Abstract
Ethiopia and South Africa are among the few countries to still implement indoor residual spraying with dichloro-diphenyl-trichloroethane (DDT) for malaria vector control. In this study, we investigated the levels and ecological risks of DDT and its metabolites in liver tissues of house rat, as a sentinel animal, for providing an early warning system for public health and wildlife intervention from Ethiopia and South Africa. The results showed that ΣDDT concentration ranged from 127 to 9155 μg/kg wet weight, and the distribution order of DDT and its metabolites in the analyzed liver samples was p,p′-DDD > p,p′-DDE >> p,p′-DDT, o,p′-DDT, and o,p′-DDD. The risk assessment indicated a potential adverse impact on humans, especially for pregnant women and children, because they spend majority of their time in a DDT-sprayed house. The ecological assessment also showed a concern for birds of prey and amphibians like frogs. This study is the first report on DDT contamination in liver tissues of house rats from Ethiopia and South Africa, and henceforth, the data will serve as a reference data for future studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rachel Carson’s book “Silent Spring” first drew attention to the effects of the widespread extensive use of pesticides, especially dichloro-diphenyl-trichloroethane (DDT), on the environment and human health (Carson 1962). Due to their high persistence and lipophilicity, DDTs tend to bioaccumulate in fatty tissues of living organisms and are toxic to humans and wildlife. Exposure to DDT and its metabolites has been associated with both estrogenic potentials and anti-androgenic effects (Crews et al. 2000). Both p,p′-DDT and o,p′-DDT promote estrogenic activity (Bhatia et al. 2005), while p,p′-DDE is a potent androgen receptor antagonist (You 2000). The effects of DDT-induced endocrine disruption such as infertility, and reduced reproductive success in birds and wildlife, are also documented (Fisk et al. 2005; Giesy et al. 2003; Tyler et al. 1998). Thus, the necessity for continual monitoring and surveillance of DDTs in natural surroundings has been recognized.
Humans and wildlife can expose to DDT either through direct contact or through secondary exposure via ingesting and inhalation. House rat, also called as the black rat or roof rat, is a good indicator of human exposure to environmental chemicals, because they live in proximity and exposed to many of the same influences as human beings (Ardizzone et al. 2014; Ishizuka et al. 2005). They are potential sinks for accumulation of indoor pesticides from the food supply and/or inhalation of pesticides while used for vector control indoors and outdoors. They are also food for a lot of natural predators, like foxes and cats just to name a few, including birds of prey. Therefore, the knowledge on the contamination status in house rat is not only to estimate the magnitude of environmental pollution but also to predict human health and wildlife risks.
Currently, DDT is produced in India and exported as a pure or as a commercially formulated product to other, mostly African, countries (UNEP 2016). South Africa formulated DDT with the technical product and exports the formulated product to other African countries. In Ethiopia, the Adami Tulu Pesticide Processing Plant has been operating since 1992 in the formulation of pesticides, including DDT. The plant imports technical grade material and has been supplying formulated DDT to the Ministry of Health since 2001 (UNEP 2016). Ethiopia and South Africa are among the others African countries which have used DDT for indoor residual spraying (IRS) for malaria vector control and is still the insecticide of choice (Van den Berg 2009; WHO 2011). As a consequence, DDT residues have been measured at concerning levels in biotic including humans (Gerber et al. 2016; Van Dyk et al. 2010; Yohannes et al. 2014a, b, 2017) and abiotic compartments (Barnhoorn et al. 2009; Yohannes et al. 2013) in these two countries.
With this in mind, the aim of this study was to investigate the levels of DDT and its metabolites in liver tissues of house rats from Ethiopia and South Africa. This study further investigates the potential human and ecological risks pose by the DDTs. To the authors’ knowledge, this is the first report on DDT contamination in house rats from Africa and to document house rats as sentinel of DDT contamination in indoor residual spraying environment.
Materials and methods
Sampling site
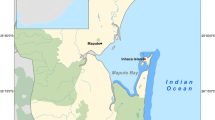
The sample stations were Ziway town from Ethiopia and Pongola river basin in South Africa (Fig. 1). Both sampling areas are known for having high incidence of malaria.
Ziway town is located 165 km from south of Addis Ababa, the capital city of Ethiopia. It is a town with a population of about 50,000 and lies on the western side of Lake Ziway. The communities are engaged in mixed farming, fishing, rearing livestock, and meet their daily water needs from the lake. The Ziway area is known for its irrigation practice where smallholder horticulture farmers and large-scale flower growing companies are located around the lake, with the use of pesticides and chemical fertilizers. The irrigation scheme, subsequently suitable habitats for vector mosquitoes, resulted in increased malaria transmission in communities throughout most of the year.
The Pongola River, with a catchment of 7000 km2 at the eastern extent of South Africa, passes through a narrow gorge between the Lebombo and the Ubombo mountains, where the Pongolapoort Dam is now situated on the border between Swaziland and South Africa. The catchment area is under large-scale agricultural activities, dominated by sugar cane, cotton, irrigated fruits, and vegetables. The surrounding area of the impoundment is classified as an malaria endemic area, and vector control has been done through indoor spraying of DDT in dwellings. Vector control based on IRS has been applied indoors on walls, ceilings, and outdoors with 75% DDT wettable powder at a dosage of 2 g active ingredient per square meter.
Collection of house rat and sample preparation
House rats were captured using gauze cage traps with food as bait in residential areas from Ziway town (n = 21), Ethiopia, in February 2013 and from KwaZulu-Natal, Pongola river basin (n = 24), South Africa, in April 2014. The traps were set indoors in the evening and collected in the next morning. Rats were euthanized, and body weight and sex were determined. Liver samples were collected from each rat and kept at − 20 °C. The frozen samples were then transported to the Laboratory of Toxicology, Graduate School of Veterinary Medicine, Hokkaido University, Japan, for analysis. All the experiments were performed using the rule approved by the Institutional Animal Care and Use Committee of Hokkaido University.
Reagents and chemicals
Mixture of DDTs (o,p′-DDT, p,p′-DDT, o,p′-DDE, p,p′-DDE, o,p′-DDD, and p,p′-DDD) was obtained from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Surrogate standard 3,3′,4,4′-tetrachlorobiphenyl (PCB 77) and syringe spike 2,4,5,6-tetrachloro-m-xylene (TCmX) solutions were purchased from Sigma-Aldrich (Tokyo, Japan) and AccuStandard, Inc. (New Haven, CT, USA), respectively. Solvents and reagents used were analytical grade and purchased from Kanto Chemical Corp. (Tokyo, Japan).
Analysis of DDT
The protocol for analysis was performed as described by Yohannes et al. (2014b). Briefly, about 2-g liver tissue was homogenized with anhydrous sodium sulfate and spiked with surrogate standard (PCB 77). Extraction was carried out with 150 mL hexane/acetone (3:1, v/v) in a Soxtherm apparatus (S306AK Automatic Extractor, Gerhardt, Germany) in hot extraction mode for 4 h. The lipid content was determined gravimetrically on an aliquot of the extract, and the remaining extract was cleaned up on a column packed with 5 g activated Florisil and topped with 1 g of anhydrous sodium sulfate. The extract was eluted with 100 mL hexane/dichloromethane (7:3, v/v). The eluate was finally concentrated to near dryness and redissolved in 100 μL n-decane. A known amount of TCmX as a syringe spike standard was added to all extracts prior to instrumental analyses.
The samples were analyzed on a Shimadzu gas chromatography equipped with a 63Ni μ–electron capture detector (GC-ECD: Shimadzu GC 2014, Kyoto, Japan). An ENV-8MS capillary column (30 m × 0.25 mm × 0.25 μm) was used for separation. The oven initial temperature was 100 °C (1 min hold), raised to 200 °C at a rate of 20 °C/min and then at 3 °C/min to 260 °C (10 min hold). Injector and detector temperatures were 250 and 310 °C, respectively. Helium at a flow rate of 1 mL/min and nitrogen at a flow rate of 45 mL/min were used as carrier and make-up gases, respectively. A 1-μL volume of samples was injected in splitless mode.
Quality assurance/quality control
DDT and its metabolites were identified by a comparison of their retention time to the peaks from the calibration standards. Multiple-level calibration curves were created for the quantification, and good linearity (r 2 > 0.995) was achieved. QC and QA were performed by analyses of procedural blanks, spiked blanks, and blind duplicate samples and consisted in daily calibration curves check. Recoveries for individual DDTs in spiked blanks were between 90 and 105%, and average recovery for PCB 77 was 90 ± 9%. The limit of detection (LOD) calculated as three times the signal-to-noise ratio was < 0.1 μg/kg for all DDTs. Confirmation of residues on representative samples using an ENV-8MS capillary column was carried on a Thermo Scientific DSQ II single stage quadrupole GC–MS system.
Statistical analysis
Concentrations of DDTs were tested for homogeneity of variance and log transformed to approximate a normal distribution of the data. As few samples had undetectable values for op′-DDD, op′, and pp′-DDT, geometrical mean calculation was impossible. Therefore, samples with concentrations below the detection limits were assigned a value of one half of the detection limit for statistical analysis (Croghan and Egeghy 2003). Student’s t test was used for determining differences in the concentrations of DDTs between both sites and sexes. The significant level was set at 0.05. All statistical analyses were performed with the JMP software version 12.0 (SAS Institute, Cary, NC, USA).
Results and discussion
Table 1 shows basic parameters of the samples. The house rats (n = 21) from Ziway area, Ethiopia, weighed between 33 and 197 g with a geometric mean of 86 g and had a fat content between 1.8 and 7.9% (geometric mean 3.7%). The house rats (n = 24) from Pongola area, South Africa, weighed from 19 to 190 g (geometric mean 73 g) and with fat content between 1.8 and 12% (geometric mean 4.1%). Of the 45 house rats, 14 from Ziway area and 12 from Pongola area were females. The female rats were generally larger than the males, while their lipid content were comparable with those of the males (Table 1). There were no significance differences (p > 0.05) in both body weight and lipid content between both sites and sexes.
Concentrations of DDTs
DDT and its metabolites residues were detected in all liver samples of rats in the present study. Concentrations of total DDTs (ΣDDTs) ranged from 127 to 9155 μg/kg wet weight (ww) (1775 to 215,748 μg/kg lipid weight (lw)) (Fig. 2). Median concentration ΣDDTs were comparable between the two sites, and concentrations were 2121 and 2214 μg/kg wet weight from Ethiopia and South Africa, respectively. In both sampling sites, high dispersion, in the levels of ΣDDTs, ranged from 667 to 9155 μg/kg ww in Ethiopia and from 127 to 7334 μg/kg ww in South Africa, was exhibited within the studied rat samples. This high variation on the magnitude of pollutants could be associated with age, metabolic capabilities, and duration of contamination. However, there was no significant difference in concentration of ΣDDTs between sites (p > 0.05).
Detailed statistical information such as geometrical mean, median, and range values of ΣDDT concentrations (based on wet weight) in the liver tissues is presented in Table 1. In both sites, female rats had higher geometrical mean concentrations of ΣDDTs than the males. This apparently contradicts the usual pattern observed in other mammals, where mature females are less polluted than males of comparable age due to the lactation transfer of lipophilic xenobiotics from mothers to offspring (Berghe et al. 2012; Lailson-Brito et al. 2012; Sørmo et al. 2003). Although the female rats had higher geometric mean/median concentrations of ΣDDTs than male in both sites, no significant difference in ΣDDT concentration was found between sexes in both sampling site (p > 0.05). Overall, the accumulation of DDTs in the studied house rats reflects widespread DDT contamination within indoor and outdoor environment in these two countries.
Lipid content and body weight are important variable for explaining the concentrations of organochlorine compounds like DDTs in the ecosystem. In this study, lipid content instead of body weight was used to avoid bias due to the increased body weight of the pregnant rats and the variation in gut fullness. Thus, log-transformed values of each DDT concentrations were regressed against lipid content (%). With all data pooled for both sites, no significant relationships (p > 0.05) between the lipid levels (%) and log-transformed DDTs (μg/kg ww) were found, even within genders (data not shown). The lack of this significant relationship and sex-related differences in the present study might be due to the great variability of the individual DDT loads detected caused by the current use of DDT for IRS in the sampling places and reduced sample size.
Composition of DDTs
Basic descriptive statistics of DDT and its metabolite concentrations in rat liver are presented in Table 1. p,p′-isomers of DDT, DDE, and DDD and o,p′-isomers of DDT and DDD were detected in the investigated samples. The metabolite present at the highest concentration in the liver of the studied rat samples was p,p′-DDD with geometric mean concentration of 1268 μg/kg ww followed by p,p′-DDE (931 μg/kg ww) in female rats from Ethiopia (Table 1). In other studies, DDE is the main metabolite of DDT found in the liver and adipose tissues of mammals and birds (Lailson-Brito et al. 2012; Sørmo et al. 2003; Tomza-Marciniak et al. 2014; Yohannes et al. 2014b, 2017).
In the environment and in vivo, DDT can be degraded to DDE and DDD. Thus, the DDT profiles or composition index in biota may provide information on input (sources) in the ecosystem or metabolism of DDT in biota. Figure 3 shows the relative (%) distribution of DDT, DDD, and DDE in liver tissues of both sexes. The profile of DDTs in the studied livers was clearly dominated by p,p′-DDD, accounting for 45 to 66%, followed by p,p′-DDE (Fig. 3). The dominance of p,p′-DDD can be explained by a rapid metabolism of p,p′-DDT to p,p′-DDD in the rat liver tissue. An exposure study by Kitamura et al. (2002) has demonstrated that the metabolism of p,p′-DDT to p,p′-DDD in rat liver was favored through two processes: (i) mainly reductive, catalyzed by the microsomal CYP system in the presence of NADPH or NADH under anaerobic conditions; and (ii) via the catalytic action of the heme group of hemoproteins in the liver microsomes of rats, it can also be reduced to p,p′-DDD in a non-enzymatically manner.
Generally, the accumulation profile of DDTs, i.e., DDD ≅ DDE >> DDT, highlighting the fact that p,p′-DDD and p,p′-DDE are resistance against degradation and elimination from the body of the rats, thus accumulated in the liver tissues. Nevertheless, p,p′-DDT was detected in almost all liver samples of the house rats, indicating the presence of fresh DDT source in these countries. Although internationally banned by the Stockholm Convention on Persistent Organic Pollutants, DDT use is exempted for IRS because of high incidence of malaria and corresponding fatalities in some African countries including Ethiopia and South Africa (WHO 2015), in addition to its unrestricted agricultural use.
Comparison with other wild terrestrial mammals
The present study data are important, because they represent, to the best of our knowledge, the first report of DDT residues in house rats from Ethiopia and South Africa, in general from Africa. The absence of data concerning residue levels in liver samples of rats rendered direct analogies extremely difficult. Thus, the residue levels of DDTs reported in other animals elsewhere in the world, and in birds and domestic chicken from the current sampling sites, are referred (Table 2). However, the species are different and interpretation should also be carefully done, as the usage of DDTs, species, nature of the sampled tissues, environment, dates, matrixes, and ecosystems differs widely. Some terrestrial animals have a great capacity to metabolize and eliminate DDTs. Recent literature data show the levels from Europe, where DDTs in wild animals (boar, badger, mongoose, etc.) are mostly detected. Concentrations of DDTs in liver tissues of house rats detected in the present study are higher than the concentrations reported in various wild mammals from Poland (2.13–1116 μg/kg ww) (Cholewa et al. 2015; Tomza-Marciniak et al. 2014) and Spain (liver: < 0.01–2736 μg/kg ww; adipose tissue: < 0.01–63,297 μg/kg lw) (Mateo et al. 2012) (Table 2). Even comparing with other high trophic level predators, levels of DDTs in the present study are higher than the levels of DDTs found in the wild birds’ liver from Ethiopia (Yohannes et al. 2017) and in egg samples of wild birds and chicken fat from South Africa (Bouwman et al. 2008; Barnhoorn et al. 2009). On the other hand, ΣDDT levels in the present study are lower than the levels of DDTs detected from domestic chicken egg (Bouwman et al. 2015) in a DDT-sprayed area from South Africa (Table 2). Care should also be taken to egg samples compared with liver, as the former samples have a higher lipid content, and also, there is transformation of DDT from liver to egg. Overall, the total DDT burden in the liver tissues of the house rats revealed that the sampling regions are contaminated more with DDT.
Potential ecological risks
House rats share the same environment with humans, where DDT is sprayed. Thus, the exposure data measured on rats can be (in)directly applied for risk assessment because many toxicological studies use rodent models. Toxic effects of DDT and its analogues, such as increment of liver weight, pathological alterations of liver, changes in serum estradiol and progesterone levels, and induction of liver cytochrome enzymes, have been studied in rats (Hojo et al. 2006; Li et al. 1995). The health of human and wildlife is relevant due to the process of bioaccumulation and biomagnification of organic pollutants through the predator–prey cycle.
Because of their persistence and excessive use in the past and the present, DDTs continue to be of serious environmental concern worldwide. In the present study, levels of DDTs ranged from 127 to 9155 μg/kg ww in the liver tissues of the house rats suggest that IRS may result in high DDT exposure in the entire population. Previous studies reported high concentrations of DDT and DDE in blood and breast milk from people residing in IRS houses (Aneck-Hahn et al. 2007; Bouwman et al. 2006; Röllin et al. 2009). Thus, the detection of high levels of DDTs in the present study provides additional input for monitoring health risk of human, in particular for pregnant women and children because they spend more time in and around the DDT-sprayed house. On the other hand, the hepatic levels of ∑DDT residues ranged from 1 to 35 μg/g reported to pose a threat to individual bird reproduction and therefore on the population (Blus 2011). Thus, it should be emphasized that birds of prey could be at high risk when preying on these rats with high levels of organic pollutants like DDTs. Wildlife and domestic animals that feed around the sprayed areas and agricultural areas could also be at risk, since DDTs can bioaccumulate and biomagnify through the predator–prey behavior on the food chain. In addition, DDT levels at a range of 5.5 to 910 ng/g ww, which is by far less than from the present study, showed a significant asymmetric testicular morphology in frogs from DDT-sprayed areas in South Africa (Viljoen et al. 2016). Generally, the DDTs that we found in the liver tissues of house rats may have contributed to DDT loads higher up in the food web. This finding supports the need to reduce and eventually terminate the use of DDT in malaria control program and to control its unrestricted agricultural use.
Conclusion
To the best of our knowledge, this is the first report on DDT and its metabolites levels in liver tissues of house rats from Ethiopia and South Africa. Although sample size was limited, the data in the present study clearly indicate the ubiquitous pollution of DDTs in the ecosystems of Ethiopia and South Africa. It stresses the need for further investigations and continuous monitoring of persistent organic pollutants such as OCPs, PCBs, and PBDEs. Even though IRS application has been regarded as an environmentally safer option in malaria vector control, the current result showed a concern for humans and wildlife. In addition, the domesticated animals kept near the homestead where DDT is applied could also be affected by the DDT used in IRS. Due to their importance for the ecosystems and their protection status, it is difficult to perform invasive tissue analyses in humans and wildlife. Thus, monitoring environmental health by using house rats as sentinels could be useful for inferring the state of higher organisms.
References
Aneck-Hahn NH, Schulenburg GW, Bornman MS, Farias P, de Jager C (2007) Impaired semen quality associated with environmental DDT exposure in young men living in a malaria area in the Limpopo Province, South Africa. J Androl 28:423–434
Ardizzone M, Vizio C, Bozzetta E, Pezzolato M, Meistro S, Dondo A, Giorgi I, Seghesio A, Mirabelli D, Capella S, Vigliaturo R, Belluso E (2014) The wild rat as sentinel animal in the environmental risk assessment of asbestos pollution: a pilot study. Sci Total Environ 479-480:31–38
Barnhoorn IEJ, Bornman MS, Jansen van Rensburg C, Bouwman H (2009) DDT residues in water, sediment, domestic and indigenous biota from a currently DDT-sprayed area. Chemosphere 77:1236–1241
Berghe MV, Weijs L, Habran S, Das K, Bugli C, Rees JF, Pomeroy P, Cocaci A, Debier C (2012) Selective transfer of persistent organic pollutants and their metabolites in grey seals during lactation. Environ Int 46:6–15
Bhatia R, Shiau R, Petreas M, Weintraub JM, Farhang L, Eskenazi B (2005) Organochlorine pesticides and male genital anomalies in the child health and development studies. Environ Health Perspect 113(2):220–224
Blus JL (2011) DDT, DDD and DDE in birds. In: Beyer WN, Meador JP (eds) Environmental contaminants in biota-interpreting tissue concentrations. CRC Press, New York, pp 425–443
Bouwman H, Sereda B, Meinhardt HM (2006) Simultaneous presence of DDT and pyrethroid residues in human breast milk from a malaria endemic area in South Africa. Environ Pollut 144:602–917
Bouwman H, Polder A, Venter B, Skaare JU (2008) Organochlorine contaminants in cormorant, darter, egret, and ibis eggs from South Africa. Chemosphere 71:227–241
Bouwman H, Bornman R, Van Dyk C, Barnhoorn I (2015) First report of the concentrations and implications of DDT residues in chicken eggs from a malaria-controlled area. Chemosphere 137:174–177
Carson R (1962) Silent spring. Houghton Mifflin, Boston
Cholewa R, Beutling D, Budzyk J, Pietrzak M, Walorczyk S (2015) Persistent organochlorine pesticides in internal organs of coypu, Myocastor coypus. J Environ Sci Health B 50:590–594
Crews D, Willingham E, Skipper JK (2000) Endocrine disruptors: present issues, future directions. Q Rev Biol 75:243–260
Croghan C, Egeghy PP (2003) Methods of dealing with values below the limit of detection using SAS. Available online: http://analytics.ncsu.edu/sesug/2003/SD08-Croghan.pdf. (Accessed 16 July 2017)
Fisk AT, de Wit CA, Wayland M, Kuzyk ZZ, Burgess N, Letcher R, Braune B, Norstrom R, Blum SP, Sandau C, Lie E, Larsen HJS, Skaar JU, Muir DCG (2005) An assessment of the toxicological significance of anthropogenic contaminants in Canadian arctic wildlife. Sci Total Environ 351–352:57–93
Gerber R, Smit NJ, van Vuren JHJ, Nakayama SMM, Yohannes YB, Ikenaka Y, Ishizuka M, Wepener V (2016) Bioaccumulation and human health risk assessment of DDT and other organochlorine pesticides in an apex aquatic predator from a premier conservation area. Sci Total Environ 550:522–533
Giesy JP, Feyk LA, Jones PD, Kannan K, Sanderson T (2003) Review of the effects of endocrine-disrupting chemical in birds. Pure Appl Chem 75:2287–2303
Hojo H, Aoyama H, Takahashi KL, Shimizu N, Araki M, Takizawa Y, Sakasai K, Kuwahara M, Saka M, Teramoto S (2006) Two-generation reproduction toxicity study in rats with 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane (p,p′-DDT). Congenit Anom Kyoto 46:105–114
Ishizuka M, Takasuga T, Senthil Kumar K, Tanikawa T, Fujita S (2005) Accumulation of persistent organochlorine pollutants and polybrominated diphenyl ether in wild rats, and toxicogenomic analyses of their effects. Organohalogen Compd 67:2435–2436
Kitamura S, Shimizu Y, Shiraga Y, Yoshida M, Sugihara K, Ohta S (2002) Reductive metabolism of p,p′-DDT and o,p′-DDT by rat liver cytochrome P450. Drug Metab Dispos 30:113–118
Lailson-Brito J, Dorneles PR, Azevedo-Silva CE, Bisi TL, Vidal LG, Legat LN, Azevedo AF, Torres JPM, Malm O (2012) Organochlorine compound accumulation in delphinids from Rio de Janeiro State, southeastern Brazilian coast. Sci Total Environ 433:123–131
Li HC, Dehal SS, Kupfer D (1995) Induction of the hepatic CYP2B and CYP3A enzymes by the proestrogenic pesticide methoxychlor and by DDT in the rat. Effects on methoxychlor metabolism. J Biochem Toxicol 10:51–61
Mateo R, Millán J, Rodríguez-Estival J, Camarero PR, Palomares F, Ortiz-Santaliestra ME (2012) Levels of organochlorine pesticides and polychlorinated biphenyls in the critically endangered Iberian lynx and other sympatric carnivores in Spain. Chemosphere 86:691–700
Röllin HB, Sandanger TM, Hansen L, Channa K, Odland JØ (2009) Concentration of selected persistent organic pollutants in blood from delivering women in South Africa. Sci Total Environ 408:146–152
Sørmo E, Skaare J, Lydersen C, Kovacs K, Hammill M, Jenssen B (2003) Partitioning of persistent organic pollutants in grey seal (Halichoerus grypus) mother–pup pairs. Sci Total Environ 302:145–155
Tomza-Marciniak A, Marciniak A, Pilarczyk B, Prokulewicz A, Bakowska M (2014) Interspecies comparison of chlorinated contaminant concentrations and profiles in wild terrestrial mammals from Northwest Poland. Arch Environ Contam Toxicol 66:491–503
Tyler CR, Jobling S, Sumpter JP (1998) Endocrine disruption in wildlife: a critical review of the evidence. Crit Rev Toxicol 28:319–361
UNEP (United Nations Environment Programme) (2016) Report of the DDT expert group on the assessment of the production and use of DDT and its alternatives for disease vector control. Sixth expert group meeting on DDT, 9 November 2016 Geneva, Switzerland. UNEP/POPS/DDT/EG.6/5
Van den Berg H (2009) Global status of DOT and its alternatives for use in vector control to prevent disease. Environ Health Perspect 17:1656–1663
Van Dyk JC, Bouwman H, Barnhoorn IEJ, Bornman MS (2010) DDT contamination from indoor residual spraying for malaria control. Sci Total Environ 408:2745–2752
Viljoen IM, Bornman R, Bouwman H (2016) DDT exposure of frogs: a case study from Limpopo Province, South Africa. Chemosphere 159:335–341
WHO (World Health Organization) (2011) The use of DDT in malaria vector control: WHO position statement. Geneva, Switzerland
WHO (World Health Organization) (2015) World Health Organization. World malaria report. Geneva
Yohannes YB, Ikenaka Y, Saengtienchai A, Watanabe KP, Nakayama SMM, Ishizuka M (2013) Occurrence, distribution, and ecological risk assessment of DDTs and heavy metals in surface sediments from Lake Awassa—Ethiopian Rift Valley Lake. Environ Sci Pollut Res 20:8663–8671
Yohannes YB, Ikenaka Y, Nakayama SMM, Ishizuka M (2014a) Concentrations and human health risk assessment of organochlorine pesticides in edible fish species from a Rift Valley lake—Lake Ziway, Ethiopia. Ecotoxicol Environ Saf 106:95–101
Yohannes YB, Ikenaka Y, Nakayama SMM, Ishizuka M (2014b) Organochlorine pesticides in bird species and their prey (fish) from the Ethiopian Rift Valley region, Ethiopia. Environ Pollut 192:121–128
Yohannes YB, Ikenaka Y, Nakayama SMM, Mizukawa H, Ishizuka M (2017) DDTs and other organochlorine pesticides in tissues of four bird species from the Rift Valley region, Ethiopia. Sci Total Environ 574:1389–1395
You L (2000) p,p′-DDE: an endocrine-active compound with the potential of multiple mechanisms of action. CIIT Activ 20:1–9
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan awarded to M. Ishizuka (no. 16H0177906), Y. Ikenaka (no. 26304043, 15H0282505, 15K1221305), S. Nakayama (16K16197), and the foundations of JSPS Core to Core Program (AA Science Platforms) and the Bilateral Joint Research Project (PG36150002 and PG36150003). We also acknowledge the financial support by the Mitsui & Co., Ltd. Environment Fund; the Sumitomo foundation for Environmental Research Projects; the Soroptimist Japan Foundation; the Nakajima Foundation; and the Inui Memorial Trust for Research on Animal Science. The authors are grateful to thank the local people for allowing us to catch the house rats and Mr. Takahiro Ichise, Ms. Mio Yagihashi, and Ms. Nagisa Hirano for their technical support and great input throughout the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the experiments were performed in accordance with the guidelines approved by the Institutional Animal Care and Use Committee of Hokkaido University.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Hongwen Sun
Rights and permissions
About this article
Cite this article
Yohannes, Y.B., Ikenaka, Y., Ito, G. et al. Assessment of DDT contamination in house rat as a possible bioindicator in DDT-sprayed areas from Ethiopia and South Africa. Environ Sci Pollut Res 24, 23763–23770 (2017). https://doi.org/10.1007/s11356-017-9911-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9911-7