Abstract
Many countries with incidence of malaria, including those surrounding Maputo Bay, use dichloro-diphenyl-trichloroethane (DDT) to reduce mosquitoes. This study is the first to estimate the human health risk associated with consumption of marine fish from Maputo Bay contaminated with DDTs. The median for ∑DDTs was 3.8 ng/g ww (maximum 280.9 ng/g ww). The overall hazard ratio for samples was 1.5 at the 75th percentile concentration and 28.2 at the 95th percentile. These calculations show increased potential cancer risks due to contamination by DDTs, data which will help policy makers perform a risk–benefit analysis of DDT use in malaria control programs in the region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Some 90% of global malaria cases occur in the African Region, necessitating control measures (WHO 2016). Indoor residual spraying (IRS) with pesticides such as dichloro-diphenyl-trichloroethane (DDT) is commonly used under WHO recommendation to control mosquito vectors in many countries. This pesticide contaminates the environment and is persistent for many years. The ecological risk of DDTs on fish and other wildlife has been common knowledge for over seven decades (Cottam and Higgins 1946). Although the mechanisms of toxicity are still unclear, DDT has now been classed by the International Agency for Research on Cancer as Group 2A, an agent probably carcinogenic to humans (IARC 2017).
Maputo Bay is an important environmental site as water originating from the Phongolo/Maputo River Basin in three countries – Mozambique, Swaziland and South Africa – enters the Indian Ocean here. During the rainy season, mosquito populations and malaria cases increase. Thus DDT is applied annually just before commencement of the rainy summer season (WHO 2016). DDTs are hydrophobic, but travel within waterways either adsorbed to sediment or within biota. Previous studies have confirmed contamination of freshwater fish within the Phongolo flood plain, without significant seasonal variation (Bouwman et al. 1990; McHugh et al. 2011). Despite the use of IRS for malaria control in these countries, there is limited information from literature assessing the impacts on the environment and residents (Blumberg and Frean 2007). The objective of this study was therefore to estimate the human health risk associated with consumption of marine fish from Maputo Bay contaminated with DDT and its metabolites.
Materials and Methods
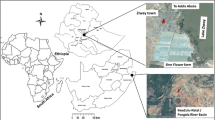
Marine species caught by fishermen in Maputo Bay were purchased from local markets on Inhaca Island, Mozambique (Fig. 1). The species were mainly reef fish, with various dietary behaviours (Heemstra and Heemstra 2004). Samples included: rockcod (Epinephelus spp., n = 7), blacktip kingfish (Caranx heberi, n = 5), spadefish (Tripterodon orbis, n = 4), delagoa threadfin bream (Nemipterus bipunctatus, n = 3), blue-lined barenose (Gymnocranius grandoculis, n = 2) and great barracuda (Sphyraena barracuda, n = 2). Muscle samples were collected from each fish, placed into clean plastic containers, and transported to the Laboratory of Toxicology, Graduate School of Veterinary Medicine, Hokkaido University, Japan. They were stored at − 20°C in a deep freezer until analysis.
DDTs were extracted and analysed using a modified protocol (Yohannes et al. 2013). Approximately 5 g muscle sample was homogenized with anhydrous sodium sulfate, before extraction with hexane:acetone (3:1 v/v) in a Soxhlet extractor (SOX416 macro SOXTHERM unit, Gerhardt, Germany). An aliquot of extract was used for gravimetric lipid determination. The surrogate standard 3,3′,4,4′-tetrachlorobiphenyl (PCB 77) was used to spike the sample; then the extract was concentrated prior to clean-up in a glass column packed with activated florisil and eluted with hexane:dichloromethane (7:3 v/v). After further concentration, 2,4,5,6-tetrachloro-m-xylene was added as a syringe spike. Final analysis was conducted using a gas-chromatograph with 63Ni electron capture detector (GC-ECD: Shimadzu GC-2014, Kyoto, Japan). Chemical identification in samples was performed by comparison of retention times with those of standards (Dr Ehrenstorfer GmbH, Germany), quantifying concentrations in samples from peak areas compared to the internal standard. Multi-level calibration curves had correction coefficients (R2) greater than 0.99. Detection limits were between 0.16 and 0.45 ng/g, based on a signal to noise ratio (S/N) of 3:1. In order to assess precision and accuracy, a standard reference material (SRM 1947 Lake Michigan Fish Tissue) was analysed with the same method; recoveries were between 85% and 105% with RSD < 12%.
Potential human health risk from consumption of fish meat was assessed. Using detected concentrations (C, ng/g ww) of DDTs, the estimated daily intake (EDI) was calculated using Eq. (1). DR is the average daily consumption of fish (23.3 g/d), according to published national consumption values (FAO 2013). BW is body weight (kg), set at 60 kg. EDIs were calculated at 25th, 50th, 75th and 95th percentiles of DDT concentrations, expressed as nanogram per kilogram body weight per day (ng/kg/bw/d). Then cancer risk estimates and hazard ratios (HR) were calculated using US EPA guidelines. For an acceptable lifetime cancer risk set at one in a million, i.e. 10−6, the cancer benchmark concentration (CBC) for carcinogenic effects represents the lifetime exposure concentration. A risk level greater than 10−4 is considered unacceptable, while the area of concern is set between 10−4 and 10−6. The cancer slope factor (CSF) for DDTs is set according to the Integrated Risk Information System (IRIS) database to 0.34 per mg/kg/d (IRIS 1987), and CBC calculated using Eq. (2). The HR for cancer risks was calculated by comparing EDI with CBC [Eq. (3)]. With this definition, an HR of greater than one implies a greater than one in a million lifetime cancer risk (Dougherty et al. 2000).
Statistical analysis was performed using JMP Pro software, Version 12 (SAS Institute). Concentration of DDTs data are shown as median and range values in ng/g wet weight (ww) of tissue.
Results and Discussion
Contamination levels differed among fish species. The median ∑DDTs by species ranged from 2.35 ng/g ww in T. orbis to 11.62 ng/g ww in Epinephelus spp. (Table 1). The highest value of ∑DDTs detected in an Epinephelus sample was 280.91 ng/g ww. Previously it has been shown that biota at higher trophic levels have higher accumulation of DDTs due to bioaccumulation and biomagnification effects (Yohannes et al. 2013). There is a diet overlap in fish analysed for this study (Heemstra and Heemstra 2004). Further fish and environmental samples should be analysed to investigate this relationship in the study area. Considering all samples, the median ∑DDTs was 3.77 ng/g ww. A previous study on freshwater tigerfish (Hydrocynus vittatus) from Lake Pongolapoort, which feeds into the Phonogolo River, showed contamination by DDTs of 5400–6000 ng/g lipid weight (Wepener et al. 2012). Although a few (4/23) samples in this study from Maputo Bay exceeded that level of contamination, the median for all fish was 922.7 ng/g lipid weight.
Of the DDT congeners analysed (o,p′-DDT, p,p′-DDT, o,p′-DDE, p,p′-DDE, o,p′-DDD and p,p′-DDD), o,p′-DDD was detected in only two fish samples, and o,p′-DDE in none. The most common congeners detected were p,p′-DDT (in N. bipunctatus and G. grandoculis), p,p′-DDE (C. heberi, T. orbis and S. barracuda), and p,p′-DDD (Epinephelus spp.) (Fig. 2). The highest concentration of p,p′-DDD, 210.8 ng/g ww, was detected in an Epinephelus sp sample. This species is a major predator, and thus relatively higher contamination levels are expected. Based on concentrations, the order of magnitude for abundance of congeners detected is: DDE > DDT > DDD. DDT is rapidly degraded both biotically and abiotically (Boul 1995). DDE is the most common metabolite of DDT detected in many species, and has been linked to toxic side effects including testicular tumors, eggshell thinning, and impaired neurodevelopment (Mrema et al. 2013). The p,p′-DDT congener was present in all but two fish samples, and the DDT/DDE ratio greater than one in nine samples, suggesting recent exposure to the parent DDT compound.
When all samples were considered and EDIs calculated, HR greater than one were found above the 75th percentile (HR of 1.5 at 75th and 28.2 at 95th percentile) (Table 2). These equate to 1.5 to 28.2 × 10−4 (1.5–28.2 chances in 10,000 people) risk of cancer associated with consumption of the fish. Calculations for S. barracuda alone did not show an increased risk. As expected, the greatest risk was associated with consumption of Epinephelus spp. (HR of 1.5 at 50% and 34.9 at 95% percentile, or 1.5–34.9 chances in 10,000 people).
Fish is a very important part of the diet for many local people around Maputo Bay. As contamination and congener profiles vary between fish species, it is necessary to consider not only how much fish is consumed but also the species. All species sampled are fished for consumption, but discussions with Inhaca locals suggested they were more likely to consume smaller fish species. Official reports by parties for the Stockholm Convention show annual use of DDT in 2014 of 12 metric tons of active ingredient applied in Mozambique and 18 metric tons in South Africa. Also not recently reported, independent data sources indicate that DDT has been used in Swaziland (Van Den Berg et al. 2017). Unregulated use of obsolete or illegal stockpiles may occur.
In summary, findings from this study suggest that historical and ongoing use of DDT results in contamination of the environment and biota contained therein. Thus, we investigated concentrations of DDTs in marine fish species in Maputo Bay, Mozambique, and assessed the possible health risk through consumption. Results revealed concentrations of DDTs ranging from ND to 280.9 ng/g ww. Albeit from a small sample size, results confirmed contamination of marine species that are a potential health risk not only for wildlife but also people. Assessment of human health risk from consumption of fish meat shows that people eating Epinephelus spp. in particular should be made aware of higher contamination and thus greater potential health risk from regular consumption of this species. This data will help policy makers perform a risk–benefit analysis of DDT use in malaria control programs in the region. Future research should focus on alternatives to DDT use in vector control programs, as well as remediation methods for DDT and its metabolites in the environment and biota.
References
[FAO] Food and Agriculture Organization of the United Nations (2013) National Aquaculture Sector Overview: Mozambique. http://www.fao.org/fishery/countrysector/naso_mozambique/en Accessed 1 May 2017
[IARC] International Agency for Research on Cancer (2017) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, list of classifications, volumes 1-117. International Agency for Research on Cancer. http://monographs.iarc.fr/ENG/Classification/latest_classif.php. Accessed 18 Jan 2017
[IRIS] Integrated Risk Information System (1987) U.S. Environmental Protection Agency. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/ 0147_summary.pdf. Accessed 18 Jan 2016
Blumberg L, Frean J (2007) Malaria control in South Africa-challenges and successes. S Afr Med J 97(11):1193–1197
Boul HL (1995) DDT residues in the environment—a review with a New Zealand perspective. N Z J Agric Res 38(2):257–277
Bouwman H, Coetzee A, Schutte CHJ (1990) Environmental and health implications of DDT-contaminated fish from the Pongolo flood plain. J Afr Zool 104(4):275–286
Cottam C, Higgins E (1946) DDT and its effect on fish and wildlife. J Econ Entom 39(1):44–52
Dougherty CP, Holtz SH, Reinert JC, Panyacosit L, Axelrad DA, Woodruff TJ (2000) Dietary exposures to food contaminants across the United States. Environ Res 84(2):170–185
Heemstra PC, Heemstra E (2004) Coastal fishes of southern Africa. NISC (PTY) LTD, Grahamstown
McHugh KJ, Smit NJ, Van Vuren JHJ, Van Dyk JC, Bervoets L, Covaci A, Wepener V (2011) A histology-based fish health assessment of the tigerfish, Hydrocynus vittatus from a DDT-affected area. Phys Chem Earth 36(14):895–904
Mrema EJ, Rubino FM, Brambilla G, Moretto A, Tsatsakis AM, Colosio C (2013) Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology 307:74–88
Van Den Berg H, Manuweera G, Konradsen F (2017) Global trends in the production and use of DDT for control of malaria and other vector-borne diseases. Malaria J 16(1):401
Wepener V, Smit N, Covaci A, Dyke S, Bervoets L (2012) Seasonal bioaccumulation of organohalogens in tigerfish, Hydrocynus vittatus Castelnau, from Lake Pongolapoort, South Africa. Bull Environ Contam Toxicol 88(2):277–282
WHO (2016) World Malaria Report 2016. http://apps.who.int/iris/ bitstream/10665/252038/1/9789241511711-eng.pdf?ua = 1. Accessed 19 Jan 2017
Yohannes YB, Ikenaka Y, Nakayama SM, Saengtienchai A, Watanabe K, Ishizuka M (2013) Organochlorine pesticides and heavy metals in fish from Lake Awassa, Ethiopia: insights from stable isotope analysis. Chemosphere 91(6):857–863
Acknowledgements
This study was supported by the Leading Program at Hokkaido University and the Japan Society for the Promotion of Science (JSPS KAKENHI Grant Number 16J02013). Also thanks to Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Nos. 16H0177906, 15H0282505 and 15K1221305), and the foundation of the Soroptimist Japan, the Nakajima, the Sumitomo, and JSPS Core-to-Core Program (AA Science Platforms) and Bilateral Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thompson, L.A., Ikenaka, Y., Yohannes, Y.B. et al. Human Health Risk from Consumption of Marine Fish Contaminated with DDT and Its Metabolites in Maputo Bay, Mozambique. Bull Environ Contam Toxicol 100, 672–676 (2018). https://doi.org/10.1007/s00128-018-2323-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-018-2323-7