 34:3079–3085
Tuduri L, Harner T, Blanchard P, Li YF, Poissant L, Waite DT, et al. (2006) A review of currently used pesticides (CUPs) in Canadian air and precipitation: Part 1: Lindane and endosulfans. Atmos Environ 40:1563–1578
Tyler CR, Jobling S, Sumpter JP (1998) Endocrine disruption in wildlife—A critical review of the evidence. Crit Rev Toxicol 28:319–361
Van Metre PC, Mahler BJ (2005) Trends in hydrophobic organic contaminants in urban and reference lake sediments across the United States, 1970–2001. Environ Sci Technol 39:5567–5574
Wania F, Mackay D (1996) Tracking the distribution of persistent organic pollutants. Environ Sci Technol 30:390A–396A
White LM, Ernst WR, Julien G, Garron C, Leger M (2006) Ambient air concentrations of pesticides used in potato cultivation in Prince Edward Island, Canada. Pest Manag Sci 62:126–136
Zaugg SD, Sandstrom MW, Smith SG, Fehlberg KM (1995) Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of pesticides in water by C-18 solid-phase extraction and capillary-column gas chromatography/mass spectrometry with selected-ion monitoring. United States Geological Survey Open-File Report 95–181
Acknowledgments
Field assistance for the project was provided by D. Campbell, D. Clow, K. Holzer, G. Ingersoll, D. Manthorne, E. Mast, L. Nanus, B. Reardon, and J. Winterringer. Analytical assistance was provided by L. Greaser, C. Lindley, J. Madsen, D. Markovchick, L. Oasheim, and F. Wiebe. Funding was provided by the USGS/NPS Water Quality Partnership Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mast, M.A., Foreman, W.T. & Skaates, S.V. Current-Use Pesticides and Organochlorine Compounds in Precipitation and Lake Sediment from Two High-Elevation National Parks in the Western United States. Arch Environ Contam Toxicol 52, 294–305 (2007). https://doi.org/10.1007/s00244-006-0096-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-006-0096-1