Abstract
Bacterial systems have drawn an increasing amount of attention on lignin valorization due to their rapid growth and powerful environmental adaptability. In this study, Klebsiella pneumoniae NX-1, Pseudomonas putida NX-1, and Ochrobactrum tritici NX-1 with ligninolytic potential were isolated from leaf mold samples. Their ligninolytic capabilities were determined by measuring (1) the cell growth on kraft lignin as the sole carbon source, (2) the decolorization of kraft lignin and lignin-mimicking dyes, (3) the micro-morphology changes and transformations of chemical groups in kraft lignin, and (4) the ligninolytic enzyme activities of these three isolates. To the best of our knowledge, this is the first report that Ochrobactrum tritici species can depolymerize and metabolize lignin. Moreover, laccase, lignin peroxidase, and Mn-peroxidase showed high activities in P. putida NX-1. Due to their excellent ligninolytic capabilities, these three bacteria are important supplements to ligninolytic bacteria library and could be valuable in lignin valorization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Growing concerns on global climate change and energy security caused by fossil resource consumption increase the demand for renewable fuels and chemicals. Lignocellulose, the largest organic polymer reservoir in ecosystems, is a potential treasury alternative to fossil resources. The main components of lignocellulose are cellulose, hemicellulose, and lignin. Lignin and hemicellulose constitute a complex, three-dimensional structure that wraps cellulose to prevent solvents, catalysts, and organisms from being in contact with the cellulose (Asina et al. 2017; Ragauskas et al. 2014). With the advances of biorefinery technologies via biochemical routes, the cellulose and hemicellulose in lignocellulose can be converted to biofuels and various chemicals through pretreatment, enzymatic hydrolysis, and microbial fermentation. The remaining lignin is projected to be burned for powering the biorefinery plant (Humbird et al. 2011).
The U.S. Energy Security and Independence Act of 2007 mandated the production of 6 × 1010 gal of lignocellulosic biofuels annually by 2030. In this case, 7.5 × 108 tons of biomass will be consumed, and approximately 2.25 × 108 tons of lignin will be produced (Holladay et al. 2007). Moreover, lignin is also generated in pulp and paper-making processes at approximately 5 × 107 tons per year globally (Bruijnincx et al. 2015). Despite this huge production, lignin is still not be efficiently utilized and is mainly burned as a low-quality solid fuel and/or used as adhesive for flakeboard (Chandel et al. 2015). In addition, lignin waste stream is often directly discharged into the environment, thereby causing significant pollution. With further development of biofuels and other chemicals from lignocellulosic biomass, the production of lignin will increase. Thus, efforts should be urgently exerted for its valorization. Lignin valorization is believed to be the key for successful development of lignocellulosic biorefinery (Bruijnincx et al. 2015; Ragauskas et al. 2014).
By now, several strategies have emerged for lignin valorization, including thermochemical treatments, homogeneous catalysis, and heterogeneous catalysis (Farag and Chaouki 2015; Li et al. 2015). However, the multi-structure of lignin leads to diversiform product streams, which require extensive separation, purification, and recovery processes. Moreover, thermochemical treatments also require large amounts of energy to break the recalcitrant structure of lignin. The conversion of lignin by microorganisms is an alternative option for lignin valorization because this method enables low cost and generates environment friendly products (Hermosilla et al. 2017; Ragauskas et al. 2014). Although lignin is recalcitrant, it still can be decomposed by some microbes. Many studies have focused on lignin biodegradation by wood-rotting basidiomycetes, especially white-rot fungi, which have been investigated for decades with certain research achievements. However, few commercial lignin biodegradation processes by fungi have been developed. New processes are required for lignin valorization.
Recently, bacterial systems have drawn an increasing attention for lignin valorization due to their rapid growth, biochemical versatility, and powerful environmental adaptability. Some researchers even claimed that bacteria may play a leading role in lignin deterioration and degradation in the natural environment (Morii et al. 1995). In fact, bacteria that can depolymerize lignin have already been discovered in compost soil, rainforest, eroded bamboo slips, sludge of pulp paper mill, and intestines of wood-feeding insects (Bandounas et al. 2011; Chai et al. 2014; Mathews et al. 2016; Paliwal et al. 2015; Raj et al. 2007a, b; Suman et al. 2016; Tian et al. 2014). Some of these bacteria have already been used in bio-product synthesis, such as polyhydroxyalkanoate, lipid, vanillin, and other high-value compounds (Cannatelli and Ragauskas 2016; Lin et al. 2016; Shi et al. 2017; Shields-Menard et al. 2017). Lignin-degrading bacteria have also been applied in the treatment of sewages from rayon grade pulp industry (Yadav and Chandra 2015). However, most of these reported bacteria require additional carbon sources for cell growth and ligninolytic enzyme production, and their ligninolytic capability was much lower than that of fungi, which impedes their industrial application. Lignin valorization by bacteria is still in the research stage, and efficient bacteria that can biodegrade and valorize lignin are still needed to enrich the bacterial library (Chen and Wan 2017; Raj et al. 2007b).
Here, we report the isolation and characterization of three potential lignin-degrading bacteria from leaf mold samples. These bacteria exhibited rapid growth in a medium that contains kraft lignin as the sole carbon source. The lignin-degrading capability of these three isolates was determined, and their activities of ligninolytic enzymes were also analyzed. The isolates obtained will be valuable in lignin valorization due to their excellent growth capability and powerful ligninolytic enzymes.
Materials and methods
Isolation and identification of lignin-degrading bacteria
Leaf mold samples of the Purple Mountain (118° 50′ E, 32° 04′ N) and Laoshan National Forest Park (118° 30′ E, 30° 40′ N) were collected to screen lignin-degrading bacteria. In brief, 1 g sample was dissolved in 100 mL sterile saline solution (0.9% NaCl) to form a soil solution. Afterward, 1 mL soil solution was added into 50 mL modified M9 medium (MM9 medium), which consisted of Na2HPO4 12.8 g/L, KH2PO4 3.0 g/L, NaCl 0.5 g/L, (NH4)2SO4 0.5 g/L, and kraft lignin 10 g/L. The culture broth was cultivated aerobically at 30 °C, 200 rpm for 24 h, and then, 1 mL culture broth was trans-inoculated into a fresh MM9 medium. After repeating this process for 7 days, the final culture broth was diluted 10−9 times for single-colony plotting on a agar plate. The obtained single colonies were re-streaked at least three times to obtain a pure colony for strain identification and ligninolytic activity characterization.
The micro-morphology and 16s rRNA gene of the isolates were determined to verify the identity of the screened isolates. The micro-morphology of the screened isolates was observed via scanning electron microscopy (SEM) (JEOL, JSM-6380, Japan). The target nucleotide fragments for 16s rRNA gene identification were amplified with bacterial universal primers 27F (AGAGTTTGATCCTGGCTCAG) and 1492R (GGTTACCTTGTTACGACTT). The obtained sequences were aligned with the multiple sequence alignment software CLUSTALW X2 and the BLAST sequence analysis tool (http://blast.ncbi.nlm.nih.gov/). The phylogenetic tree was constructed by the neighbor-joining method using Mega 6.0.
Determination of bacterial growth and lignin-degrading capability
Cell growth and lignin degradation were initially determined to test the ligninolytic activity of the screened bacteria. A loop of single colony was inoculated into LB medium and incubated for 12 h at 30 °C, 200 rpm. The culture broth was sequentially centrifuged for 5 min at 4000 rpm to collect the bacterial cells. The cell pellets were washed with sterile saline solution (0.9% NaCl) three times. The washed cells were inoculated into MM9 medium (containing 1 g/L kraft lignin) with the initial OD600 = 0.1. The lignin biodegradation experiment was performed at 30 °C, 200 rpm for 7 days. In brief, 1 mL sample was withdrawn every 24 h to measure cell growth and lignin degradation. Cell growth was determined by colony-forming unit (CFU) counts instead of OD600 determination because there was still an absorption of the MM9 medium without bacteria at 600 nm (OD600 was approximately 0.228 when 1 g/L lignin was applied). Cell growth in MM9 medium that contains glucose or sucrose instead of kraft lignin was used as the control. Lignin degradation was evaluated by monitoring the decrease in A280 with a TU-1810 spectrophotometer (Purkinje General Instrument Co., Ltd., Beijing), and an uninoculated medium was used as the control (Chai et al. 2014; Kumar et al. 2015; Raj et al. 2007a). Degradation ratio was calculated by the equation:\( \mathrm{Degradation}\%=\frac{A_{\mathrm{control}}-{A}_{\mathrm{sample}}}{A_{\mathrm{control}}}\times 100\% \). All of the experiments were conducted in triplicates.
Characterization of the decolorization capability for aromatic dyes
The capability of the screened isolates to decolorize the aromatic dyes whose structures are close to lignin fragments was determined to further understand their role in lignin degradation. The following dyes were selected: Malachite Green (MG), Methylene Blue (MB), Remazol Brilliant Blue R (RBBR), Toluidene Blue O (TB), and Azure B (AB) (Fig. S1). Dye decolorization by the isolates was conducted following the method of Bandounas et al. with slight modification (Bandounas et al. 2011). Each of the isolates was separately inoculated in LB medium and cultivated at 30 °C, 200 rpm until OD600 = 1.0. Different lignin-mimicking dye solutions were added into the culture broth to achieve the final concentration of 50 mg/L. The cultivations were maintained for another 72 h. The LB medium without bacteria was used as the blank control for spontaneous dye decolorization. At the end of the incubation, the samples were centrifuged at 12,000 rpm for 10 min and the supernatants were used to determine aromatic dyes decolorization. The decolorization of a specific dye was measured and calculated at λmax (MG, 470 nm; MB, 665 nm; RBBR, 595 nm; TB 635 nm; AB, 650 nm). The decolorization ratio was calculated with the same method of lignin degradation as follows: \( \mathrm{Decolorization}\%=\frac{A_{\mathrm{control}}-{A}_{\mathrm{sample}}}{A_{\mathrm{control}}}\times 100\% \) (Tian et al. 2016).
SEM and Fourier transform infrared spectroscopy analysis
The changes in morphology and chemical bonds of lignin were observed with SEM and Fourier transform infrared spectroscopy (FTIR), respectively, to visually confirm the depolymerization of lignin by the isolates. Samples were withdrawn after 7 days of incubation in MM9 medium. The samples were centrifuged at 12,000 rpm for 10 min to remove bacterial cells. Then, the supernatant was dried to constant weight with a vacuum freeze-drying apparatus (LGJ-10D, Four-Ring Science Instrument Beijing Co., Ltd., China). The dried powder samples were used for SEM and FTIR analysis. The morphology of related samples was examined via SEM (JEOL, JSM-6380, Japan). FTIR spectra were measured in the 4000–400-cm−1 region by a Nicolet iS5 FTIR spectrometer using an iD7 diamond attenuated total reflectance optical base with a resolution of 1.0 cm−1. In the SEM and FTIR analysis, the untreated lignin and the lignin treated by incubation without bacteria were applied as the controls.
Enzyme assays
As the isolates were all cellular bacterium, the kraft lignin cannot be taken up into the cell directly. Therefore, these bacteria had to secrete extracellular ligninolytic enzymes to degrade lignin. Thus, the extracellular enzyme activities of the isolates were assayed. Laccase (Lac) activity was determined by monitoring the oxidation of 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) to ABTS radical at 420 nm (ε420 = 36,000 mol−1 cm−1). The reaction mixture (1 mL) contained 50 mM HAc-NaAc (pH 4.5), 1 mM ABTS, and 100 μL cell-free supernatant (Sakamoto et al. 2008). Lignin peroxidase (LiP) activity was determined by monitoring the oxidation of veratryl alcohol to veratraldehyde at 310 nm (ε310 = 9300 mol−1 cm−1). The reaction mixture (1 mL) contained 50 mM HAc-NaAc (pH 4.5), 1 mM veratryl alcohol, and 100 μL cell-free supernatant (Kapich et al. 2004). Mn-peroxidase (MnP) activity was determined by monitoring the oxidation of 2,6-dimethyl phenol (2,6-DMP) to coerulignone at 469 nm (ε469 = 49,600 mol−1 cm−1). The reaction mixture (1 mL) contained 50 mM HAc-NaAc (pH 4.5), 1 mM MnSO4, 0.5 mM 2,6-DMP, and 100 μL cell-free supernatant (Kapich et al. 2004). The reactions of LiP and MnP were started with 0.1 mM H2O2. One unit of enzyme activity was defined as the amount of enzyme required to produce 1 μmol product per min. All assays were performed in triplicate.
Results and discussion
Isolation and identification of lignin-degrading bacteria
The microorganisms living in leaf molds have been identified as fast decomposers of plant materials. Rapid nutrient cycling of dead wood and leaf litter by microorganisms is thought to support the bio-diversity of forests (Carles et al. 2017). Thus, we attempted to screen for bacteria with high ligninolytic capacity from the leaf mold samples. To obtain lignin-degrading bacteria, 10 g/L kraft lignin was added in MM9 medium. The microorganisms that cannot depolymerize kraft lignin and utilize the degradation compounds as the carbon and energy sources would be weeded out because no other carbon source exists in the medium. Furthermore, the high concentration of kraft lignin can also eliminate most microorganisms that cannot tolerate aromatic compounds derived from lignin decomposition because these aromatic compounds can induce membrane disruption, enzyme inhibition, and DNA damage (Zeng et al. 2014). After 7-day repeated cultivation, culture broth was diluted for 10−9 times and plated on a MM9 agar plate and the three most abundant bacteria were obtained based on colonial morphology difference. To identify the species of the screened isolates, the 16S rRNA gene sequences were determined and submitted to NCBI with accession numbers of MF093730, KY593170, and MF093751. On the basis of 16S rRNA gene sequence homology, these three isolates were closely matched to Klebsiella pneumoniae strain CAV1042 (99%), Pseudomonas putida strain PC2 (99%), and Ochrobactrum tritici TA93 (99%) (Table 1). To further confirm the species of the screened bacteria, the micro-morphologies of the isolates were observed by SEM (Fig. S2), and the result was consistent with that of 16S rRNA gene blast. Thus, these three isolates were identified and named as Klebsiella pneumoniae NX-1, Pseudomonas putida NX-1, and Ochrobactrum tritici NX-1 (Fig. 1).
K. pneumoniae, a gram-negative bacterium that belongs to γ-Proteobacteria, is widely distributed in the environment. In industrial biotechnology, K. pneumoniae is an important strain for many bio-products, such as 2,3-butanediol, muconic acid, and some exopolysaccarides. To the best of our knowledge, only one study was conducted on lignin degradation by K. pneumoniae, which was co-cultivated with a Bacillus subtilis (Yadav and Chandra 2015). However, several studies reported its degradation capability of aromatic substances, such as N,N′-dimethyl-p-phenylenediamine, herbicide bromoxynil, and methyl red (Wong and Yuen 1996, 1998). The degradation capability of K. pneumoniae for aromatic substances indicated that this species contains abundant oxidoreductases, which are also important components for lignin degradation. P. putida is commonly considered as an innocuous environmental microorganism with an enormous potential for biotechnological applications due to its well-developed genetic system and metabolic versatility. P. putida has been applied to produce various bio-products, such as arginine deiminase, biosurfactant, and indigoids (Patil et al. 2017). Except for synthesizing many valuable bio-products, P. putida has also been applied to degrade multitudinous hazardous compounds, especially heterocyclic compounds and aromatic compounds, for example, nicotine, lignin, phenol, polycyclic pesticide, methyl parathion, and γ-hexachlorocyclohexane (Lin et al. 2016; Ravi et al. 2017). Despite limited available reports, some researchers have found the powerful capability of O. tritici to biodegrade pyrethroids and nicotine (Wang et al. 2011; Yu et al. 2015). As an opportunistic pathogen of wheat, the pathogenicity of O. tritici may be greatly correlated with its lignin-degrading capability because lignin is a critical protective layer for protecting plants from being damaged by microorganisms and insects. To the best of our knowledge, this is the first report with O. tritici species capable of degrading lignin.
The lignin-degrading bacteria reported in the literature fall into several classes: Proteobacteria, Actinobacteria, Firmicutes, Archaea, and some Bacteroidetes. These bacteria were isolated from compost soil, sediments, sludge of pulp paper mill, eroded bamboo slips, and insect guts. Among these strains, Bacillus subtilis, Paenibacillus sp., K. pneumonia, and Aneurinibacillus aneurinilyticus have been applied for the treatment of pulp mill effluent (Mathews et al. 2014; Raj et al. 2007a; Yadav and Chandra 2015). Some strains have already been applied in pretreatment to accelerate cellulase performance (Bugg et al. 2011; Chang et al. 2014), and some bacteria can even produce valuable compounds from lignin (Johnson and Beckham 2015; Vardon et al. 2015; Zhao et al. 2016). These studies illustrated the abilities of bacteria in lignin treatment. We believe that the screened isolates in this study are excellent additions to the knowledge of lignin valorization by bacteria.
Cell growth of the isolates using lignin as the sole carbon source
As reported, the lignin-degrading bacteria could be classified into two categories based on their carbon source utilization: those that could utilize lignin as the sole carbon source for cell growth and other physiological metabolic activities, and those that require additional carbon sources for cell growth and lignin decomposition (Bandounas et al. 2011). In this study, no additional carbon source in MM9 medium is available, and thus, all isolates could utilize lignin as the sole carbon source. To determine the cell growth of the isolates on lignin as the sole carbon source, the bacterial counts were determined in the 3rd and 7th days. As shown in Table 2, all of the three isolates reached 1010–1011 CFUs/mL in the MM9 medium. In previous study, lignin-degrading bacteria achieved only 105–109 CFUs/mL in the lignin media. For example, Rhodococcus opacus DSM 1069R achieved approximately 107 CFUs/mL in a medium that contains ethanol organosolv lignin (Kosa and Ragauskas 2013). Rhizobium sp. strain YS-1r achieved approximately 107 CFUs/mL in alkali lignin medium (Jackson et al. 2017). Pseudomonas sp. LD002 and Bacillus sp. LD003 achieved 109–1010 CFUs/mL in the lignin medium (Bandounas et al. 2011). Compared with those in previous studies, the three isolates in the present study exhibited advantage in cell growth when using lignin as the carbon source. The growth superiority of these three isolates indicated their potential industrial application. However, there is still a great room for improvement because these isolates’ cell density could reach as high as 1015–1016 CFUs/mL when glucose was used as the carbon source (Table 2).
Lignin and aromatic dye degradation by the screened bacteria
The decrease in A280 could be used to characterize the degradation of aromatic compounds due to the intense absorption of benzene ring at 280 nm. Thus, the A280 of incubation was detected daily to determine the rate of lignin degradation (Chai et al. 2014; Kumar et al. 2015; Raj et al. 2007a) (the standard curve of “Abs280 and lignin concentration” was presented in the supplementary materials (Fig. S3)). The lignin degradation results, which are illustrated as the reduction of A280, are shown in Fig. 2. A280 was reduced by 23.8, 28.5, and 19.4% during the 7-day incubation with K. pneumoniae NX-1, P. putida NX-1, and O. tritici NX-1, respectively. All of these three isolates rapidly degrade kraft lignin in the initial 4 days. This finding indicated that the isolates could break down lignin in the primary metabolic stage. The same result is also mentioned in the study of lignin degradation by Comamonas sp. (Chai et al. 2014). The loss of lignin content can be directly correlated with the cell growth of the isolates because no additional carbon source exists in the medium. Although the growth rates of the isolates slowed down in the 5th–7th days, considerable amount of lignin was degraded. This phenomenon occurred because these bacteria utilized lignin not only for their growth but also for other metabolic activities. Moreover, the slow degradation rates in the 5th–7th days are probably caused by the special obstinate components which cannot be degraded directly by the screened isolates.
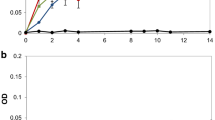
The decolorization of dyes, whose structure is close to that of lignin fragments, was used as indicator of lignin-degrading activity because dye decolorization is direct and easy to visualize. Among the five types of the aromatic dyes used in the experiments, MG showed a great extent of spontaneous dye decolorization (54.58%) in 72 h even without any bacteria. Spontaneous dye degradation was also observed by Bandounas (Bandounas et al. 2011; dos Santos et al. 2014), and the spontaneous dye degradation may be caused by pH and light fluctuation. The decolorization rates of the screened bacteria are shown in Fig. 3. In general, each isolate exhibited a certain degradation capability for MB, MG, TB, and AB. K. pneumoniae NX-1, P. putida NX-1, and O. tritici NX-1 degraded MG with maximum decolorization rate of 93.9, 87.7, and 93.1%, respectively. In particular, only K. pneumoniae NX-1 significantly decolorized RBBR (17.3%), indicating that some special enzymes exist in K. pneumoniae NX-1 as compared with P. putida NX-1 and O. tritici NX-1. The undecolorization for RBBR of P. putida NX-1 indicated that it lacked related enzymes for RBBR degradation. However, P. putida NX-1 must have other powerful enzymes for lignin degradation because it exhibited the most powerful degradation capability for lignin as compared with K. pneumoniae NX-1, and O. tritici NX-1. As mentioned in the introduction part, some bacteria display versatile metabolic pathways and various enzymes to degrade and metabolize heterocyclic compounds and aromatic compounds, from simple phenols to xenobiotic substances and complex lignins, and these bacteria are great treasures for target enzyme mining. Therefore, further research may focus on investigating the related genes and enzymes involved in the decolorization of lignin and related dyes in these three isolates. Moreover, these three isolates could also be applied in the decolorization and degradation of reactive textile dyes, similar to Phanerochaete chrysosporium (Koyani et al. 2013).
Micro-morphology and chemical group analysis for lignin degradation
To confirm the degradation of lignin visually, the change in its morphology was observed by SEM. The SEM images of the related kraft lignin are presented in Fig. 4. The figure exhibited that the untreated kraft lignin consisted of small balls, ovals, and spherical fragments (Fig. 4(a)), which have become irregular fragments after 7 days of incubation even without any bacterium (Fig. 4(b)). The particle sizes of lignin treated with the isolates were smaller compared with that of lignin without microbial treatment. The lignin incubated without isolates has large bigger particle sizes (within the range of 100–200 μm). When incubated with the isolates, the fragment size ranges of lignin were reduced evidently by different degrees. In particular, the fragment size of the lignin treated with K. pneumoniae NX-1 was reduced to 10–30 μm (Fig. 4(c)). The change in lignin fragment size indicated that lignin was physically and/or chemically decomposed into small parts. Thus, these three isolates were visually confirmed as capable of lignin degradation.
FTIR could exhibit a visualized and rapid indication of the changes in chemical bonds, and many researchers have investigated the structural changes of lignin given its infrared absorption characteristics (Chen et al. 2015; Liu et al. 2014). The FTIR spectra of related kraft lignin samples are presented in Fig. 5. The figure shows indicated that the appearance of lignin was changed from round to flakes when incubated in the liquid medium for 7 days. However, the change in its chemical bond was slight. Moreover, the FTIR spectra of kraft lignin changed evidently under the action of the isolates and the main change ranged from 1600 to 500 cm−1, especially at 1580, 1410, 1080, 858, and 618 cm−1. The band at 1580 cm−1 was attributed to the stretching vibration of aromatic rings (Chai et al. 2014); the band at 1410 cm−1 was attributed to carboxylic anionic groups, carbonate, and CH2 (Cohen and Gabriele 1982); the band at 1080 cm−1 may be attributed to C–O vibrations in aliphatic ethers and/or in secondary alcohols (Carvalho et al. 2008); the band at 858 cm−1 was assigned to the C–H out-of-plane vibrations of guaiacyl units (Wang et al. 2015); the band at 618 cm−1 was assigned to the stretching vibrations of the C–S bond linked to the aromatic ring (Rumyantseva et al. 1994). The variation in sizes and chemical groups is consistent with the decrease in A280 in the above experiment, further illustrating that lignin was chemically broken by the screened bacterial strains.
Analyses of enzyme activities related to lignin biodegradation
With the decrease in A280 of culture broth, the decolorization of aromatic dyes, and the variation in lignin sizes and chemical groups, there must be related enzymes secreted by the isolates. It is commonly accepted that Lac, LiP, and MnP are the three major enzymes involved in lignin-degrading processes. Lac is a class of multicopper oxidases that can catalyze the oxidation of a variety of organic compounds, including oxidative coupling of phenolic compounds, aryl-ring cleavage, and degradation of polymers (Huang et al. 2013). LiP is a hemeprotein which catalyzes the H2O2-dependent oxidation of lignin derivative-based polymers. Compared with the common LiP, MnP is a specific heme enzyme, whose catalysis depended on the oxidation of Mn2+ to Mn3+ (Xu et al. 2017). In this research, the cell-free supernatant was used to measure the ligninolytic enzyme activity. The activity of related ligninolytic enzymes from these three isolates is presented in Fig. 6. The results show that all three isolates secreted considerable reactive LiP under tested conditions. However, only P. putida NX-1 secreted Lac and MnP efficiently. For P. putita NX-1, the activities of Lac, LiP, and MnP reached the maximum values of 635.9, 6497.2, and 599.7 U/L, respectively, during the first day followed by evident decrease in the second day. Then, the related activity fluctuated in a definitive range in the 3rd–7th days, indicating that ligninolytic enzymes were secreted during the entire life cycle of P. putita NX-1, including the primary and secondary metabolism stages. However, P. putita NX-1 did not exhibit any capability to decolorize RBBR (Fig. 3), which could be degraded by Lac secreted by cyanobacteria and white-rot fungi. For K. pneumoniae NX-1, the activities of Lac and LiP were detected during the first 5 days, whereas low MnP activity was only detected in the 6th and 7th days. In contrast to that in P. putita NX-1 and K. pneumoniae NX-1, only high LiP activity was detected in O. tritici NX-1, and the Lac and MnP activities were extremely low during incubation. The enzyme activity assays revealed that P. putita NX-1 exhibited the best ligninolytic capability among the three isolates. The result of lignin morphology also yielded the same conclusion, because the lignin treated with P. putita NX-1 had the smallest particle size (Fig. 4(d)).
In previous studies, some research results indicated that bacteria cannot secrete extracellular ligninolytic enzymes and thus could only use the low molecular weight portion of lignin instead of depolymerizing lignin. However, in 1988, Ramachandra et al. reported that Streptomyces viridosporus T7A could secrete extracellular peroxidases for lignin degradation (Ramachandra et al. 1988). Since then, other ligninolytic enzymes, such as oxygen-utilizing Lac and MnP from Rhodococcus jostii RHA1 and Comamonas sp. B-9, were found successively (Chai et al. 2014; Chen et al. 2012). In some cases, the characteristics, such temperature-, pH-, and salt-tolerance, of bacterial-origin ligninolytic enzymes are superior to their fungal analogs and confirm that their potential industrial applications are intriguing. For example, some Lac found in Bacillus halodurans exhibited excellent characteristics in alkaline- and chloride-contaminated environment (Ruijssenaars and Hartmans 2004). Thus, it is interesting to characterize the ligninolytic enzymes of these three isolates and evaluate their applications in bio-bleaching, bio-pulping, bio-remediation, and other industrial processes in the future studies.
Conclusion
In this study, three lignin-degrading bacteria were isolated from leaf molds. All three isolates exhibited substantial growth on kraft lignin. The ligninolytic capabilities of the three isolates were evaluated, and their related ligninolytic enzyme activities were detected. The results showed that P. putida NX-1 exhibited the best lignin-degrading capability and could efficiently secrete all of Lac, LiP, and MnP. We believe that these three bacteria are important supplements to ligninolytic bacteria library due to their excellent ligninolytic capabilities, and these isolates may also be valuable in lignin valorization.
References
Asina F, Brzonova I, Kozliak E, Kubátová A, Ji Y (2017) Microbial treatment of industrial lignin: successes, problems and challenges. Renew Sust Energ Rev 77:1179–1205
Bandounas L, Wierckx NJ, de Winde JH, Ruijssenaars HJ (2011) Isolation and characterization of novel bacterial strains exhibiting ligninolytic potential. BMC Biotechnol 11:94
Bruijnincx PC, Rinaldi R, Weckhuysen BM (2015) Unlocking the potential of a sleeping giant: lignins as sustainable raw materials for renewable fuels, chemicals and materials. Green Chem 17:4860–4861
Bugg TD, Ahmad M, Hardiman EM, Singh R (2011) The emerging role for bacteria in lignin degradation and bio-product formation. Curr Opin Biotechnol 22:394–400
Cannatelli MD, Ragauskas AJ (2016) Conversion of lignin into value-added materials and chemicals via laccase-assisted copolymerization. Appl Microbiol Biotechnol 100:8685–8691
Carles L, Rossi F, Joly M, Besse-Hoggan P, Batisson I, Artigas J (2017) Biotransformation of herbicides by aquatic microbial communities associated to submerged leaves. Environ Sci Pollut Res 24:3664–3674
Carvalho SI, Otero M, Duarte AC, Santos EB (2008) Spectroscopic changes on fulvic acids from a kraft pulp mill effluent caused by sun irradiation. Chemosphere 73:1845–1852
Chai LY, Chen YH, Tang CJ, Yang ZH, Zheng Y, Shi Y (2014) Depolymerization and decolorization of kraft lignin by bacterium Comamonas sp. B-9. Appl Microbiol Biotechnol 98:1907–1912
Chandel AK, Gonçalves BC, Strap JL, da Silva SS (2015) Biodelignification of lignocellulose substrates: an intrinsic and sustainable pretreatment strategy for clean energy production. Crit Rev Biotechnol 35:281–293
Chang YC, Choi D, Takamizawa K, Kikuchi S (2014) Isolation of Bacillus sp. strains capable of decomposing alkali lignin and their application in combination with lactic acid bacteria for enhancing cellulase performance. Bioresour Technol 152:429–436
Chen Z, Wan C (2017) Biological valorization strategies for converting lignin into fuels and chemicals. Renew Sust Energ Rev 73:610–621
Chen YH, Chai LY, Zhu YH, Yang ZH, Zheng Y, Zhang H (2012) Biodegradation of kraft lignin by a bacterial strain Comamonas sp. B-9 isolated from eroded bamboo slips. J Appl Microbiol 112:900–906
Chen Y, Huang J, Li Y, Zeng G, Zhang J, Huang A, Zhang J, Ma S, Tan X, Xu W, Zhou W (2015) Study of the rice straw biodegradation in mixed culture of Trichoderma viride and Aspergillus niger by GC-MS and FTIR. Environ Sci Pollut Res 22:9807–9815
Cohen MS, Gabriele PD (1982) Degradation of coal by the fungi Polyporus versicolor and Poria monticola. Appl Microbiol Biotechnol 44:23–27
dos Santos TC, Zocolo GJ, Morales DA, de Aragão Umbuzeiro G, Zanoni MVB (2014) Assessment of the breakdown products of solar/UV induced photolytic degradation of food dye tartrazine. Food Chem Toxicol 68:307–315
Farag S, Chaouki J (2015) Economics evaluation for on-site pyrolysis of kraft lignin to value-added chemicals. Bioresour Technol 175:254–261
Hermosilla E, Schalchli H, Mutis A, Diez MC (2017) Combined effect of enzyme inducers and nitrate on selective lignin degradation in wheat straw by Ganoderma lobatum. Environ Sci Pollut Res 24:21984–21996. https://doi.org/10.1007/s11356-017-9841-4
Holladay JE, White JF, Bozell JJ, Johnson D (2007) Top value-added chemicals from biomass—volume II. Results of screening for potential candidates from biorefinery lignin (No. PNNL-16983). Pacific Northwest National Lab.(PNNL), Richland, WA (United States); National Renewable Energy Laboratory (NREL), Golden, CO (United States)
Huang XF, Santhanam N, Badri DV, Hunter WJ, Manter DK, Decker SR, Vivanco JM, Reardon KF (2013) Isolation and characterization of lignin-degrading bacteria from rainforest soils. Biotechnol Bioeng 110:1616–1626
Humbird D, Davis R, Tao L, Kinchin C, Hsu D, Aden A (2011) Process design and economics for biochemical conversion of lignocellulosic biomass to ethanol. NREL technical report, NREL/TP-5100-47764: 1–114
Jackson CA, Couger MB, Prabhakaran M, Ramachandriya KD, Canaan P, Fathepure BZ (2017) Isolation and characterization of Rhizobium sp. strain YS-1r that degrades lignin in plant biomass. J Appl Microbiol 122:940–952
Johnson CW, Beckham GT (2015) Aromatic catabolic pathway selection for optimal production of pyruvate and lactate from lignin. Metab Eng 28:240–247
Kapich AN, Prior BA, Botha A, Galkin S, Lundell T, Hatakka A (2004) Effect of lignocellulose-containing substrates on production of ligninolytic peroxidases in submerged cultures of Phanerochaete chrysosporium ME-446. Enzym Microb Technol 34(2):187–195
Kosa M, Ragauskas AJ (2013) Lignin to lipid bioconversion by oleaginous Rhodococci. Green Chem 15:2070–2074
Koyani RD, Sanghvi GV, Sharma RK, Rajput KS (2013) Contribution of lignin degrading enzymes in decolourisation and degradation of reactive textile dyes. Int Biodeterior Biodegrad 77:1–9
Kumar M, Singh J, Singh MK, Singhal A, Thakur IS (2015) Investigating the degradation process of kraft lignin by β-proteobacterium, Pandoraea sp. ISTKB. Environ Sci Pollut Res 22:15690–15702
Li C, Zhao X, Wang A, Huber GW, Zhang T (2015) Catalytic transformation of lignin for the production of chemicals and fuels. Chem Rev 115:11559–11624
Lin L, Cheng Y, Pu Y, Sun S, Li X, Jin M, Pierson EA, Gross DC, Dale BE, Dai SY, Ragauskas AJ, Yuan JS (2016) Systems biology-guided biodesign of consolidated lignin conversion. Green Chem 18:5536–5547
Liu Y, Hu T, Wu Z, Zeng G, Huang D, Shen Y, He X, Lai M, He Y (2014) Study on biodegradation process of lignin by FTIR and DSC. Environ Sci Pollut Res 21(24):14004–14013
Mathews SL, Pawlak JJ, Grunden AM (2014) Isolation of Paenibacillus glucanolyticus from pulp mill sources with potential to deconstruct pulping waste. Bioresour Technol 164:100–105
Mathews SL, Grunden AM, Pawlak J (2016) Degradation of lignocellulose and lignin by Paenibacillus glucanolyticus. Int Biodeter Biodegr 110:79–86
Morii H, Nakamiya K, Kinoshita S (1995) Isolation of a lignin-decolorizing bacterium. J Biosci Bioeng 80:296–299
Paliwal R, Uniyal S, Rai JPN (2015) Evaluating the potential of immobilized bacterial consortium for black liquor biodegradation. Environ Sci Pollut Res 22:6842–6853
Patil MD, Dev MJ, Tangadpalliwar S, Patel G, Garg P, Chisti Y, Banerjee UC (2017) Ultrasonic disruption of Pseudomonas putida for the release of arginine deiminase: kinetics and predictive models. Bioresour Technol 233:74–83
Ragauskas AJ, Beckham GT, Biddy MJ, Chandra R, Chen F, Davis MF, Davison BH, Dixon RA, Gilna P, Keller M, Langan P, Naskar AK, Saddler JN, Tschaplinski TJ, Tuskan GA, Wyman CE (2014) Lignin valorization: improving lignin processing in the biorefinery. Science 344:1246843
Raj A, Chandra R, Reddy MMK, Purohit HJ, Kapley A (2007a) Biodegradation of kraft lignin by a newly isolated bacterial strain, Aneurinibacillus aneurinilyticus from the sludge of a pulp paper mill. World J Microbiol Biotechnol 23:793–799
Raj A, Reddy MK, Chandra R, Purohit HJ, Kapley A (2007b) Biodegradation of kraft-lignin by Bacillus sp. isolated from sludge of pulp and paper mill. Biodegradation 18:783–792
Ramachandra M, Crawford DL, Hertel G (1988) Characterization of an extracellular lignin peroxidase of the lignocellulolytic actinomycete Streptomyces viridosporus. Appl Environ Microbiol 54(12):3057–3063
Ravi K, García-Hidalgo J, Gorwa-Grauslund MF, Lidén G (2017) Conversion of lignin model compounds by Pseudomonas putida KT2440 and isolates from compost. Appl Microbiol Biotechnol 101:5059–5070
Ruijssenaars HJ, Hartmans S (2004) A cloned Bacillus halodurans multicopper oxidase exhibiting alkaline laccase activity. Appl Microbiol Biotechnol 65:177–182
Rumyantseva YI, Zhbankov RG, Marhevka R, Rataiczak H (1994) IR spectra and structure of alkaline lignin and thiolignin. J Appl Spectrosc 61:699–703
Sakamoto Y, Nakade K, Yano A, Nakagawa Y, Hirano T, Irie T, Watanabe H, Nagai M, Sato T (2008) Heterologous expression of lcc1 from Lentinula edodes in tobacco BY-2 cells results in the production an active, secreted form of fungal laccase. Appl Microbiol Biotechnol 79:971–980
Shi Y, Yan X, Li Q, Wang X, Xie S, Chai L, Yuan J (2017) Directed bioconversion of Kraft lignin to polyhydroxyalkanoate by Cupriavidus basilensis B-8 without any pretreatment. Process Biochem 52:238–242
Shields-Menard SA, AmirSadeghi M, Green M, Womack E, Sparks DL, Blake J, Edelmann M, Ding X, Sukhbaatar B, Hernandez R, Donaldson JR, French TR (2017) The effects of model aromatic lignin compounds on growth and lipid accumulation of Rhodococcus rhodochrous. Int Biodeterior Biodegrad 121:79–90
Suman SK, Dhawaria M, Tripathi D, Raturi V, Adhikari DK, Kanaujia PK (2016) Investigation of lignin biodegradation by Trabulsiella sp. isolated from termite gut. Int Biodeterior Biodegrad 112:12–17
Tian JH, Pourcher AM, Bouchez T, Gelhaye E, Peu P (2014) Occurrence of lignin degradation genotypes and phenotypes among prokaryotes. Appl Microbiol Biotechnol 98:9527–9544
Tian JH, Pourcher AM, Peu P (2016) Isolation of bacterial strains able to metabolize lignin and lignin-related compounds. Lett Appl Microbiol 63:30–37
Vardon DR, Franden MA, Johnson CW, Karp EM, Guarnieri MT, Linger JG, Salm MJ, Strathmann TJ, Beckham GT (2015) Adipic acid production from lignin. Energy Environ Sci 8:617–628
Wang BZ, Ma Y, Zhou WY, Zheng JW, Zhu JC, He J, Li SP (2011) Biodegradation of synthetic pyrethroids by Ochrobactrum tritici strain pyd-1. World J Microbiol Biotechnol 27:2315–2324
Wang S, Ru B, Lin H, Sun W, Luo Z (2015) Pyrolysis behaviors of four lignin polymers isolated from the same pine wood. Bioresour Technol 182:120–127
Wong PK, Yuen PY (1996) Decolorization and biodegradation of methyl red by Klebsiella pneumoniae RS-13. Water Res 30:1736–1744
Wong PK, Yuen PY (1998) Decolorization and biodegradation of N, N′-dimethyl-p-phenylenediamine by Klebsiella pneumoniae RS-13 and Acetobacter liquefaciens S-1. Lett Appl Microbiol 85:79–87
Xu H, Guo MY, Gao YH, Bai XH, Zhou XW (2017) Expression and characteristics of manganese peroxidase from Ganoderma lucidum in Pichia pastoris and its application in the degradation of four dyes and phenol. BMC Biotechnol 17:19
Yadav S, Chandra R (2015) Syntrophic co-culture of Bacillus subtilis and Klebsiella pneumonia for degradation of kraft lignin discharged from rayon grade pulp industry. J Environ Sci 33:229–238
Yu H, Tang H, Li Y, Xu P (2015) Molybdenum-containing nicotine hydroxylase genes in a nicotine degradation pathway that is a variant of the pyridine and pyrrolidine pathways. Appl Environ Microbiol 81:8330–8338
Zeng Y, Zhao S, Yang S, Ding SY (2014) Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels. Curr Opin Biotechnol 27:38–45
Zhao C, Xie S, Pu Y, Zhang R, Huang F, Ragauskas AJ, Yuan JS (2016) Synergistic enzymatic and microbial lignin conversion. Green Chem 18:1306–1312
Acknowledgements
This work was supported by the National Key R&D Program of China (grant number 2016YFE0105400), National Natural Science Foundation of China (grant number 21606132), Natural Science Foundation of Jiangsu Province (grant numbers BK20160823 and BK20170829), and Fundamental Research Funds for the Central Universities (grant numbers 30916011202 and 30917011307).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 780 kb)
Rights and permissions
About this article
Cite this article
Xu, Z., Qin, L., Cai, M. et al. Biodegradation of kraft lignin by newly isolated Klebsiella pneumoniae, Pseudomonas putida, and Ochrobactrum tritici strains. Environ Sci Pollut Res 25, 14171–14181 (2018). https://doi.org/10.1007/s11356-018-1633-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1633-y