Abstract
There is no commercial or industrial-scale process for the remediation of black liquor using microorganisms to date. One of the most important causes is that most microorganisms are not able to use lignin as their principal metabolic carbon or energy source. The bacterial strain Comamonas sp. B-9 has shown remarkable ability to degrade kraft lignin and decolorize black liquor using lignin as its principal metabolic carbon and energy source. This report looks at the depolymerization and decolorization of kraft lignin by Comamonas sp. B-9. The degradation, decolorization, and total carbon removal reached 45, 54, and 47.3 %, respectively, after 7 days treatment. Comamonas sp. B-9 was capable of depolymerizing kraft lignin effectively as analyzed by gel permeation chromatography and decolorization via degrading benzene ring structures as shown using Fourier transform infrared spectroscopy analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the pulp and paper industry, the alkaline effluent from alkaline sulfide treatment process, also known as black liquor (BL), accounts for only 15 % of the wastewater amount but contributes 90–95 % of the total pollution load (Pokhrel and Viraraghavan 2004). Kraft lignin (KL) is the main contaminant that leads to high chroma, toxicity, and chemical oxygen demand (COD) of BL. KL differs from natural lignin as it undergoes a conventional kraft cooking with aqueous solution of sodium hydroxide and sodium sulfide. During this treatment, the hydroxide and hydrosulfide anions react with the lignin, including aryl-alkyl cleavages, strong modification of side chains, and various ill-defined condensation reactions causing the polymer to fall into smaller water/alkali-soluble fragments (Chakar and Ragauskas 2004). In spite of this, KL is still a large molecular weight polymer that is resistant to biodegradation.
The white-rot fungi are considered to be the most effective lignin-degrading microorganism and studies on microbial degradation of lignin have primarily focused on breakdown by fungi (Sánchez 2009). However, there is as yet no commercial biocatalytic process for lignin depolymerization by fungus (Bugg et al. 2010) and no white-rot fungus has been convincingly shown to grow on as well as degrade lignin using lignin as its principal metabolic carbon or energy source (Archibald et al. 1990). In previous studies, some bacteria and actinomyces were found capable of degrading a variety of lignin and lignin-related compounds including KL but the degradation was limited with efficiency less than 20 % (Vicuna 1988; Perestelo et al. 1994, 1996). In recent studies, some bacteria such as Paenibacillus sp. and Bacillus sp. were reported to have much stronger degradation of KL, which was close to 40 % (Raj et al. 2007; Chandra et al. 2007, 2008). However, the degradation of lignin by these strains was performed under mild conditions (neutral pH, low concentration of lignin, and COD). Besides, the additional carbon source (glycerol, glucose or starch, etc.) must be provided for these strains to degrade lignin. Therefore, the degradation of lignin by these reported strains has been fuelled by additional carbon source, but a practical industrial-scale process would have to employ a substrate that was much less expensive.
The bacterium Comamonas sp. B-9 (CGMCC No. 4251) was confirmed capable of degrading KL as the sole carbon in our previous study (Chen et al. 2012). In addition, Comamonas sp. B-9 was used to treat BL directly under high alkaline pH and COD load conditions (pH 10, COD 23,000 mg l−1) without any additional carbon or energy source (Zheng et al. 2013a); the enhanced remediation of BL was realized by activated sludge bioaugmented with Comamonas sp. B-9 (Zheng et al. 2013b). In these studies, Comamonas sp. B-9 has been demonstrated to possess a remarkable ability in decolorization, lignin degradation, and COD removal. Especially, no additional carbon source is required for the treatment of KL or BL by Comamonas sp. B-9, which could be a great advantage in practical industrial-scale application. To understand the role of Comamonas sp. B-9 in lignin degradation, the decolorization and depolymerization of KL by Comamonas sp. B-9 were further performed in the present study.
Materials and methods
Bacterial growth conditions and experimental procedures
Pure Comamonas sp. B-9 colonies were inoculated in Luria–Bertani broth medium and incubated overnight till the A600 (absorbance at 600 nm) of inoculum was approximately 1.2 at 30 °C under continuous rotary shaking condition (120 rpm). Five milliliter of the inoculum was centrifuged to collect the bacterial cells and then the precipitate was washed with sterile water for three times. The washed cells were inoculated into a 1,000-ml conical flask containing 500 ml sterile KL mineral salt medium (KL-MSM, pH 7.0). The composition of KL-MSM was as follows (in gram per liter of deionized sterile water): KL, 3 (purchased from Sigma, average molecular weight (M w ), ∼10,000); (NH4)2SO4, 2; K2HPO4, 1; KH2PO4, 1; MgSO4, 0.2; CaCl2, 0.1; FeSO4, 0.05; and MnSO4, 0.02. The biodegradation experiment was carried out at 30 °C in rotary shaking incubator at 120 rpm for 7 days. The appropriate volumes of samples were withdrawn periodically at every 24-h interval for the measurement of lignin degradation, decolorization, and total carbon (TC) removal. Uninoculated medium was used as control. The biodegradation experiments were carried out in triplicates as parallel experiments. The values were presented as mean values and standard deviation was less than 3.7 %. Error bars are not shown in the figures.
KL degradation and decolorization measurement
For the measurement of color reduction, residual KL, samples were centrifuged at 12,000×g for 10 min. Supernatant (1 ml) was diluted by adding 2 ml phosphate buffer (pH 7.6). Degradation of KL was determined by the decrease of the absorbance at 280 nm (A280) and at 465 nm (A465) for the decolorization with a Hitachi U-4100 spectrophotometer (Chandra et al. 2007). For the measurement of TC removal, samples (1 ml) were centrifuged at 12,000×g for 10 min and the supernatant was diluted by adding 19 ml deionized water. TC removal was measured with a Shimadzu TOC-V CPH.
Analytical methods
Control and inoculated samples (50 ml for each sample) were withdrawn after 7 days of incubation. The samples were centrifuged at 12,000×g for 10 min to remove bacterial cells. Cell-free supernatant was dried to constant weight in the vacuum freeze-drying apparatus. The dried powder samples were used for scanning electron microscopy (SEM), gel permeation chromatography (GPC), and Fourier transform infrared spectroscopy (FTIR) analysis.
SEM was carried out in a JEOL JSM-6360LV microscope
GPC was performed in an AKTA purifier UPC100. The dried samples were dissolved in 0.1 mol l−1 Tris–HCl buffer (pH 7.5) with a concentration of 3 g l−1. The molecular size distribution in each sample was determined by loading 0.5 ml of solution into a Superdex 75 10/300 GL column (GE Healthcare Life Sciences) in 0.1 mol l−1 Tris–HCl buffer (pH 7.5). The flow rate was 1 ml min−1. Sample elution was monitored by measuring the absorbance at 280 nm. Sodium polystyrene sulfonate standards (American Polymer Standards Corporation) were used to obtain the calibration curve that was related to the retention time (T r , minutes) and the M w . The standard equation were obtained with Superdex 75 10/300 GL column using sodium polystyrene sulfonate standards: log (M w ) = 4.409–0.026 T r .
FTIR spectra of the samples were obtained with analysis performed on a Thermo Scientific Nicolet IS10 FTIR spectrometer in the wave number range of 4,000–800 cm−1. Of the dried powder sample, 0.001 g was mixed with KBr at a ratio of accurately 1/100 (w/w), then the mixture was ground in an agate mortar to a very fine powder. The fine powder was used to make a pellet under a continuous pressure of 30 MPa for 1 min. The pellet was analyzed immediately and the spectra were recorded by 64 scans with 4 cm−1 resolution. A pellet prepared with an equivalent quantity of pure KBr powder was used as background
Results
KL degradation and decolorization by Comamonas sp. B-9
Degradation and decolorization of KL indicated by the decrease of A280 and A465 are shown in Fig. 1. The initial absorbance of the culture medium containing 3 g l−1 KL was 4.54 at 280 nm and 0.54 at 465 nm and dropped to 2.50 and 0.25, respectively, after 7 days of incubation. Simultaneously, 47.3 % TC of the KL-MSM had been removed after 7 days of treatment. A rapid decrease was found in the initial 4 days and a slight decrease in the following 3 days. In addition, the chroma of the culture medium was observably faded and the color turned from dark brown to brown.
KL depolymerization
The SEM photos of untreated and treated KL powders are shown in Fig. 2. Figure 2a shows the magnification photo (×200) of untreated KL powder. As shown in Fig. 2a, the micromorphology of untreated KL used in this study was irregular spherical particles with porous internal structure and the diameter of the particles was approximately 100–150 μm. Figure 2b shows a high magnification photo (×3,000) of KL treated for 7 days. In Fig. 2b, instead of spherical particle, the micron-sized fragments (<10 μm) dominated in the treated KL powders.
The molecular size distribution in KL degradation by Comamonas sp. B-9 was studied by GPC. The patterns of elution from Sephadex 75 10/300 GL column at 280 nm obtained with both untreated KL and treated KL by Comamonas sp. B-9 are shown in Fig. 3. The average molecular weight for KL samples was calculated with the standard equation (Table 1). As shown in Fig. 3 and Table 1, KL in control sample had a broad molecular weight distribution between M w 15,100 and 1,655, while after treatment with Comamonas sp. B-9 for 4 days, the KL had a narrower molecular weight distribution from M w 13,767 to 6,456. Further, after 7 days of incubation, a lower molecular weight distribution from M w 12,900 to 4,810 was found. Additionally, the peaks of T r (8.85, 34.87, and 45.78) disappeared and a significant decrease in A280 of the treated sample was observed.
FTIR analysis
FTIR spectra of untreated and treated KL samples are presented in Fig. 4 and the assignments are given in Table 2 according to previous references (Boeriu et al. 2004; Thielemans and Wool 2004; Kubo and Kadla 2005; Guo and Rockstraw 2006; Liu et al. 2008; Hage et al. 2009). The bands between 3,700 and 3,000 cm−1 were assigned to the O–H groups in phenolic and aliphatic structures and the band at 2,938 cm−1 corresponded to C–H stretching in aromatic methoxyl groups and in methyl and methylene groups. The band at 1,660 cm−1 was assigned to the C=O stretching conjugation with aromatic rings. The bands between 1,600 and 1,400 cm−1 were due to aromatic skeleton vibrations. Aromatic skeleton vibration coupled with C=O at 1,596 cm−1 and the C–H deformation combined with aromatic ring vibration at 1,463 cm−1 are common for various types or forms of lignin. The bands at 1,217 and 1,128 cm−1 were the vibrations characterized by guaiacyl unit and syringyl unit, respectively.
Discussion
Due to the high pH, BOD, COD, and color, BL is significantly toxic to the environment. Besides, in terms of industrial chemical modification of lignin, the kraft pulping process is the dominant process. Hence, the adequate treatment of BL prior to its discharge into the environment is warranted. Biological treatment is a practical choice for BL treatment. Among these biological methods applied so far, most of the literature confined to a few genera of white-rot fungi and the published reports on a variety of white-rot fungi indicated that these fungi cannot use lignin as carbon or energy source for growth and further lignin degradation. During the kraft pulping, chemical reactions lead to the liberation of lignin fragments and also enhance their dissolution. Bacteria seem to play a leading role in decomposing lignin in aquatic ecosystem because of the wider tolerance of temperature, pH, and oxygen limitations as compared with fungi (Daniel and Nilsson 1998). In fact, some bacteria isolated from compost soil, Azotobacter and Serratia marcescens, were capable of degradation and decolorization of lignin (Morii et al. 1995). Bacteria such as Bacillus subtilis and Bacillus sp. also were able to degrade KL (El-Hanafy et al. 2008; Abd-Elsalam and El-Hanafy 2009). Previously, Comamonas sp. B-9 had been confirmed for the treatment of KL and BL without any exogenous carbon source (Chen et al. 2012; Zheng et al. 2013a, b). The present study focused on the KL depolymerization and decolorization by Comamonas sp. B-9. As the results show in Fig. 1, the degradation, decolorization, and TC removal reached 45.06, 53.97, and 47.3 %, respectively, after 7 days treatment. However, degradation and decolorization occurred mainly in the initial 4 days indicating that Comamonas sp. B-9 broke down KL in the primary metabolic stage as described in the previous report (Chen et al. 2012).
It is well accepted that the decrease of A280 could be used to characterize the degradation of aromatic rings. In Figs. 1 and 3, A280 of the treated sample reduced many fold revealing that a large number of benzene ring structures in KL were decomposed by Comamonas sp. B-9. The values in Fig. 3 showed a significant decline in the high molecular weight peaks (M w > 8,000) and a slight increase in the medium molecular weight peaks (5,000 < M w < 8,000). In addition, the untreated sample had a wider molecular weight distribution but the molecular weight of the major fragments was approximately 10,000. There was still a higher molecular weight distribution in the treated sample; however, the amount of high and medium molecular weight fragments reduced demonstrably. These results suggested that the high molecular weight fragments were probably depolymerized to medium molecular weight fragments. These residual fragments were possibly responsible for most of the residual A280 and color in treated sample. Simultaneously, the low molecular weight peaks (T r , 34.87 and 45.78) disappeared, indicating some of which were decomposed to compounds with small molecular weight. The results of previous study also showed that Comamonas sp. B-9 was able to degrade KL polymer into monomer benzene compounds and aliphatic compounds (Chen et al. 2012). In conclusion, Comamonas sp. B-9 was capable of depolymerizing KL effectively.
The color associated with the pulping effluent is primarily due to the chromophores existing in lignin and its various derivative structures. Alkaline pulps are generally much darker than other pulps, which may be due to the presence of more highly condensed and unsaturated lignin and phenolic materials. Although the exact nature of color-causing structures of KL is still uncertain, it is believed that the chromophoric structures, such as carbonyl conjugated with the aromatic rings, quinine methides, quinones, and free radicals are responsible for the color of the KL (Hon and Glasser 1979; Garg and Modi 1999). FTIR can give a quick and qualitative indication of the extent of lignin. Many researchers have studied the infrared absorption characteristics of the lignin (Boeriu et al. 2004; Thielemans and Wool 2004; Kubo and Kadla 2005; Guo and Rockstraw 2006; Liu et al. 2008; Hage et al. 2009). The FTIR spectrum of the untreated sample was relatively simple and there were only four distinct peaks (Fig. 4 and Table 2). The band at 1,660 cm−1 was assigned to the C=O stretching conjugation with aromatic rings which was the primary chromophores in lignin. The results in Polein and Rapson's research showed that the pulp color was proportional to the absorption around 1,670 cm−1 (Polein and Rapson 1971). In the FTIR spectrum of treated sample, the peak at 1,660 cm−1 had blue shift to 1,596 cm−1, which was the potential main reason for KL decolorization by Comamonas sp. B-9. In addition, the spectrum of the treated sample in the aromatic skeleton vibration region (1,600–1,400 cm−1) was complex and the intensity of the bands was weak suggesting that the aromatic ring structures in KL were modified and even degraded by Comamonas sp. B-9.
Since lignin is a phenylpropanoid polymer, a number of aromatic and phenolic sites and activated aliphatic locations capable of participating in decoloration reactions also exist. Obviously, the complexity of structures and components as well as reactions hindered the investigation of potential chromophoric groups in lignin. In general, color problems encountered in pulps arise either from color already present in wood or from chemical changes which take place during grinding, cooking, refining, and bleaching processes. Alkaline pulps are generally much darker than other pulps, which may be due to the presence of more highly condensed and unsaturated lignin and phenolic materials (Hon and Glasser 1979). Therefore, further research is required to investigate the nature of the chromophores in KL and the related mechanisms of depolymerization and decolorization by Comamonas sp. B-9. The native lignin undergoes various types of chemical reactions during the kraft pulping process, resulting in an increase of the solubility of lignin and decrease of molecular weight. However, KL is still a heterogeneous polymer with a complex structure and a variety of functional groups. In that way, it is a better choice to use KL model compounds rather than KL to study the mechanism of depolymerization and decolorization.
Ether bonds are the most important linkage between the benzene structural units in lignin. The cleavage of ether bonds is the critical process in lignin degradation. Moreover, the C=O stretching conjugated with aromatic ring was the main chromophore in lignin. It is critical to identify the related enzymes and encoding genes in Comamonas sp. B-9 which are responsible for KL degradation and decolorization. In that case, a practical industrial-scale application of BL biotreatment by Comamonas sp. B-9 is to be expected.
References
Abd-Elsalam HA, El-Hanafy AA (2009) Lignin biodegradation with ligninolytic bacterial strain and comparison of Bacillus subtilis and Bacillus sp. isolated from Egyptian soil. Am Eurasian J Agric Environ Sci 5:39–44
Archibald F, Paice MG, Jurasek L (1990) Decolorization of kraft bleachery effluent chromophores by Coriolus (Trametes) versicolor. Enzyme Microb Technol 12:846–853. doi:10.1016/0141-0229(90)90021-H
Boeriu CG, Bravo D, Gosselink RJA, van Dam JEG (2004) Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind Crop Prod 20:205–218. doi:10.1016/j.indcrop.2004.04.022
Bugg TD, Ahmad M, Hardiman EM, Singh R (2010) The emerging role for bacteria in lignin degradation and bio-product formation. Curr Opin Biotechnol 22:1–7. doi:10.1016/j.copbio.2010.10.009
Chakar FS, Ragauskas AJ (2004) Review of current and future softwood kraft lignin process chemistry. Ind Crop Prod 20:131–141. doi:10.1016/j.indcrop.2004.04. 016
Chandra R, Raj A, Purohit HJ, Kapley A (2007) Characterisation and optimisation of three potential aerobic bacterial strains for kraft lignin degradation from pulp paper waste. Chemosphere 67:839–846. doi:10.1016/j.chemosphere.2006.10.011
Chandra R, Singh S, Reddy MMK, Patel DK, Purohit HJ, Kapley A (2008) Isolation and characterization of bacterial strains Paenibacillus sp. and Bacillus sp. for kraft lignin decolorization from pulp paper mill waste. J Gen Appl Microbiol 54:399–407
Chen YH, Chai LY, Zhu YH, Yang ZH, Zheng Y, Zhang H (2012) Biodegradation of kraft lignin by a bacterial strain Comamonas sp. B-9 isolated from eroded bamboo slips. J Appl Microbiol 112:900–906. doi:10.1111/j.1365-2672.2012.052 75.x
Daniel G, Nilsson T (1998) Developments in the study of soft rot and bacterial decay. In: Bruce A, Palfreyman JW (eds) Forest products biotechnology. Taylor & Francis, London, pp 37–62
El-Hanafy AA, Abd-Elsalam HE, Hafez EE (2008) Molecular characterization of two native Egyptian ligninolytic bacterial strains. J Appl Sci Res 4:1291–1296
Garg SK, Modi DR (1999) Decolorization of pulp-paper mill effluents by white-rot fungi. Crit Rev Biotechnol 19:85–112. doi:10.1080/0738-859991229206
Guo Y, Rockstraw DA (2006) Physical and chemical properties of carbons synthesized from xylan, cellulose, and kraft lignin by H3PO4 activation. Carbon 44:1464–1475. doi:10.1016/j.carbon.2005.12.002
Hage RE, Brosse N, Chrusciel L, Sanchez C, Sannigrahi P, Ragauskas A (2009) Characterization of milled wood lignin and ethanol organosolv lignin from Miscanthus. Polym Degrad Stabil 94:1632–1638. doi:10.1016/j.polymdegradstab. 2009.07.007
Hon DN-S, Glasser W (1979) On possible chromophoric structures in wood and pulpsa survey of the present state of knowledge. Polymer Plast Tech Eng 12:159–179. doi:10.1080/03602557908067670
Kubo S, Kadla JF (2005) Hydrogen bonding in lignin: a Fourier transform infrared model compound study. Biomacromolecules 6:2815–2821. doi:10.1021/bm 050 288q
Liu Q, Wang SR, Zheng Y, Luo ZY, Cen KF (2008) Mechanism study of wood lignin pyrolysis by using TG-FTIR analysis. J Anal Appl Pyrol 82:170–177. doi:10.1016/j.jaap.2008.03.007
Morii H, Nakamiya K, Kinoshita S (1995) Isolation of lignin-decolorizing bacterium. J Ferment Bioeng 80:296–299. doi:10.1016/0922-338X(95)90835-N
Perestelo F, Falcon MA, Carnicero A, Rodrfguez A, Fuente G (1994) Limited degradation of industrial, synthetic and natural lignins by Serratia marcescens. Biotechnol Lett 16:299–302. doi:10.1007/BF00134629
Perestelo F, Rodriguez A, Phez R, Carnicero A, Fuente G, Falch MA (1996) Isolation of a bacterium capable of limited degradation of industrial and labeled natural and synthetic lignins. World J Microbiol Biotechnol 12:111–112. doi:10.1007/BF00327817
Pokhrel D, Viraraghavan T (2004) Treatment of pulp and paper mill wastewater—a review. Sci Total Environ 333:37–58. doi:10.1016/j.scitotenv.2004.05.017
Polein J, Rapson WH (1971) Effect of bleaching agents on the absorption of lignin in groundwood pulps. Part II. Oxidative-reductive bleaching. Pulp Paper Mag Can 72:80–91
Raj A, Reddy MMK, Chandra R (2007) Identification of low molecular weight aromatic compounds by gas chromatography–mass spectrometry (GC–MS) from kraft lignin degradation by three Bacillus sp. Int Biodeter Biodegr 59:292–296. doi:10.1016/j.ibiod.2006.09.006
Sánchez C (2009) Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol Adv 27:185–194. doi:10.1016/j.biotechadv.2008.11.001
Thielemans W, Wool RP (2004) Butyrated kraft lignin as compatibilizing agent for natural fiber reinforced thermoset composites. Compos: Part A 35:327–338. doi:10.1016/j.compositesa.2003.09.011
Vicuna R (1988) Bacterial degradation of lignin. Enzyme Microb Technol 10:646–655
Zheng Y, Chai LY, Yang ZH, Chen YH, Shi Y, Wang YY (2013a) Environmentally safe treatment of black liquor with Comamonas sp. B-9 under high alkaline conditions. J Basic Microb. doi:10.1002/jobm.201200340
Zheng Y, Chai LY, Yang ZH, Tang CJ, Chen YH, Shi Y (2013b) Enhanced remediation of black liquor by activated sludge bioaugmented with a novel exogenous microorganism culture. Appl Microb Biotechnol 97:6525–6535. doi:10.1007/s00253-012-4453-x
Acknowledgments
This work was supported by National Funds for Distinguished Young Scientists of China (50925417), National High Technology Research and Development Program of China (2011AA061001), and National Research Funding for the Public Benefit of Environmental Protection (2011467062).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chai, Ly., Chen, Yh., Tang, Cj. et al. Depolymerization and decolorization of kraft lignin by bacterium Comamonas sp. B-9. Appl Microbiol Biotechnol 98, 1907–1912 (2014). https://doi.org/10.1007/s00253-013-5166-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5166-5



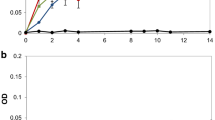

 ), decolorization (
), decolorization ( ), and TC removal (
), and TC removal ( ) by Comamonas sp. B-9
) by Comamonas sp. B-9

