Abstract
Dichlorodiphenyltrichloroethane (DDT) is a well-known insecticide that was introduced and widely used during World War II. In total more than 4.5 million tonnes DDT have been produced. Although its use and production stopped worldwide during the 1970s, it was reintroduced in the 2000s as a malaria vector control by the World Health Organization (WHO). DDT is toxic to animals and humans. Its main characteristics are its persistence, lipophilicity, and bioaccumulative potential. DDT and its metabolites are normally determined in organisms, sediments, or soil by gas chromatography combined with either electron capture detection or, preferably, mass spectrometry. Many interlaboratory studies have been carried out on the analysis of DDT and its metabolites, and certified reference materials are now available. DDT and its metabolites have been found in air, water, sediment, and biota from all over the world. As a consequence of global fractionation and cold condensation, DDT and its metabolites accumulate in the Arctic. Since the reintroduction of DDT against malaria in Africa in 2005, monitoring of DDT compounds is again highly relevant to detect changes in environmental levels of DDT. Such monitoring is particularly needed in Africa. The first indications show an increase of DDT in humans and wildlife.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
1,1,1-Trichloro-2,2-bis(p-chlorophenyl)ethane (DDT) was one of the first synthetic pesticides used. It was first synthesized by an Austrian chemist, Othmar Zeidler, in 1874 [1], but its insecticidal properties were not discovered until 1939, by Paul Muller, a Swiss entomologist, who won the Nobel Prize for this discovery [2]. Its first use as an insecticide was during World War II for the protection of the Allied forces from diseases such as typhus and malaria, which were spread by mosquitoes and lice to humans [2, 3]. This breakthrough, in addition to its effectiveness and low price, led to its commercialization and use for agricultural purposes [1, 2]. One of its great successes was the elimination of malaria from Europe and the USA [1].
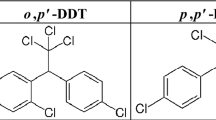
According to the World Health Organization (WHO) specifications for public health pesticides, technical DDT is a white- or cream-colored powder consisting of DDT and manufacturing impurities. Its mixture consists of 80% p,p′-DDT and 20% o,p′-DDT [4].
Dicofol (Fig. 1) is a widely used pesticide, synthesized from DDT. It contains DDT as impurity which must not be above 0.1% according to European legislation [5].
The mass production of DDT started in 1944, and only that year, in the USA alone, 4,366 t were produced, reaching its peak in 1963 (81,154 t) because of the export market. According to the Organization for Economic Cooperation and Development (OECD), the worldwide annual production of DDT in 1974 was 60,000 t [6]. In the former Soviet Union, the mass production of DDT began in 1946 in Moscow, and during the 1950s–1970s, 20,000 t per year were produced. Despite its ban, the production of DDT still continued in the former USSR, and in 1986 10,000 t per year were recorded [7]. According to Li and Macdonald [8], the total worldwide production of DDT from the 1940s until now is estimated to be 4.5 million tonnes, which is almost 3.5 times higher than the estimated total PCB production, according to Breivik et al. [9, 10].
Despite its advantages, DDT was banned in most developed countries during the 1970s. This was due to its harmful effects on the environment and wildlife [11]. Properties such as chemical stability, lipophilicity, and bioaccumulation in the food chain caused a rising concern on its negative effect on humans and wildlife. These properties were also characteristic for 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE), one of DDT’s metabolites [1].
Rachel Carson’s book Silent Spring, in which the author outlined the global character of the DDT problem, had a great impact on the environmental movement and initiated changes in laws that protected the environment [11]. By the mid-1950s, several studies showed that DDT and its metabolites had adverse effects on the reproduction of many animals. For example, thinning of eggshells due to hormonal effects and changes in calcium metabolism by DDT [1] resulted in the potential extinction of the bald eagle, as nesting females were accidentally crushing their eggs [12]. High mortality in fish was also observed, such as young salmon and trout, species that feed on small insects [11]. Also, a mass decline in seal population was observed with their population dropping from 3,000 to 500 within two decades, due to consumption of polluted fish [13]. The wide use of DDT resulted not only in the eradication of malicious pests but also in the death of beneficial insects. As a result, populations of previously minor insects arouse [2].
In January 1970, Sweden was the first country to ban the use of DDT due to its effect on the environment, and in the same year, the former USSR prohibited DDT for agricultural purposes. Despite its ban, DDT production and use continued illegally at many places until 1986. In the early 1970s, the USA banned the use of DDT apart from emergency public health use, and later in 1970, DDT was also banned in Norway as a general pesticide.
Now, many years after its ban, DDT is recommended again by the WHO (World Health Organization) as a malaria vector control in many African countries. As a result, the use and production of DDT is continued in countries such as Mexico, India, and several countries in Africa (e.g., Ethiopia, Mozambique, Zambia, Zimbabwe, South Africa), with India being the largest producer of DDT (4,100–4,500 t for the period 2003–2007) [14, 15]. However, until now and in spite of substantial investments in research, no other alternatives to DDT have been found with the same efficiency.
2 Toxicology
2.1 Human Toxicity
Not many cases of direct human poisoning have been reported, but there is a high concern about the chronic effects of DDT and its metabolites [2]. As a result, many studies have been conducted on the association of DDT with illnesses such as leukemia, brain cancer, prostate cancer, liver cancer, diabetes, and breast cancer [1]. The negative impact on humans of DDT and its metabolites is a rather controversial subject.
Everett et al. [16] reported an association between diabetes and DDT, not only with diagnosed diabetes but also with total diabetes [16].
In vitro studies have shown that DDT shows estrogenic activity in humans, while DDE acts as an androgen antagonist. Some of these studies suggest that DDT is also responsible for the decrease of semen quality [17–19]. Due to their lipophilic nature, DDT and its metabolites are found in relatively high concentrations in breast milk, leading to exposure of children when breast-feeding. This can turn out to be an important contamination factor for infants, especially because earlier studies showed that in Africa, mothers breast-feed their children for up to 2 years [14, 20]. In utero exposure to DDT, even in low concentrations, is shown to alternate thyroid hormones, decrease cognitive skills, and increase asthma in infants [21]. Many researchers also suggest that, due to its bioaccumulative properties, DDT is also responsible for premature death of humans, infant mortality, and effects on the neurological development of infants [2, 21].
After the early 1990s, many studies have focused on the correlation between DDT and breast cancer. Many of them suggested that DDT and its metabolites act as tumor promoters. On the other hand, many reviews pointed out that no correlation between breast cancer and DDT exists [22–25]. Due to these inconsistent data, more research is still needed on this topic [1, 2].
The acceptable daily intake (ADI) of ΣDDT (which consists of p,p′-DDT, p,p′-DDE, p,p′-DDD) is 20 μg/kg/day as established by the FAO/WHO in 1984 [20]. According to the European Union pesticides database, the maximum residue level (MRL) for ΣDDT is 0.05 mg/kg, as set by the Commission Regulation (EU) No. 212/2013. The Codex Alimentarius recently set the MRL for ΣDDT in milk at 20 μg/kg [20].
The International Agency for Research on Cancer (IARC) and the US Environmental Protection Agency (EPA) have categorized DDT as a 2B classification (possibly carcinogenic to humans with sufficient evidence from animal bioassay data, with little or no human data), according to the IARC monograph volumes and EPA’s carcinogen risk assessment guidelines.
2.2 Ecotoxicity
Toxic effects of DDT and its metabolites in animals include a disruption of the endocrine function causing, e.g., feminization of male embryos of many bird species and transformation of male fish embryos to females, among other non-desirable effects [2].
Recently, a correlation between DDT and the size of certain brain regions in birds, which are responsible for mating behavior and song, was reported. Especially in male songbirds, DDT and DDT metabolites reduced brain nuclei that are responsible for the reproductive success, reproductive behavior, and parental care with increasing levels of DDT. A significant reduction of brain and forebrain size was found after exposure to DDT during early development [26].
DDT has also an effect on many animals’ main nervous system. Animals such as rats, guinea pigs, rabbits, and cats, treated with DDT, start to show tremor, ataxia, and finally epileptiform convulsions. Death is noticed due to respiratory failure. In many species DDT may cause arrhythmia and ultimately death due to ventricular fibrillation. The liver is also affected by DDT. Tests that were conducted in mice and rats showed that if fed for long periods with DDT (>2 mg/kg for mice and >5 mg/kg for rats), hypertrophy of the liver has been reported, the first changes being observed after 4 days of administration (exposure times not mentioned) [6].
2.3 Acute Toxicity
Acute toxicity of DDT to mammals is considered moderate. Oral LD50 of DDT in rats ranges from 113 to 118 and 150 to 300 mg/kg in mice, for oral administration in oil. It is less toxic for these animals when exposed via the skin, with an LD50 of 3,000 mg/kg in rats and 1,000–1,500 mg/kg in mice [6, 27]. It mainly affects the central nervous system and the liver. Low levels of DDT in humans may cause nausea, diarrhea, increased liver enzyme activity, and irritation of the eyes, nose, and/or throat [6].
The LD50 in rats of p,p′-DDD is 2,400 and 575 mg/kg of dicofol [6].
3 Analysis
DDT and its main metabolites can be analyzed by gas chromatography (GC) after extraction and cleanup, which should be optimized for each matrix.
3.1 Sampling, Extraction, and Cleanup
3.1.1 Air
Air samples are usually collected with denuder sampling devices, which concentrate the vapor insecticide in a single step. Materials such as polyurethane foam (PUF), Texan, or PUF/Texan are the most common absorbents for the collection and concentration of DDT [28, 29]. Solvent extraction is required before the sample is ready for GC analysis. Thermal desorption is an alternative method with which solvent extraction steps can be avoided [29]. Martin et al. [28] have developed this desorption method for the quantification of DDT in air. One important limitation of this method is the DDT degradation to DDE and DDD due to high temperatures during thermal desorption, which makes it difficult to measure the DDT degradation that may occur in the environment [28].
3.1.2 Water
The usual method for DDT extraction from water samples is solid-phase extraction (SPE). It is used as either an extraction technique or a cleanup technique [30, 31]. Zhou et al. [32] optimized this technique, using silica as an adsorbent. In another study, a one-step extraction of DDT and its metabolites using microwave-assisted headspace controlled-temperature liquid-phase micro-extraction was developed. Results from tests on various water samples showed that this is a rapid, sensitive, cost-effective, and eco-friendly preparation method for the determination of DDT and its metabolites in environmental water samples [32]. Solid-phase micro-extraction (SPME) is also used for the extraction and cleanup of DDT and its metabolites in water samples. Its main advantages are the reduction of sample handling and the use of small amounts of solvents [31]. An interlaboratory study was carried out on the suitability of SPME as a sample preparation method for water samples in pesticide analysis. Eleven laboratories participated. The results showed coefficients of variation (RSD) from 24.7% to 73.3%, with 4 out of 12 results being outliers and two stragglers [33, 34]. Probably due to its limited reproducibility, SPME is not broadly used [31].
3.1.3 Soil, Sediment, and Biota
Soil and sediment are more complex matrices as these can contain sulfur and many other interfering compounds. Soxhlet extraction is the most common method of choice for the extraction of organochlorine pesticides (OCPs) in general [35, 36]. The use of a mixture of polar/nonpolar solvents is essential for the extraction of DDT that is adsorbed to organic carbon in the samples. Other techniques that are being used for DDT extraction from soil samples are supercritical fluid extraction (SFE); accelerated soil extraction (ASE), also known as pressurized liquid extraction (PLE); and microwave-assisted solvent extraction (MASE). Methods such as saponification and sulfuric acid treatment should not be used as degradation may readily occur. Several studies showed that p,p′-DDE degrades immediately after sulfuric acid treatment, and DDT and DDD convert to DDE after saponification [35]. Taylor et al. [37] used PLE for the determination of 13 OCPs, DDT and its metabolites included. Hexane–acetone (1:1 v/v) was considered to be the optimum solvent mixture for the extraction. The removal of sulfur from soil and sediment samples is important as sulfur appears as a broad peak in the chromatograms, thus disturbing the determination of DDT. Several methods can be used to remove sulfur from the extracts, such as copper rods, copper powder, mercury (although not environmentally friendly!), gel permeation chromatography (GPC), and AgNO3-modified silica columns, with the last one being the most effective one, as it removes both elemental sulfur and organosulfur compounds [37, 38].
Extraction and cleanup techniques for most biota are comparable to those used for sediments. Instead of sulfur, fat is the most confounding factor. The solvent mixture is an essential factor to quantitatively extract the OCPs with the lipids from the sample [35]. In most cleanup methods, Florisil, alumina, and silica gel column chromatography are used, as they are suitable for the separation of fat and plant waxes from the samples [35, 39].
3.2 Gas Chromatography
The method of choice for the final chromatographic separation of DDT and its metabolites is capillary GC [32]. The most common detectors are the electron capture detector (ECD) and a number of mass spectrometers (MS) [28, 35, 36]. Most of the environmental sample extracts are complex mixtures. As a result co-elution of compounds may easily occur. Heart-cut multidimensional gas chromatography (MDGC) and comprehensive two-dimensional gas chromatography (GCxGC) are more effective in separation [40]. In GCxGC two ovens can be used, and specific software provides two-dimensional chromatograms [41, 42]. The splitless mode is used most frequently for injection in order to improve sensitivity [30, 32, 35]. Attention must be paid to the temperatures used with this technique, as high temperatures in the splitless mode may result in conversion of DDT into DDD. Due to dirty glass inserts and due to the use of glass wool in the liner conversion of DDT into DDE during GC injection may also occur. Open liners are, therefore, strongly recommended. Degradation over 10% was mentioned as a consequence of a dirty glass liner [44].
Nowadays, fused silica capillary columns are almost exclusively used for the determination of OCPs in general and DDT in particular. The length is usually 25–50 m with an internal diameter of 0.15–0.25 mm. The film thickness should be >0.15 mm to prevent on-column degradation. Fifty meter columns provide a better separation of the analytes but result in longer retention times, thus increasing on-column degradation [35]. Using 50 m columns, PCBs may be separated from DDT, but this needs to be validated. Alternatively, PCBs can be separated from DDT and other OCPs using silica gel column chromatography prior to the GC analysis [45]. The most common capillary column used for the detection of DDT and its metabolites is the HP5-MS column [30, 32, 46].
During GC analysis, interferences may be present. For example, toxaphene may be present in some samples. Toxaphene is a pesticide consisting of a complex mixture of chlorinated camphenes, bornanes, and bornenes, and the peaks may interfere with DDT and its metabolites, hindering their quantification. Numerous studies showed that it is very difficult to separate these compounds in a mixture, even when using different columns in GC. Toxaphene is an aliphatic mixture, which means that the response factors with ECD are much lower than those of the aromatic DDT compounds. That means that in samples from most areas, toxaphene is not a very serious interference. Attention should however be paid to samples from areas that are highly polluted with toxaphene, such as samples from the Arctic. GCxGC may also help for such samples.
The combination of GC and ECD is a valuable and relatively inexpensive method for the determination of DDT and its metabolites, as ECD has a high sensitivity for halogenated aromatic compounds such as DDT [35, 47, 48]. The so-called micro-ECDs are even more sensitive. It is a very common detector, especially for routine environmental analysis, and it is much more affordable than a mass spectrometric (MS) detector [41]. Its only drawback is its poor linearity especially in the low pg range. This problem can be solved with multilevel calibration [35].
GC coupled to MS is another useful technique for the identification of DDT and its metabolites. The MS linearity is much better than that of ECD [35]. It is suitable for qualitative and quantitative analysis of DDT, as it has the ability to detect ultra-trace concentration levels of these compounds [32]. The only disadvantage of GC–MS is its relatively high running cost for a laboratory that carries out routine analysis in environmental samples. However, there has been a major development in MS during the last decades. Sensitivity has increased a lot, whereas prices have dropped considerably. High-resolution sector MS systems are not needed any longer to obtain enough sensitivity for many compounds including DDT. Benchtop quadrupole MS systems, time-of-flight (ToF) MS, orbitraps, and many other systems are nowadays available. These are still more expensive than ECD, but they do offer a wealth of possibilities, including a very high resolution and great sensitivity, which are all very useful for the analysis of DDT, in particular in combination with many other compounds. Another possibility is tandem mass spectrometry, which monitors specific collision-induced dissociation reactions, thus lowering the chemical background and increasing the signal to noise ratio. Also in GC–MS, DDT degradation can occur.
When using a mass spectrometry analysis, much interference from the matrix of the sample can occur. The internal standard method is the ideal one, in order to overcome such interferences. 13C-labeled standards in an isotope dilution can be used for best results, as they have the same physicochemical properties and chromatographical behavior as the target compound, but different m/z ratio [49].
3.3 Interlaboratory Studies and Certified Reference Materials (CRMs)
Since the late 1970s, the analysis of DDT compounds has been evaluated in many interlaboratory tests. There are numerous organizations that conduct such tests, among which the United Nations Environment Programme (UNEP, Geneva, Switzerland); SETOC/WEPAL, Wageningen, Netherlands; and QUASIMEME (Quality Assurance of Information for Marine Environmental Monitoring in Europe), also in Wageningen, Netherlands. A recent interlaboratory study with a participation of 103 laboratories from all over the world was conducted by UNEP for the evaluation of the level of performance on persistent organic pollutant (POP) analysis. The laboratories were requested to analyze two test solutions and fish, sediment, fly ash, and human milk. The extraction and cleanup protocol, spiking schemes, standards, and internal QA/QC were left to the choice of the participating laboratories, but validation of their methods was very much emphasized. The only mandatory parameter was the use of capillary gas chromatography columns, to achieve the right separation for the determination of the analytes. Table 1 shows the results of this study.
The percentage of satisfactory results is given in Fig. 2. The best z-scores were obtained for the standard solution. From this study it became clear that not all laboratories perform well in the analysis of all compounds, but many were specialized in specific categories [43]. It should be noted that many of the participants were from developing countries who participated in such a test for the first time, sometimes after having received only a basic training. The QUASIMEME proficiency tests show a better comparability, caused by the participation of more experienced laboratories. In general GC–MS results are better than those from ECD, due to the higher resolution of GC-MS and lower specificity of ECD [43].
Percentage of laboratories with satisfactory z-scores (i.e., z ± 2) in the test solution, sediment, milk, and fish for ∑DDT [43]
The concern on the presence of DDT and OCPs in general and their effect on the environment has led to an increasing demand for certified reference materials (CRMs) or standard reference materials (SRMs). However, there are not many CRMs/SRMs available for these compounds, as their production and certification is a relatively expensive and slow process. The European Institute for Reference Materials and Measurements (IRMM, Geel, Belgium), the US National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA), and the Canadian National Research Council (NRC, Ottawa, Canada) are the three main producers in the world of CRMs. For OCPs, eight CRMs are available in biota: two cod liver oils, four mussel tissues, whale blubber, and a sea plant homogenate, which are shown in Table 2 [50].
4 Environmental Occurrence
4.1 Air
The most common DDT disposal into the atmosphere is by agricultural spray drift, post-application vitalization, and wind erosion [51]. A study conducted in China showed that DDT and DDT metabolite concentrations were higher in urban areas than in nonurban areas [47]. The concentrations were measured in the gaseous and particular phase and showed that the DDT compounds mainly occur in the gaseous phase (Fig. 3) [47].
Average concentrations of DDTs (sum of o,p′- and p,p′-DDT, DDE, and DDD) in (a) vapor phase, (b) particulate phase, and (c) air samples (gaseous + particulate phases) from Dongguan (DG) and Shunde (SD) obtained in dry and wet weather seasons [47]
Seasonal influences play a role in the DDT monitoring in air, as DDT levels are usually higher in summer than winter (Fig. 4) [47, 51].
Levels of DDTs (mean ± standard deviation) in air of (a) the Pearl River Delta (b) Hong Kong and (c) Guangzhou at an urban site, Guangzhou Branch, Chinese Academy of Science (GCAS), and a suburban site, South China Botanical Garden (SCBG) [51]
The Global Monitoring Plan (GMP) for persistent organic pollutants (POPs) was built with the support of the United Nations Environment Programme (UNEP)/Global Environmental Facility (GEF) involving countries from West and East Africa, the Caribbean, Latin America, and the South Pacific. Air was sampled by the use of PUF disks for passive sampling, and DDT and its metabolites were often the compounds occurring in the highest concentrations (Table 3) [46].
4.2 Water
DDT concentrations in water are very low, as these compounds are highly hydrophobic. Much higher levels are found in particulate matter than in the dissolved phase [48, 49]. The most common sources of DDT in water are from former use in agriculture, atmospheric deposition, and diffuse pollution from erosion processes [49]. o,p′-DDT is more persistent than p,p′-DDT and found in higher concentrations in water samples [48, 49]. Iwata et al. [52] performed an impressive cruise during which they sampled air and analyzed ocean water for several organochlorine contaminants. They found total DDT concentrations to be much lower levels than HCH levels, obviously also related to the higher water solubility of HCHs. Furthermore, they found DDT concentrations much higher in tropical waters and air from southern latitudes compared to northern areas.
4.3 Sediments and Suspended Particular Matter
Suspended particular matter and bottom sediments are the main reservoir of DDT in marine, lake, and river ecosystems [48, 49, 51]. The main inputs of DDT in sediments are agricultural runoff, sewage discharge, atmosphere disposition, and wastewater. Pham et al. [49] conducted a study on suspended sediment in the St. Lawrence River in Canada. ΣDDT concentration in 93 samples was found from non-detectable to 0.34 μg/g, with the most contaminated sediment coming from the Great Lakes and the Ottawa River. Higher concentrations were found during spring probably due to surface runoff and erosion [49]. Another study conducted in Greenland showed that concentrations of DDT in sediment from the Greenland coast are comparable with those from other Arctic regions. On the other hand, data from European estuaries for DDTs were much higher than those found in Greenland [53]. Kelderman et al. [54] studied the pollution of Delft canal sediments and found that DDT and its metabolites were the dominant contaminants. Although DDT was banned in the Netherlands since 1973, these high concentrations of DDT are presumed to be due to their strong persistence in the environment [54].
4.4 Soil
In a study that took place in Guangzhou, China, surface and subsurface vegetable soil samples showed a large variation in DDT levels (3.6–831 ng/g) [51].
Depending on the conditions of the soil, two pathways of degradation may occur: aerobic (p,p′-DDE as a degradation product) and anaerobic (p,p′-DDD as a degradation product) [4]. A study conducted by Wang [55] showed that DDT concentrations in soil are dependent of its agricultural use. After growing specific plant species in DDT-contaminated soil, the total amount of DDT and DDE in soil had decreased, and this decrease was equal to the total amount of DDT and DDE measured in the plants [55]. In this way, phytoremediation of DDT-contaminated soil can take place. Another important factor for DDT degradation in soil is the pH value. There seems to be a strong correlation between pH and concentrations of DDT and its metabolites in soil. A study showed that at pH < 8.0, DDT metabolites were present in higher concentrations than at pH > 8.0. This may be caused by the change of the humic acid structure from fibrous to smaller sheetlike structure, at pH > 7.0 [4].
4.5 Aquatic Organisms
Fishes are generally used for environmental monitoring, as the contaminant loading in fish can reflect the state of their ecosystem [51]. A study conducted in Baltic herring and pike showed large variations in DDT levels (ND–10 mg/kg), depending on the differences in the fat content of the fish. Also, DDT levels vary between tissues of muscle and some organs. For example, in the same study, kidney of pike appeared to be a possible storage site [56]. The fat content of the various tissues is determining the final concentrations of DDT. For example, in cod muscle tissue with a fat content of ca. 1%, the DDT concentrations are much lower than in cod liver, which has an average fat content of 50% [57]. On the contrary, in eel, the DDT concentrations are much higher in the muscle tissue, which has a fat content of 5–40%, compared to those in the liver of the eel with a fat content of <5% [58]. DDT levels in farmed fish (freshwater and seawater) vary with the contamination of the DDT level in their feed [51].
Marine shellfish, especially mussels, are often used as local indicators of DDT contamination, as these species are highly bioaccumulative and do not migrate. In a study on OCPs in mussels and oysters collected from the east coast of Thailand, DDT was the contaminant with the highest concentrations in both organisms. This study also concluded that there is a decreasing trend of DDT compounds in mussels since 1979 [59].
5 Time Trends
Several publications have appeared on temporal trends of DDT and its metabolites. A study on DDT and its metabolites in cod liver oil from the Baltic Sea during the period 1971–1989 showed that DDT concentrations were rapidly declining from 1974 onwards. ΣDDT ranged from 0.4 to 25 μg/g (on a lipid weight (lw) basis). p,p′-DDE was the dominant compound followed by p,p′-DDD and p,p′-DDT [60]. The NOAA mussel watch program shows a decline of total DDT concentrations (mainly DDE) from 1,600 to 300 ng/g dw over the period 1986–2002 in Palos Verdes, CA, USA [61]. Macgregor et al. [62] reported a declining trend in eel in most, but not all, locations in Scotland. On the other hand, Suns et al. [63], after studying ΣDDT temporal trends in spottail shiners from the Great Lakes over the period 1975–1990, concluded that although there has been a decline in DDT concentrations during these years, they tend to stabilize after the 1980s, especially DDE [63]. Zhang et al. [64] reported increasing DDT levels in sediment cores from the Pearl River Delta, China. They assumed that excessive soil runoff enhanced by the large-scale land transform and regional flooding in the Pearl River Delta might have contributed to the transport of organochlorine pesticides from soil to the sedimentary system in the early 1990s. They also found increasing DDE/DDT ratios, pointing to former rather than recent use of DDT. An atmospheric monitoring program (1993–2006) in the Arctic showed, in general, low levels of DDT-related compounds during the entire period. Although there was a reduction in concentration of these compounds in the Arctic during the 1990s, this reduction was lower during later years, and concentrations of DDT and its metabolites are almost stable now. The only exception was during the summer of 2004 when a summer forest fire occurred in Yukon, Canada. During that period, high concentrations of p,p′-DDE and o,p′-DDE were observed at two stations, probably due to biomass burning. No seasonal profiles were observed, apart for p,p′-DDE, in which concentrations were higher during the winter period [65]. Guglielmo et al. [66] studied the global environmental cycling of DDT in the 1980s for one decade. Figure 5 shows that multiple cycles are responsible for the DDT burden in soil, vegetation, ocean water, and sea ice [66]. The study concludes that after 10 years, approximately 12% of DDT would have accumulated in the Arctic. Assuming continuing low DDT emissions, Schenker et al. [67] showed in a model that concentrations will decrease by a factor of 30 in temperate regions and by a factor of 100 in the Arctic, as compared to the concentrations in the 1960s and 1970s. In the tropics, levels decrease by a factor of 5 to 10, only.
DDT global mass fractions (%) in atmosphere (gray), soil (orange), vegetation (green), and ocean and sea ice (blue) in first cycle (single hop, full line) and during subsequent cycles (multi-hop, dashed line) [67]
In 2002, a POP assessment in the Arctic occurred by the Arctic Monitoring and Assessment Programme (AMAP). In this assessment, temporal trends of POPs in air, water, sediment, and Arctic biota were examined. Many interesting conclusions came out of this assessment. Data of temporal trends of POPs in Arctic air were available from five air monitoring stations in the Arctic. Data was collected from 1993 to 2000. The main DDT metabolites that were found were o,p′-DDE and p,p′-DDE. Seasonal variation was present at all sites. According to Hung et al. [68], p,p′-DDE is decreasing slightly at the Alert station, while a slight increase of o,p′-DDT during the period 1993–1998 was also reported. This could be due to an unknown source causing technical DDT entering the Arctic [68].
There are a number of temporal trend data of DDT in freshwater and sediments in the Arctic. Zhulidov et al. [69] reported ΣDDT changes in water and sediments from eight Russian Arctic rivers during the period 1988–1994. Concentrations of ΣDDT declined over the years, reaching detection limits of ng/L magnitude by 1992 (Fig. 6) [69].
Temporal changes in DDT, DDD, and DDE concentrations (mean ± S.E.) in the water and sediments of eight Russian rivers [69]
All temporal trend studies carried out in the Arctic indicate that, although there has been a substantial decrease of DDT and its metabolites in biota since the 1970s, concentrations of ΣDDT stabilized from the 1990s to the 2000s [70].
Research on POPs was not only conducted in the Arctic ecosystem but also in Antarctica. Most of the studies concluded that p,p′-DDE in particular is the most persistent organic contaminant in Antarctic biota [71–73]. Trends of DDT and its metabolites in Antarctic biota showed a decreasing pattern during the period 1980–1995, but increased again in 2000–2002 [74]. The main reasons that these compounds are found in the Arctic and the Antarctic are the global fractionation and cold condensation processes [75]. Comparing data from research in the Arctic and the Antarctic shows that concentrations of DDT found in the Arctic are much higher than those found in the Antarctic. This is mainly due to geographical features. The Antarctic region is surrounded by the Southern Ocean, which can act as a boundary for POPs. Therefore, they can reach the Antarctic only through air mass transport [74, 75]. The Southern Hemisphere contains less land mass, which is also relatively far away from the South Pole. In addition, the population density at the Southern Hemisphere is relatively low, while, on the contrary, the dense population of the Northern Hemisphere causes a higher DDT level in the Arctic [74]. The increase of DDT in Antarctica is, therefore, very interesting, as the question is if this is related to the renewed application of DDT in southern Africa. Although based on a limited data set, Alava et al. [15] reported a significant increase in DDT concentrations in Galapagos sea lion pups between 2005 and 2008 (280–530 μg/kg lipid weight). These levels are able to affect the immune system and population dynamics during periods of nutritional stress such as El Niño events. The authors concluded that DDT and associated health risks in wildlife are generally believed to be declining, but this may no longer be the case in tropical countries where DDT is increasingly used. Lopez-Carillo et al. [76] expressed their concern in 1996 about relatively high DDT levels in Mexico, higher than in other Latin-American countries. Women living in Mexico City showed up to 0.6 mg/kg lipid weight in their breast milk. Increasing DDT trends were also suggested by Manaca et al. [77]. Examination of the distributions of DDT and its metabolites in the walls of 43 dwellings from Manhiça (Mozambique) showed median levels of 19, 130, and 23 ng/g for o,p′-DDT, p,p′-DDT, and p,p′-DDE, respectively, directly after indoor residual spraying (IRS). The concentrations of these compounds at the onset of the IRS campaign (n = 48) were 5.5, 47, and 2.2 ng/g, respectively. In another study, Manaca et al. [78] reported concentrations of DDT compounds in the cord blood of 214 children born between 2003 and 2006, so before IRS, in Manhiça (Mozambique) of 0.8 and 0.4 ng/ml for p,p′-DDE and p,p′-DDT, respectively, were similar to those found in western countries. However, the p,p′-DDT/p,p′-DDE ratio was high indicating an association with recent use of DDT. A significant increase in p,p′-DDT and its main metabolite, p,p′-DDE, in mother’s milk samples from Manhiça was observed between 2002 (median values 2.4 and 0.9 ng/ml, respectively) and 2006 (7.3 and 2.6 ng/ml, respectively). Grimalt [79] reports a further significant increase of DDT and its metabolites in the same mother and newborn cohort in 2010. Channa et al. [17] found elevated p,p′-DDT/p,p′-DDE ratios in coastal KwaZulu-Natal, also outside the endemic malaria areas, which indicates recent ongoing and possibly illegal use, since DDT use is only allowed in designated areas. The DDT concentrations reported by Manaca et al. [77] and Grimalt [79] are above the thresholds for deleterious effects in cognitive skill observed in children at 4 years of age. This was also confirmed by Bouwman et al. [80] who reported DDT levels in 163 breast milk samples from South Africa. Mean ΣDDT levels in breast milk were 18, 11, and 9.5 mg/kg lipid weight from three DDT-sprayed villages, respectively, including the highest ΣDDT level ever reported for breast milk from South Africa (140 mg/kg lipid weight). A provisional tolerable daily intake for DDT by infants was significantly exceeded. Van Dyk et al. [81] reported elevated DDT levels in the Limpopo province in South Africa and raise concern on the potential health effects in residents living in the immediate environment following DDT IRS.
6 Conclusions
At present, DDT’s use is mainly as an insecticide in African countries for the fight against malaria. However, its effect on future generations is concerning many researchers [82]. Its persistence, estrogenic activity, lipophilic nature, and ability to bioaccumulate in higher organisms of the food chain are the main factors of concern as DDT, and its metabolites seem to be responsible for illnesses such as diabetes, asthma, and cancer. More than 75 years after its introduction, DDT and its metabolites are still present in many organisms and abiotic environmental compartments all over the world which shows the extreme persistence of this compound. As its main use is for malaria vector control, there is a need to search for alternatives of DDT, which would be less toxic to living organisms and more environmentally friendly. The two main alternatives are indoor residual spraying and insecticide-treated bed nets with pyrethroids instead of DDT [14]. The main drawback of pyrethroids is that some mosquito breeding, particularly in Africa, seems to be resistant to this particular insecticide [14]. Another alternative that has the prospect to eliminate completely malaria is malaria vaccination. Much research has been done on this matter for more than 30 years [83–85], but only recently tests and clinical trials have been conducted [84]. DDT is still present in the environment and it is unknown for how long it will exist. Most trend studies show that there has been a decreasing trend of DDT concentrations in the environment, but these concentrations seem to stabilize over the last decade. This may be caused by its reintroduction as a malaria vector control in many African countries, but this needs further evidence. There is no concrete evidence that DDT and its metabolites can be eliminated completely from the environment. Ongoing environmental monitoring is important, especially in Africa, to detect a possible increase in DDT levels in the environment, as is suggested by a study in sea lion pups from the Galapagos Archipelago [15] and several studies recently carried out in Africa [21, 77, 78, 80, 86]. The Global Monitoring Plan of the Stockholm Convention may enable such monitoring in the near future [46].
References
Beard J (2006) DDT and human health. Sci Total Environ 355:78–89. doi:10.1016/j.scitotenv.2005.02.022
Walker K, Ricciardone M, Jensen J (2003) Developing an international consensus on DDT: a balance of environmental protection and disease control. Hyg Environ Health 206:423–435
Purnomo A, Mori T, Kamei I, Kondo R (2011) Basic studies and applications on bioremediation of DDT: a review. Int Biodeterior Biodegrad 65:921–930. doi:10.1016/j.ibiod.2011.07.011
Gong ZM, Tao S, Xu FL et al (2004) Level and distribution of DDT in surface soils from Tianjin, China. Chemosphere 54:1247–1253. doi:10.1016/j.chemosphere.2003.10.021
Turgut C, Gokbulut C, Cutright TJ (2009) Contents and sources of DDT impurities in dicofol formulations in Turkey. Environ Sci Pollut Res Int 16:214–217. doi:10.1007/s11356-008-0083-3
World Health organization (1979) Environmental health criteria 9: DDT and its derivatives. 194. World Health Organization, Geneva, pp 10
Fedorov L (1999) Persistent organic chemicals in the former Soviet Union. Environ Pollut 105:283–287
Li YF, Macdonald RW (2005) Sources and pathways of selected organochlorine pesticides to the Arctic and the effect of pathway divergence on HCH trends in biota: a review. Sci Total Environ 342:87–106. doi:10.1016/j.scitotenv.2004.12.027
Breivik K (2004) Primary sources of selected POPs: regional and global scale emission inventories. Environ Pollut 128:3–16. doi:10.1016/j.envpol.2003.08.031
Lohmann R, Breivik K, Dachs J, Muir D (2007) Global fate of POPs: current and future research directions. Environ Pollut 150:150–165. doi:10.1016/j.envpol.2007.06.051
Carson R (1962) Silent spring. Houghton Mifflin, Boston
Stokstad E (2007) Can the bald eagle still soar after it is delisted? Science 316:1689–1690
Reijnders P (1986) Reproductive failure in common seals feeding on fish from polluted coastal waters. Nature 324:456–457
Van den Berg H (2009) Global status of DDT and its alternatives for use in vector control to prevent disease. Environ Health Perspect 117:1656–1663. doi:10.1289/ehp.0900785
Alava JJ, Salazar S, Cruz M et al (2011) DDT strikes back: Galapagos sea lions face increasing health risks. Ambio 40:425–430. doi:10.1007/s13280-011-0136-6
Everett CJ, Frithsen IL, Diaz V et al (2007) Association of a polychlorinated dibenzo-p-dioxin, a polychlorinated biphenyl, and DDT with diabetes in the 1999–2002 National Health and Nutrition Examination Survey. Environ Res 103:413–418. doi:10.1016/j.envres.2006.11.002
Channa K, Röllin H, Nøst T (2012) Prenatal exposure to DDT in malaria endemic region following indoor residual spraying and in non-malaria coastal regions of South Africa. Sci Total Environ 429:183–190. doi:10.1016/j.scitotenv.2012.03.073
Phillips KP, Tanphaichitr N (2008) Human exposure to endocrine disrupters and semen quality. J Toxicol Environ Health B Crit Rev 11:188–220. doi:10.1080/10937400701873472
Tavares RS, Mansell S, Barratt CLR et al (2013) p,p′-DDE activates CatSper and compromises human sperm function at environmentally relevant concentrations. Hum Reprod 28:3167–3177. doi:10.1093/humrep/det372
Bouwman H, Sereda B, Meinhardt HM (2006) Simultaneous presence of DDT and pyrethroid residues in human breast milk from a malaria endemic area in South Africa. Environ Pollut 144:902–917. doi:10.1016/j.envpol.2006.02.002
Manaca M, Grimalt J, Sunyer J (2011) Concentration of DDT compounds in breast milk from African women (Manhiça, Mozambique) at the early stages of domestic indoor spraying with this insecticide. Chemosphere 85:307–314. doi:10.1016/j.chemosphere.2011.06.015
Calle EE, Frumkin H, Henley SJ et al (1977) Organochlorines and breast cancer risk. CA Cancer J Clin 52:301–309
Snedeker SM (2001) Pesticides and breast cancer risk: a review of DDT, DDE, and dieldrin. Environ Health Perspect 109(Suppl 1):35–47
Charlier C, Albert A, Herman P et al (2003) Breast cancer and serum organochlorine residues. Occup Environ Med 60:348–351
Wolff MS, Berkowitz GS, Brower S et al (2000) Organochlorine exposures and breast cancer risk in New York City women. Environ Res 84:151–161. doi:10.1006/enrs.2000.4075
Iwaniuk AN, Koperski DT, Cheng KM et al (2006) The effects of environmental exposure to DDT on the brain of a songbird: changes in structures associated with mating and song. Behav Brain Res 173:1–10. doi:10.1016/j.bbr.2006.05.026
Faroon O, Harris M, Llados F, Swarts S (2002) Toxicological profile for DDT, DDE, and DDD. Agency for Toxic Substances and Disease Registry, Atlanta, p 403
Martin NJ, Smith P, Brown CW et al (2012) Dichlorodiphenyltrichloroethane determination in air by thermal desorption gas chromatography–mass spectrometry. Pest Manag Sci 68:1360–1367. doi:10.1002/ps.3313
Naudé Y, Rohwer ER (2012) Novel method for determining DDT in vapour and particulate phases within contaminated indoor air in a malaria area of South Africa. Anal Chim Acta 730:112–119. doi:10.1016/j.aca.2012.02.054
Sánchez-Avila J, Fernandez-Sanjuan M, Vicente J, Lacorte S (2011) Development of a multi-residue method for the determination of organic micropollutants in water, sediment and mussels using gas chromatography-tandem mass spectrometry. J Chromatogr A 1218:6799–6811. doi:10.1016/j.chroma.2011.07.056
Martínez Vidal JL, Plaza-Bolaños P, Romero-González R, Garrido Frenich A (2009) Determination of pesticide transformation products: a review of extraction and detection methods. J Chromatogr A 1216:6767–6788. doi:10.1016/j.chroma.2009.08.013
Zhou Q, Wu W, Xie G (2013) Solid phase extraction with silicon dioxide microsphere adsorbents in combination with gas chromatography-electron capture detection for the determination of DDT and its metabolites in water samples. J Environ Sci Health B 48:191–197. doi:10.1080/03601234.2013.730289
Vinoth Kumar P, Jen J-F (2011) Rapid determination of dichlorodiphenyltrichloroethane and its main metabolites in aqueous samples by one-step microwave-assisted headspace controlled-temperature liquid-phase microextraction and gas chromatography with electron capture detection. Chemosphere 83:200–207. doi:10.1016/j.chemosphere.2010.12.041
Doong R, Liao PL (2001) Determination of organochlorine pesticides and their metabolites in soil samples using headspace solid-phase microextraction. J Chromatogr A 918:177–188
Górecki T, Mindrup R, Pawliszyn J (1996) Pesticides by solid-phase microextraction. Results of a round robin test. Analyst 121:1381–1386
De Boer J, Law RJ (2003) Developments in the use of chromatographic techniques in marine laboratories for the determination of halogenated contaminants and polycyclic aromatic hydrocarbons. J Chromatogr A 1000:223–251
Taylor P, Moreno DV, Ferrera ZS, Rodríguez JJS (2008) Determination of organochlorine pesticides from agricultural soils using pressurized liquid extraction. Soil Sediment Contam 17:1–11. doi:10.1080/15320380701741263
You J, Lydy MJ (2004) Evaluation of desulfuration methods for pyrethroid, organophosphate, and organochlorine pesticides in sediment with high sulfur content. Arch Environ Contam Toxicol 47:148–153. doi:10.1007/s00244-003-3166-7
Van der Hoff GR, van Zoonen P (1999) Trace analysis of pesticides by gas chromatography. J Chromatogr A 843:301–322
Naudé Y, Rohwer ER (2012) Two multidimensional chromatographic methods for enantiomeric analysis of o, p′-DDT and o, p′-DDD in contaminated soil and air in a malaria area of South Africa. Anal Chim Acta 730:120–126. doi:10.1016/j.aca.2012.03.028
Korytár P, Leonards PEG, de Boer J, Brinkman UAT (2005) Group separation of organohalogenated compounds by means of comprehensive two-dimensional gas chromatography. J Chromatogr A 1086:29–44. doi:10.1016/j.chroma.2005.05.087
Korytár P, Parera J, Leonards PEG et al (2005) Quadrupole mass spectrometer operating in the electron-capture negative ion mode as detector for comprehensive two-dimensional gas chromatography. J Chromatogr A 1067:255–264. doi:10.1016/j.chroma.2004.11.045
Van Leeuwen SPJ, De Boer J, Van Bavel B (2013) First worldwide UNEP interlaboratory study on persistent organic pollutants (POPs), with data on polychlorinated biphenyls and organochlorine pesticides. TrAC Trends Anal Chem 46:110–117. doi:10.1016/j.trac.2012.12.020
Gfrerer M, Lankmayr E (2005) DDT degradation during enhanced solid–liquid extractions. J Chromatogr A 1072:117–125. doi:10.1016/j.chroma.2005.03.024
De Boer J, Wells DE (1996) The 1994 QUASIMEME laboratory-performance studies: chlorobiphenyls and organochlorine pesticides in fish and sediment. Mar Pollut Bull 32:654–666. doi:10.1016/0025-326X(96)00074-4
Leslie H, van Bavel B, Abad E, de Boer J (2013) Towards comparable POPs data worldwide with global monitoring data and analytical capacity building in Africa, Central and Latin America, and the South Pacific. TrAC Trends Anal Chem 46:85–97. doi:10.1016/j.trac.2013.01.009
Yue Q, Zhang K, Zhang B-Z et al (2011) Occurrence, phase distribution and depositional intensity of dichlorodiphenyltrichloroethane (DDT) and its metabolites in air and precipitation of the Pearl River Delta, China. Chemosphere 84:446–451. doi:10.1016/j.chemosphere.2011.03.045
Pham T, Lum K, Lemieux C (1996) Seasonal variation of DDT and its metabolites in the St. Lawrence River (Canada) and four of its tributaries. Sci Total Environ 179:17–26
Pham T, Lum K, Lemieux C (1993) The occurrence, distribution and sources of DDT in the St. Lawrence River, Quebec (Canada). Chemosphere 26:1595–1606
De Boer J, McGovern E (2001) Certified reference materials for organic contaminants for use in monitoring of the aquatic environment. TrAC Trends Anal Chem 20:140–159
Guo Y, Yu H-Y, Zeng EY (2009) Occurrence, source diagnosis, and biological effect assessment of DDT and its metabolites in various environmental compartments of the Pearl River Delta, South China: a review. Environ Pollut 157:1753–1763. doi:10.1016/j.envpol.2008.12.026
Iwata H, Tanabe S, Sakai N, Tatsukawa R (1993) Distribution of persistent organochlorines in the oceanic air and surface seawater and the role of ocean on their global transport and fate. Environ Sci Technol 27:1080–1098. doi:10.1021/es00043a007
Cleemann M, Riget F, Paulsen GB et al (2000) Organochlorines in Greenland marine fish, mussels and sediments. Sci Total Environ 245:87–102
Kelderman P, Drossaert W, Min Z (2000) Pollution assessment of the canal sediments in the city of Delft (the Netherlands). Water Res 34:936–944
Wang H (2008) Plant growth-promoting rhizobacteria (PGPR) enhanced phytoremediation of DDT contaminated soil. MSc thesis, p 117, University of Waterloo, Canada
Linko R, Kaitaranta J (1974) Occurrence of DDT and PCB compounds in Baltic herring and pike from the Turku archipelago. Environ Pollut 7:193–207
de Boer J (1988) Trends in chlorobiphenyl contents in livers of Atlantic cod (Gadus morhua) from the North Sea, 1979–1987. Chemosphere 17:1811–1819
Belpaire CGJ, Goemans G, Geeraerts C et al (2009) Decreasing eel stocks: survival of the fattest? Ecol Freshw Fish 18:197–214. doi:10.1111/j.1600-0633.2008.00337.x
Cheevaporn V, Duangkaew K, Tangkrock-Olan N (2005) Environmental occurrence of organochlorines in the East Coast of Thailand. J Health Sci 51:80–88. doi:10.1248/jhs.51.80
Kannan K, Falandysz J, Yamashita N et al (1992) Temporal trends of organochlorine concentrations in cod-liver oil from the southern Baltic proper, 1971–1989. Mar Pollut Bull 24:358–363. doi:10.1016/0025-326X(92)90373-E
The National Centers for Coastal Ocean Science (NCCOS) https://noaaoceanscience.wordpress.com/2012/02/16/changes-over-time-mussel-watch/, 6 March 2015
Macgregor K, Oliver IW, Harris L, Ridgway IM (2010) Persistent organic pollutants (PCB, DDT, HCH, HCB & BDE) in eels (Anguilla anguilla) in Scotland: current levels and temporal trends. Environ Pollut 158:2402–2411. doi:10.1016/j.envpol.2010.04.005
Suns KR, Hitchin GG, Toner D (1993) Spatial and temporal trends of organochlorine contaminants in spottail shiners from selected sites in the Great Lakes (1975–1990). J Great Lakes Res 19:703–714. doi:10.1016/S0380-1330(93)71258-7
Zhang G, Parker A, House A et al (2002) Sedimentary records of DDT and HCH in the Pearl River Delta, South China. Environ Sci Technol 36:3671–3677. doi:10.1021/es0102888
Hung H, Kallenborn R, Breivik K et al (2010) Atmospheric monitoring of organic pollutants in the Arctic under the Arctic Monitoring and Assessment Programme (AMAP): 1993–2006. Sci Total Environ 408:2854–2873. doi:10.1016/j.scitotenv.2009.10.044
Guglielmo F, Lammel G, Maier-Reimer E (2009) Global environmental cycling of gamma-HCH and DDT in the 1980s–a study using a coupled atmosphere and ocean general circulation model. Chemosphere 76:1509–1517. doi:10.1016/j.chemosphere.2009.06.024
Schenker U, Scheringer M, Hungerbühler K (2008) Investigating the global fate of DDT: model evaluation and estimation of future trends. Environ Sci Technol 42:1178–1184. doi:10.1021/es070870h
Hung H, Halsall CJ, Blanchard P et al (2002) Temporal trends of organochlorine pesticides in the Canadian Arctic atmosphere. Environ Sci Technol 36:862–868
Zhulidov AV, Robarts RD, Headley JV et al (2002) Levels of DDT and hexachlorocyclohexane in burbot (Lota lota l.) from Russian Arctic rivers. Sci Total Environ 292:231–246
AMAP, 2004. AMAP Assessment (2002) persistent organic pollutants in the Arctic. Arctic Monitoring and Assessment Programme (AMAP), Oslo, 310 pp
Schiavone A, Corsolini S, Borghesi N, Focardi S (2009) Contamination profiles of selected PCB congeners, chlorinated pesticides, PCDD/Fs in Antarctic fur seal pups and penguin eggs. Chemosphere 76:264–269. doi:10.1016/j.chemosphere.2009.03.007
Corsolini S, Covaci A, Ademollo N et al (2006) Occurrence of organochlorine pesticides (OCPs) and their enantiomeric signatures, and concentrations of polybrominated diphenyl ethers (PBDEs) in the Adélie penguin food web, Antarctica. Environ Pollut 140:371–382. doi:10.1016/j.envpol.2005.04.039
Weber K, Goerke H (1996) Organochlorine compounds in fish off the Antarctic Peninsula. Chemosphere 33:377–392. doi:10.1016/0045-6535(96)00204-4
Corsolini S (2009) Industrial contaminants in Antarctic biota. J Chromatogr A 1216:598–612. doi:10.1016/j.chroma.2008.08.012
Wania F, Mackay D (1993) Global fractionation and cold condensation of low volatility organochlorine compounds in polar regions. Ambio 22:10–18
López-Carrillo L, Torres-Arreola L, Torres-Sánchez L et al (1996) Is DDT use a public health problem in Mexico? Environ Health Perspect 104:584–588. doi:10.1289/ehp.96104584
Manaca MN, Grimalt JO, Gari M et al (2012) Assessment of exposure to DDT and metabolites after indoor residual spraying through the analysis of thatch material from rural African dwellings. Environ Sci Pollut Res Int 19:756–762. doi:10.1007/s11356-011-0601-6
Manaca MN, Grimalt JO, Sunyer J et al (2013) Population characteristics of young African women influencing prenatal exposure to DDT (Manhiça, Mozambique). Environ Sci Pollut Res Int 20:3472–3479. doi:10.1007/s11356-012-1293-2
Grimalt JO (2014) Impacts and potential effects of DDT reintroduction against malaria in African populations. In: International symposium on halogenated persistent pollutants, Madrid, 31 August–5 September. http://www.dioxin2014.org
Bouwman H, Kylin H, Sereda B, Bornman R (2012) High levels of DDT in breast milk: intake, risk, lactation duration, and involvement of gender. Environ Pollut 170:63–70. doi:10.1016/j.envpol.2012.06.009
Van Dyk JC, Bouwman H, Barnhoorn IEJ, Bornman MS (2010) DDT contamination from indoor residual spraying for malaria control. Sci Total Environ 408:2745–2752. doi:10.1016/j.scitotenv.2010.03.002
Bouwman H, Van den Berg H, Kylin H (2011) DDT and malaria prevention: addressing the paradox. Environ Health Perspect 119:744–747
Greenwood B (2008) Progress in malaria control in endemic areas. Travel Med Infect Dis 6:173–176. doi:10.1016/j.tmaid.2007.11.003
Boggild HK, Kain KC (2008) Protozoan diseases: malaria clinical features, management, and prevention. Int Encycl Public Health 371–382. doi:10.1016/B978-012373960-5.00490-1
Moss WJ, Shah RHM, Morrow RH (2008) The history of malaria and its control. Int Encycl Public Health 389–398. doi:10.1016/B978-012373960-5.00374-9
Valvi D, Mendez M (2012) Prenatal concentrations of polychlorinated biphenyls, DDE, and DDT and overweight in children: a prospective birth cohort study. Environ Health Perspect 120:451–457
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Mirmigkou, S., de Boer, J. (2015). DDT and Metabolites. In: Alaee, M. (eds) Dioxin and Related Compounds. The Handbook of Environmental Chemistry, vol 49. Springer, Cham. https://doi.org/10.1007/698_2015_367
Download citation
DOI: https://doi.org/10.1007/698_2015_367
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23888-3
Online ISBN: 978-3-319-23889-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)