Abstract
The biodegradation process of lignin by Penicillium simplicissimum was studied to reveal the lignin biodegradation mechanisms. The biodegradation products of lignin were detected using Fourier transform infrared spectroscopy (FTIR), UV–Vis spectrophotometer, different scanning calorimeter (DSC), and stereoscopic microscope. The analysis of FTIR spectrum showed the cleavage of various ether linkages (1,365 and 1,110 cm−1), oxidation, and demethylation (2,847 cm−1) by comparing the different peak values in the corresponding curve of each sample. Moreover, the differences (Tm and ΔHm values) between the DSC curves indirectly verified the FTIR analysis of biodegradation process. In addition, the effects of adding hydrogen peroxide (H2O2) to lignin biodegradation process were analyzed, which indicated that H2O2 could accelerate the secretion of the MnP and LiP and improve the enzymes activity. What is more, lignin peroxidase and manganese peroxidase catalyzed the lignin degradation effectively only when H2O2 was presented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignin is a natural polymer found in plant cell walls, which is composed of three basic units: p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol (Cai et al. 2010; Liu et al. 2008). It is an irregular network biopolymer that was made up of oxidative coupling of the three major units linked by ether and carbon–carbon bonds, such as β-O-4,4-O-5,β-β,β-1,β-5 and 5-5′(Zhang et al. 2010). Different from most other natural polymers (such as cellulose and hemicellulose), the lignin cannot be cleaved by hydrogen enzymes due to its bond types and irregular linked ways (Hofrichter 2002). The agricultural organic residues and papermaking wastewater which contain large amounts of lignin may cause serious environment pollution if they were randomly stored and discharged without any effective treatment measures.
At present, the lignin disposal could be achieved by either chemical or biological method. Compared to the two methods, the biological technology is safe and economical, which could be the promising direction in the future (Sun et al. 2013), but it is difficult to explore the biodegradation mechanism in lignin due to its irregular network structure. Currently, white rot fungus, brown rot fungus, and soft rot fungus are the major microorganisms that are widely used in the study of the lignin biodegradation. Compared with the other two kinds of fungi, the white rot fungi (especially Phanerochaete chrysosporium) have been known as the most popular fungi for lignin biodegradation. The relevant works about the delignifying of P. chrysosporium are focused on the enzyme production that could provide indirect evidences for the degradation of lignin or the determination of its model compounds (Arora et al. 2002). In present literatures, Penicillium simplicissimum was found to be capable of secreting three kinds of ligninolytic enzymes: cellulase, hemicellulase, and laccase (Lac), but rarely secreting lignin peroxidase (LiP) and manganese peroxidase (MnP) (Zeng et al. 2006). In this research, P. simplicissimum was found to secret LiP and MnP in the case of adding H2O2 during the cultivation when it was isolated from the soil samples. LiP, MnP, and Lac that were secreted by P. simplicissimum in the lignin degrading process were oxidized by the electron acceptors (H2O2 and O2) (Janusz et al. 2013; Yu et al. 2005). For these three enzymes, LiP and MnP are able to oxidize non-phenolic lignin units, while Lac can oxidize phenolic lignin units (Sigoillot et al. 2012). There is good synergies among these enzymes during the degrading process which involved demethylation, side-chain oxidation, and aromatic ring cleavage (Mousavioun et al. 2012), so P. simplicissimum has a great application potential in agricultural wasting treatment and bioremediation (Tang et al. 2006; Zeng et al. 2013; Hua et al. 2013).
As mentioned above, most of the literatures about lignin degradation were focused on the ligninolytic enzyme production in synthetic substrate, which contains the model compounds like veratryl alcohol instead of real lignin, but few were about the natural lignin as the degradation material, structure, and properties of lignin varied with the method of extraction and origin. Therefore, this work was designed to investigate the lignin degradation mechanism in P. simplicissimum, and the used lignin was extracted from poplar by organic solvent which could mainly maintain its natural attributes.
Fourier transform infrared spectroscopy (FTIR) and different scanning calorimetric analysis (DSC) are especially performed to explore the biodegradation process of lignin too, which has been proven to be effective techniques for investigating the chemical and structural changes of lignin, but it is rarely used in biodegradation research of lignin in spite being often used in the study on pyrolysis mechanism of lignin. Both methods also require minimum sample quantities for analysis when compared to other conventional gravimetric techniques in which several grams of lignin are needed for each analysis, but their precision was much higher (Xu et al. 2013), so the whole degradation process in this research was monitored by detecting three kinds of ligninolytic enzymes activity and analyzing the spectrum differences between non-degradation and degradation of lignin by a set of comprehensive analytical methods including FTIR, DSC, UV–Vis, and stereoscopic microscope.
Materials and methods
Materials
P. simplicissimum
The strain of P. simplicissimum (Oudem.) Thom BGA was isolated and screened from humus soil in wild nature (Yu et al. 2005). The strain (P. simplicissimum) was cultured on potato dextrose agar at 30 °C. After 7 days’ growth, the spores on the agar surface were scraped and blended into 30-ml sterile water, and the spores’ concentration was determined by the turbidimeter. All the other chemicals used in this work were of analytical reagent grade.
Lignin purifying
One hundred-milligram poplar wood chip was grinded for 1 h and then steeped and dissolved in 500-ml mixed liquid (dioxane/water = 4:1) (Guo et al. 2013). The mixed liquor was filtered three times, and then the filtrate was evaporated and dried at 50 °C under vacuum condition to remove the corresponding solvent and formed brown powder product. Subsequently, the brown powder product was defined as the crude lignin. The crude lignin was freeze-dried and further purified by dissolution in 20 ml 90 % acetic acid and then precipitated in 400 ml distilled water. Finally, the lignin precipitated was dried under vacuum freezing condition for 24 h (El Hage et al. 2010).
Methods
Fermentation methods
Purified lignin of 0.5 g was evenly spread on the solid agar medium which was covered with a semi-permeable membrane, and then each culture medium was inoculated with 0.5-ml spore suspension. Finally, the entire culture medium was placed into the constant temperature and humidity incubator; these could ensure the source of moisture for the fungi to survive and undergo fermentation. The fermentation time ranged from 5 to 25 days according to the experimental solutions.
In crude enzyme solution of solid-state fermentation, 0.5 ± 0.005 g of solid sample was taken out after a period of solid-state fermentation, then placed into the 100-ml conical flask in which 20-ml distilled water was added, and then put in 150 r/min oscillation incubator to be oscillated and dissolved for 1 h. After the oscillation, the liquid was centrifuged at 10,000 r/min for 10 min to form the crude enzyme solution that removed spores and is used for the determination of enzymatic activity by UV–Vis spectrophotometer.
Fourier transform infrared spectroscopy analysis
The sample was a mixture of 2-mg dried lignin powder and 200-mg KBr, which was milled and homogenized carefully. Then, the sample was dried again and compressed at 10 t/cm−2 pressures for 1 min to form a thin KBr wafer (Zhang et al. 2013). The prepared sample wafer was determined at room temperature by a Fourier transform infrared spectrophotometry (Nicolet 6700/8700, Thermo Scientific, USA) in the spectral range of 4,000 to 500 cm−1 using 64 scans at 4 cm−1 resolution with a mirror velocity of 0.6329 cm/s, and the measurement time for each sample was around 60 s. Relative changes in the intensity of lignin characteristic bands were determined at different periods.
Differential scanning calorimetry analysis
Five milligrams of the sample was precisely weighed and then sealed in an aluminum pan. The sample in the pan was heated and determined from 75 to 550 °C at the heating rate of 10 °C/min (Pyris Diamond DSC, Perkin Elmer Corporation, USA).
Stereo microscope analysis
The morphology of the lignin samples in various degradation periods by P. simplicissimum was viewed and analyzed by using a stereo microscope (KH-7700, HIRO, Japan).
Analysis of enzyme activities by UV–Vis spectrophotometer
LiP enzyme activity was measured based on the oxidation of dye Azure B monitored at 651 nm on a UV–Vis spectrophotometer (UV-2550, Shimadzu, Japan) (Arora and Gill 2001). MnP enzyme activity was measured based on the oxidation of Mn2+ monitored at 240 nm.
H2O2 content analysis
The level of H2O2 secreted by P. simplicissimum was measured following the method of Su et al. with some modifications. After the extraction liquid was prepared from a sample, its absorbance was measured at 415 nm, and the absorbance values were calibrated to a standard graph generated with known concentration of H2O2 (Su et al. 2005).
All the experiments were performed in triplicate, and the standard deviation was lower than 5 %. Controls were run to check the volatilization and adsorption of phenol to the glass walls of the flasks during the experimental course.
Results and discussion
Degradation products analysis by FTIR
FTIR analysis was used to examine qualitative and quantitative changes in lignin and carbohydrate components in the study. The results of FTIR determining to decayed lignin samples and non-decayed samples were both presented to investigate the detailed changes of the chemical components of lignin. Figure 1 shows the FTIR spectra of lignin degraded by P. simplicissimum for different durations (0, 5, 10, 15, 20, and 25 days). The FTIR spectrum of sample 1# was shown in Fig. 1. In Fig. 1, a strong absorption was seen around 3,400 cm−1 which is assigned to the stretching vibration of aromatic and aliphatic –OH in the lignin (Mousavioun et al. 2012) and a prominent absorption peak around 2,990 cm−1 which is assigned to the stretching vibration of C–H band in CH2, CH3, and CH3O groups of the lignin structure (Sun et al. 2013). While the lignin degradation processes continue, the absorption peaks of the bonds at their respective corresponding location mentioned above was gradually reducing compared with the non-decayed one, indicating that some methoxyls may be removed from the aromatic ring during the biodegradation process. In addition, there are also existed many well-defined peaks in the fingerprint region between 1,800 and 600 cm−1 (Pandey and Pitman 2003). Corresponding to Figs. 2 and 3 (the fingerprint region), more clear differences can be detected in the infrared spectra, both in the absorbencies and shapes of the bands. The most representative bands studied within this spectra range are summarized in Table 1 (Colom et al. 2003). The peaks in the fingerprint are assigned: 1,740 cm−1 for C═O stretching of acetyl and 1,650 cm−1 for absorbed O–H and conjugated C–O. The peak values at 1,598 and 1,510 cm−1 are often attributable to the contribution of lignin since it arises purely due to aromatic skeletal vibration (C═C) in the lignin. The peak values at 1,465 and 1,426 cm−1 are caused by the stretching in the phenol–ether bonds of the lignin (Xu et al. 2013), while the 1,268 cm−1 peak is for C–O stretching in the lignin and C–O linkage in guaiacyl aromatic methoxyl groups, and the 1,244 cm−1 peak is for syringyl ring and C–O stretching in the lignin (Pandey and Pitman 2003).
FTIR spectra of lignin samples with various days of degradation by Penicillium simplicissimum: a the spectra between 1,700 and 1,400 cm−1; b the spectra between 1,400 and 1,000 cm−1. 1# undegraded sample, 2# degraded for 5 days, 3# degraded for 10 days, 4# degraded for 15 days, 5# degraded for 20 days, 6# degraded for 25 days
By the contrasting analysis between the infrared spectrum of the samples of 10 and 20 days, we can draw the conclusion that the decreasing of the peak at 1,660 cm−1 means that the breakage or replacing of the aromatic ring chain reaction happened in the lignin structure during the degradation process, which indicates the reduction of benzene ring structure units, that is, to say, the lignin was degraded to some extent. The decreasing of the peak at 1,110 cm−1 means that –O– asymmetric stretching vibration in the ether bond and C–O stretching vibration are also decreased; this may be due to the generation of more easily degradable materials after the lignin side chain oxidation and demethylation. The appearance of the bands in the range of 1,260–1,270 and 1,330–1,375 cm−1 shows the existence of guaiacyl and syringyl groups in the lignin, while the single appearance of band in 1,330–1,375 cm−1 means that there is only the syringyl group in the lignin (Liu et al. 2008). The obvious difference can be found in Fig. 3b that the sharp peak in the range of 1,330–1,375 cm−1 is gradually flat, and the peak in the range of 1,260–1,270 cm−1 is gradually disappearing as the degradation processes was continuing, indicating that guaiacyl and syringyl groups in the lignin are destroyed by P. simplicissimum, and the degraded speed of guaiacyl was faster than syringyl.
In general, the direct quantitative analysis of lignin contents by FTIR spectra are not very accurate, but the absorbance values of these wave numbers (1,598, 1,510, and 1,268 cm−1) in the lignin are correlated linearly with the lignin contents that were determined by their chemical properties (Gelbrich et al. 2012). According to Fig. 4, the highest correlation coefficient (R 2 = 0.9472) was found at the wave number 1,510 cm−1 because of the aromatic skeletal vibration absorbance of the benzene ring in lignin (Pandey 1999), while the others assigned to lignin were derived from other functional groups which could be influenced by overlapping effects, so the lignin contents of these samples were calculated based on the transmittance values at 1,510 cm−1, and the undegraded one was as the lignin content background value. Using this method, we can evaluate the degradation degree of the lignin by P. simplicissimum, and the results were presented in Table 2. It shows that the degradation percent rate was 11.98, 33.76, 44.96, 49.89, and 57.08 % respectively. The lignin samples were disintegrated and lost 11.98 % of mass within 5 days, then the value increased rapidly to 33.76 % in the next 5 days. After 15 days, the degradation speed was slowed down to 51.2 and 57.08 %. These results were similar with the weight lost of the samples (13.04, 35.86, 41.02, 46.34, and 50.12 %) for the corresponding degradation time.
Analysis of the lignin degradation process by DSC
Differential scanning calorimetry is sensitive to slight composition changes during the thermal degradation process of lignin materials. In order to complement and verify the above results analyzed by FTIR, differential scanning calorimetry (DSC) was further used to monitor and analyze the degradation process of lignin. DSC analysis results show the curve change in degradation process (as in Fig. 5): (1#) undegraded sample, (2#) sample degraded for 5 days, (3#) sample degraded for 10 days, (4#) sample degraded for 15 days, (5#) sample degraded for 20 days, and (6#) sample degraded for 25 days, which are similar and show endothermic events 200 °C up to 275 °C and superimposed exothermic peaks at higher temperatures with a maximum at 550 °C. These analysis results are related to either the decomposition or the condensation of the aromatic ring (Fernandes et al. 2006). From Fig. 5, we can see that all the curves were initiated with endothermic regions due to the required heat for evaporation of moisture before the heat flows turned from endothermic to exothermic. We also found that lignin was slowly decomposed in a wider temperature range, but not as cellulose or hemicellulose. Lignin decomposition was associated with exothermic heat flow (Haykiri-Acma et al. 2010). Figure 5 shows that the melting enthalpies change the degradation process of the samples too, which matches the values in literature (Mousavioun et al. 2013). 1# lignin sample takes place the melting change between 225 and 275 °C and that thermal degradation starts at 275 °C. In addition, 1# lignin sample showed an additional degradation peak at 229 °C due to the grafted vinyl acetate polymer chains produced in lignin biodegradation process (Panesar et al. 2013). As the degradation time continued, the peak at its respective corresponding location gradually disappeared. From Fig. 5, it can also be seen that the onset of the thermal degradation is shifted to a lower temperature, and the melting peak is also shifted to a lower temperature along with the degradation. These behaviors are more visualized in the peak temperature data that was shown in Table 3. When the components of the lignin sample are amorphous or semi-crystalline polymers during the degradation process, the melting point depression is indicative of favored breakage interactions between the polymer chains (Castro et al. 2003). The lower ΔH and Tm values may also be associated with the breakup of aromatic polymers chains (Mousavioun et al. 2012).
DSC spectra of lignin samples with various days of degradation by Penicillium simplicissimum: a the spectra between 75 and 550 °C; b the spectra between 200 and 275 °C. 1# undegraded sample, 2# degraded for 5 days, 3# degraded for 10 days, 4# degraded for 15 days, 5# degraded for 20 days, 6# degraded for 25 days
Surface macroscopic analysis of the degradation process
To check the morphological changes of the lignin that existed in the degradation process, the samples were further evaluated by stereomicroscope (the photographs were shown in Fig. 6; Fig. 6a was a blank filter paper). The stereoscopic microscopy of the lignin samples revealed the presence of heterogeneous particles and surface changing in the lignin degraded by P. simplicissimum. The particle of non-degraded lignin (Fig. 6b) has a smooth surface and deep color, which presents a significant difference compared with the degraded lignin samples. As the degradation processes continued, the color of the lignin was gradually weaker, and the surface of the lignin particle fragmentation was also rougher. It can be concluded that the degradation of lignin takes place steadily throughout the process.
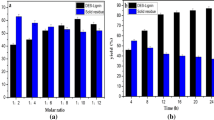
The influence of H2O2 in ligninolytic enzyme activity in the degradation process
P. simplicissimum usually secretes a lot of Lac, cellulase, and hemicellulase, but the secretion of LiP and MnP is little and the enzyme activity is low. In this research, we found that adding H2O2 could accelerate the secretion of MnP and LiP during the cultivation process, and the enzyme activity was also increased (Fig. 7). By the experiment, we also found that the P. simplicissimum can synthesize corresponding enzymes which secret hydrogen peroxide (H2O2); this discovery is consistent with the previous literature (Guillén et al. 1990). LiP and MnP both need to be activated by H2O2. There are two types of enzymes which can secrete H2O2, one is glucose oxidase that existed intracellularly and the other is glyoxalase that existed extracellularly. Under the participation of molecular oxygen, these enzymes secreted H2O2 by oxidating their corresponding substrates, glucose and glyoxal respectively, and then oxidate Fe3+ that existed in the LiP and MnP to start the two oxidase catalytic cycle (Fig. 8). It is a series of free radical chain reaction. Firstly, high activity enzyme intermediates are generated and oxidate lignin into many different free radicals (R·) including hydroxyl radicals (·OH) that have great oxidation ability; secondly, the lignin free radical intermediates with chemical instability are generated; and thirdly, a series of spontaneous degradation reaction are produced, which mainly occurred in the secondary metabolic stage. This process is shown in Fig. 9. LiP and MnP are heme-containing proteins, which catalyze the hydrogen peroxide-dependent one-electron oxidation in highly recalcitrant substrates (Tang et al. 2006), and that Lac is multi-copper phenol oxidase that can oxidize numerous phenolic compounds and aromatic amines using molecular oxygen as a terminal electrons acceptor (Janusz et al. 2013). The structural–functional properties of these three enzymes will not be discussed in depth in this study, except their activities and synergy.
The catalytic cycle of LiP and MnP (Buswell et al. 1995)
In order to further verify the role of hydrogen peroxide in lignin degradation process, this study conducted two groups of experiments for comparison. The entire medium used in this study has no glucose or its analogue, so the fermentation system itself could not produce H2O2. If there was no extra H2O2 added, the ligninolytic enzymes secreted by P. simplicissimum cannot play a role in lignin degradation. Laccase does not need H2O2 as the starting agent in the degradation process of lignin, but without the synergy of LiP and MnP, Lac alone cannot play a role in ligninolysis. According to Fig. 8, although both groups had high enzyme activity, enzyme activity of the group with added H2O2 is higher than enzyme activity of the group without added H2O2; in addition, the maintaining time of high enzyme activity of the group added with H2O2 is longer than the maintaining time of high enzyme activity of group without added H2O2. This shows that LiP and MnP are synergy in lignin degradation under the effect of H2O2, and the degradation products can basically maintain P. simplicissimum’s metabolism; thus, the ligninolytic enzyme activity can maintain at a high level for a period of time. Though the enzymes activity of the group without added H2O2 also has certain vitality in the first few days of degradation process, they did not have the catalyzing effect of the lignin degradation, so the enzyme activity declined rapidly after reaching the peak.
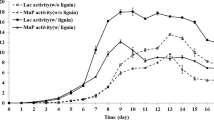
The enzyme activities during the different degradation time were shown in Fig. 7. The LiP activity on the 5th day degradation was relatively higher than that on the 10th day and then decreased significantly after the 15th day. The MnP activity was higher than the LiP activity within 15 days, and two peaks was observed on the 5th day and 15th day respectively, which is in line with previous studies on ligninolytic enzymes (Arora and Gill 2000). Reappearance of peak value of the 15th day MnP enzyme activity during later stages of lignin degradation might be attributed to fungal autolysis resulting in the release of cell membrane bound or intracellular enzymes in the medium (Arora et al. 2002), but its activity rapidly reduced and almost disappeared after 25 days. In addition, the Lac activity was relatively higher on the 15th day than the other days; it is worth noting that nearly no Lac activity was observed within the first 5 days, and it was still steady at lower value before the 10th day. Table 2 shows that the maximum loss percent rate of the lignin was on the 25th day, while maximum activities of LiP, MnP, and Lac were detected on the 5th and 15th respectively, which might suggest a synergistic effect of LiP, MnP, and Lac in ligninolysis. No single enzyme could be held responsible for ligninolysis (Arora et al. 2002).
Conclusion
The P. simplicissimum has fairly ligninolytic ability because it produces three kinds of enzymes (LiP, MnP, and Lac) which are considered to be the most important that catalyzes in the biodegrading process of lignin. The investigated results indicated that FTIR and DSC were used to effectively examine the changes in the chemical structure of lignin before and after decay of the P. simplicissimum. According to the analysis and determining of FTIR spectroscopy (with reduction of the bands at 1,598, 1,510, 1,268, and 898 cm−1), the quantity of lignin was clearly decreased and the lignin was changed into the pseudo-lignin, and it consists of carbonyl, carboxylic, aromatic, and aliphatic structures in the degradation process. According to the DSC analysis, the numerical reduction of Tm (from 246 to 230 °C) and ΔHm(from –208 to –455 J/g) which is associated with the break up of aromatic polymer chains indicated that the lignin sample had been degraded by P. simplicissimum during the process of fermentation. In addition, lignin peroxidase and manganese peroxidase catalyzed the lignin degradation effectively only when H2O2 existed. All of the detailed and laboratory-based chemical and biological studies will promote the understanding of the degradation mechanism of lignin.
References
Arora DS, Gill PK (2000) Laccase production by some white rot fungi under different nutritional conditions. Bioresour Technol 73:283–285
Arora DS, Gill PK (2001) Comparison of two assay procedures for lignin peroxidase. Enzym Microb Technol 28:602–605
Arora DS, Chander M, Gill PK (2002) Involvement of lignin peroxidase, manganese peroxidase and laccase in degradation and selective ligninolysis of wheat straw. Int Biodeterior Biodegrad 50:115–120
Buswell JA, Cai Y, Chang S (1995) Effect of nutrient nitrogen and manganese on manganese peroxidase and laccase production by Lentinula (Lentinus) edodes. FEMS Microbiol Lett 128:81–87
Cai Y, Li G, Nie J, Lin Y, Nie F, Zhang J, Xu Y (2010) Study of the structure and biosynthetic pathway of lignin in stone cells of pear. Sci Hortic 125:374–379
Castro REN, Toledo EA, Rubira AF, Muniz EC (2003) Crystallisation and miscibility of poly(ethylene oxide)/poly(vinyl chloride) blends. J Mater Sci 38:699–703
Colom X, Carrillo F, Nogus F, Garriga P (2003) Structural analysis of photodegraded wood by means of FTIR spectroscopy. Polym Degrad Stab 80:543–549
El Hage R, Brosse N, Sannigrahi P, Ragauskas A (2010) Effects of process severity on the chemical structure of Miscanthus ethanol organosolv lignin. Polym Degrad Stab 95:997–1003
Fernandes DM, Winkler Hechenleitner AA, Job AE, Radovanocic E, Gómez Pineda EA (2006) Thermal and photochemical stability of poly(vinyl alcohol)/modified lignin blends. Polym Degrad Stab 91:1192–1201
Gelbrich J, Mai C, Militz H (2012) Evaluation of bacterial wood degradation by Fourier transform infrared (FTIR) measurements. J Cult Herit 13:S135–S138
Guillén F, Martinez A, Martínez M (1990) Production of hydrogen peroxide by aryl-alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. Appl Microbiol Biotechnol 32:465–469
Guo G, Li S, Wang L, Ren S, Fang G (2013) Separation and characterization of lignin from bio-ethanol production residue. Bioresour Technol 135:738–741
Haykiri-Acma H, Yaman S, Kucukbayrak S (2010) Comparison of the thermal reactivities of isolated lignin and holocellulose during pyrolysis. Fuel Process Technol 91:759–764
Hofrichter M (2002) Review: lignin conversion by manganese peroxidase (MnP). Enzym Microb Technol 30:454–466
Hua G, Li J, Zeng G (2013) Recent development in the treatment of oily sludge from petroleum industry: a review. J Hazard Mater 261:470–490
Janusz G, Kucharzyk KH, Pawlik A, Staszczak M, Paszczynski AJ (2013) Fungal laccase, manganese peroxidase and lignin peroxidase: gene expression and regulation. Enzym Microb Technol 52:1–12
Liu Q, Wang S, Zheng Y, Luo Z, Cen K (2008) Mechanism study of wood lignin pyrolysis by using TG–FTIR analysis. J Anal Appl Pyrolysis 82:170–177
Mousavioun P, George GA, Doherty WOS (2012) Environmental degradation of lignin/poly(hydroxybutyrate) blends. Polym Degrad Stab 97:1114–1122
Mousavioun P, Halley PJ, Doherty WOS (2013) Thermophysical properties and rheology of PHB/lignin blends. Ind Crop Prod 50:270–275
Pandey KK (1999) A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J Appl Polym Sci 71:1969–1975
Pandey KK, Pitman AJ (2003) FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int Biodeterior Biodegrad 52:151–160
Panesar SS, Jacob S, Misra M, Mohanty AK (2013) Functionalization of lignin: fundamental studies on aqueous graft copolymerization with vinyl acetate. Ind Crop Prod 46:191–196
Sigoillot J, Berrin J, Bey M, Lesage-Meessen L, Levasseur A, Lomascolo A, Record E, Uzan-Boukhris E (2012) Chapter 8 - Fungal strategies for lignin degradation. in: J.A.C.L. Lise (Ed.) Advances in Botanical Research. Academic, pp. 263–308
Su G, An Z, Zhang W, Liu Y (2005) Light promotes the synthesis of lignin through the production of H2O2 mediated by diamine oxidases in soybean hypocotyls. J Plant Physiol 162:1297–1303
Sun Y, Qiu X, Liu Y (2013) Chemical reactivity of alkali lignin modified with laccase. Biomass Bioenergy 55:198–204
Tang L, Zeng G, Shen G, Zhang Y, Huang G, Li J (2006) Simultaneous amperometric determination of lignin peroxidase and manganese peroxidase activities in compost bioremediation using artificial neural networks. Anal Chim Acta 579:109–116
Xu G, Wang L, Liu J, Wu J (2013) FTIR and XPS analysis of the changes in bamboo chemical structure decayed by white-rot and brown-rot fungi. Appl Surf Sci 280:799–805
Yu HY, Zeng GM, Huang GH, Huang DL, Chen YN (2005) Lignin degradation by Penicillium simplicissimum. Environ Sci 26:167–171
Zeng G, Yu H, Huang H, Huang D, Chen Y, Huang G, Li J (2006) Laccase activities of a soil fungus Penicillium simplicissimum in relation to lignin degradation. World J Microbiol Biotechnol 22:317–324
Zeng G, Chen M, Zeng Z (2013) Shale gas: surface water also at risk. Nature 499(7457):154
Zhang A, Liu C, Sun R (2010) Fractional isolation and characterization of lignin and hemicelluloses from triploid of Populus tomentosa Carr. Ind Crop Prod 31:357–362
Zhang F, Zhu Z, Wang B, Wang P, Yu G, Wu M, Chen W, Ran W, Shen Q (2013) Optimization of Trichoderma harzianum T-E5 biomass and determining the degradation sequence of biopolymers by FTIR in solid-state fermentation. Ind Crop Prod 49:619–627
Acknowledgments
This work was supported by the Natural Science Foundation of Hunan Province (07JJ5053), the Hunan Province Open Foundation of University Innovation Platform (11K013), the National Natural Science Foundation of China (51039001,51378190), and the Program for Changjiang Scholars and Innovative Research Team in University (IRT-13R17).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Gerald Thouand
Rights and permissions
About this article
Cite this article
Liu, Y., Hu, T., Wu, Z. et al. Study on biodegradation process of lignin by FTIR and DSC. Environ Sci Pollut Res 21, 14004–14013 (2014). https://doi.org/10.1007/s11356-014-3342-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3342-5