Abstract
It is important to establish the toxicity pesticides against non-target species, especially those pesticides used in commercial formulations. Pyrethroid insecticides are widely used in agriculture despite their toxicity to aquatic animals. In this study, we determine the toxicity of commercial formulation of two pyrethroid insecticides, cypermethrin and deltamethrin, in two life stages of Physalaemus gracilis, a frog that breeds in agricultural ecosystems and has potential contact with pyrethroid pesticides. The acute toxicity test (96 h) was carried out with embryos of stage 17:18 and larvae of stages 24:25. Embryos were more resistant to both pesticides than larvae. In embryo mobility assays, we found that both pesticides caused spasmodic contractions, suggestive of neurological effects. In acute toxicity assays, we found that P. gracilis is more resistant to these insecticides than other studied species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pesticides of the pyrethroid group are the most widely used insecticides, among which are cypermethrin and deltamethrin. The low toxicity of these pyrethroids to mammals and birds and their limited persistence in the soil make them important products in agriculture (Solomon et al. 2001), veterinary medicine, and in public health (malaria control or to control other insects) (Montanha and Pimpão 2012). However, pyrethroids are considered toxic to aquatic species (Svartz et al. 2016), which might affect the environment.
Currently, organism models are used for conducting ecotoxicological studies to evaluate pesticide toxicity. In aquatic environments, mollusks, benthic worms, sponges, fish, and amphibians (about fish and amphibians, see Johnson et al. 2017) have been used for such purposes. Amphibians are good indicators because of their biphasic cycle, allowing contact with the pesticide in both aquatic (as embryos and larvae) and terrestrial environments (as young and adults; Brandão et al. 2011), their permeable skin and eggs, and their sensitivity to environmental changes caused by pesticides (Gonçalves et al. 2014). Amphibians are non-target organisms to pesticides but regularly met these substances because they tend to live near or within agricultural ecosystems.
The effect of pyrethroids on amphibians has been well studied. Several studies have addressed the effects of cypermethrin (e.g., Agostini et al. 2010; David et al. 2012; Izaguirre et al. 2000, 2006; Svartz and Pérez-Coll 2013; Svartz et al. 2016) and deltamethrin (e.g., Aydin-Sinan et al. 2012; L’Hotellier and Vincent 1986; Salibian 1992) on amphibians, but there is an absence of information for most species.
Physalaemus gracilis, also known as the crying frog, is a small frog measuring 2.7 to 3.2 cm (Borges-Martins et al. 2007; Zank et al. 2014). The geographic range is from southern Brazil and Uruguay to adjacent Argentina (Frost 2017) in fields and marshes and lentic bodies of water in preserved or environmentally disturbed areas (Borges-Martins et al. 2007), such as agroecosystems.
Here, we aimed to determine the toxicity of commercial formulation of two pyrethroid insecticides, in two life stages of P. gracilis, a frog that breeds in agricultural ecosystems and has potential contact with pyrethroid pesticides.
Materials and methods
Pesticides
The studied pesticides were deltamethrin (commercial insecticide Decis 25 emulsifiable concentrate [EC], 25 g L−1 of active ingredients and 886 g L−1 inert ingredients) and cypermethrin (commercial insecticide cypermethrin [EC], 250 g L−1 of active ingredient and 723 g L−1 inert ingredient).
Collection and acclimatization of spawns
P. gracilis spawns were collected within 24 h of oviposition at the lake of the Federal University of Fronteira Sul (latitude −27.728681°, longitude −52.285852°), the established point of local reference. Samples were collected between October 2015 and March 2016. The spawns were collected manually, stored in sterile plastic bags, and transported to the Ecology and Conservation Laboratory at the Federal University of Fronteira Sul, where they were transferred to 15-L capacity tanks containing well water and with steady aeration. The room was acclimatized with a temperature between 20 ± 2 °C with humidity between 60 and 80% and controlled lighting (12/12-h light-dark cycle). Dissolved oxygen (7 ± 1 mg L−1) and tank temperature (20 ± 2 °C) were controlled daily.
Embryo acute toxicity tests
The acute toxicity test (96 h) was carried out with embryos of stage 17:18 (according to the generalized table of frog development, Gosner 1960). Elongation of the body and development of the tail bud and muscular activity begin in stage 17:18 (Romero-Carvajal et al. 2009). The embryos were monitored daily for mortality and metamorphosis. The tests were performed in 24-well flat bottom cell culture plates (3 mL capacity per well). One embryo was placed in each well. Tests were performed in duplicate, with 40 tested individuals per concentration, and eight control individuals in each plate. In addition to the plate control, 48 individuals were used to perform the negative control plates.
To establish the lethal concentration (LC50), ten concentrations of cypermethrin were tested (25, 40, 50, 60, 80, 120, 140, 160, 180, and 200 mg L−1. For deltamethrin, eight concentrations were tested (0.5, 1.0, 1.5, 2.5, 3.5, 4.0, 4.5, and 5.0 mg L−1).
Larvae acute toxicity tests
The acute toxicity test (96 h) was carried out on larvae of stages 24:25 (Gosner 1960). During stages 24:25, the larvae can feed and swim freely, the mouthparts are essentially complete, and the operculum develops (McDiarmid and Altig 1999). Larvae were placed in sterile glass flasks containing 500 mL of the test solutions (1 larva for each 100 mL). Each flask contained five larvae in sextuplicates, totaling 30 tadpoles exposed to each test concentration. This same number of tadpoles was placed in water from the artesian well to perform the negative control.
For cypermethrin, six concentrations were tested (2, 4, 5, 6, 7, and 8 mg L−1). For deltamethrin, ten concentrations were tested (0.01, 0.05, 0.1, 0.3, 0.4, 0.5, 0.6, 0.7, 1.0, and 3.0 mg L−1). These tests were evaluated every 24 h. Dead individuals were counted and removed from the flasks.
For embryos and larvae of P. gracilis, we tested concentrations smaller than that used in this study (pretest), based on the literature (Izaguirre et al. 2000, 2006; Svartz et al. 2016; Aydin-Sinan et al. 2012). However, there was no mortality at these concentrations, and so they were excluded from the LC50 analysis.
Mobility analyses
For technical reasons, mobility analyses were performed only on embryos. The embryos started the test at stages 17:18 and ended at stages 19:20. Between stages 17 and 20, the muscular response and elongation of the tail occur, with first movements (McDiarmid and Altig 1999). This study uses the name mobility to refer to the muscular activity, that is, the first movements performed by the embryo.
The embryos were exposed to nine concentrations of cypermethrin (5.0, 5.5, 6.0, 6.5, 8.5, 9.0, 10.0, 11.0, and 12.0 mg L−1) and six concentrations of deltamethrin (0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mg L−1). The endpoints evaluated were the following: (a) mobility lower than the control, (b) mobility equal to the control, (c) mobility greater than the control, and (d) spasmodic contractions.
Statistical analyses
The results obtained in the acute toxicity assays were analyzed by the Trimmed Spearman-Karber (TSK) test. Comparisons were performed by one-way analysis of variance (ANOVA), and Tukey or Dunnet post hoc test when the P value was <0.05. For this analysis, the Statistica software was used.
Ecological risk evaluation
The ecological risk was assessed using the hazard quotient (HQ) approach, which was calculated as EEC/LC50 of lethality (acute hazard quotient [AHQ]). The estimated environmental concentration (EEC) is an estimated (or maximum) environmental contaminant concentration next to the geographic range of the species, using the maximum level of cypermethrin reported in Argentina (0.194 mg L−1) (Marino and Ronco 2005) and maximum level of deltamethrin reported in Brazil (0.005 mg L−1) (Moraes et al. 2003). After the AHQ had been calculated, it was compared to the USEPA (The United States Environmental Protection Agency) level of concern (LOC), in which the risk presumption for aquatic animals is LOC = 0.5 (high acute risk).
Results
Cypermethrin
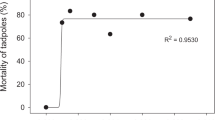
The tested commercial formulation of cypermethrin insecticide presented greater toxicity to larvae than embryos (Table 1). The LC50 96h observed for embryos was 117.41 mg L−1 (−95% = 109.02 mg L−1 and +95% = 126.44 mg L−1). For larvae, this value was 5.01 mg L−1 (4.52–5.55). The highest mortality of P. gracilis embryos occurred in the last 24 h of the test (Fig. 1a). For larvae, the highest mortality occurred within the first 24 h (Fig. 1b).
We detected no significant difference in mortality between embryos and larvae of P. gracilis exposed to cypermethrin (F 1,46 = 0.31, p = 0.58). Larvae and embryos were influenced differently by the pesticide. The concentration was significant for mortality in embryos (F 9,50 = 205.84, p < 0.01, significant from 80 mg L−1, Tukey test, p < 0.01) and larvae (F 5,29 = 164.78, p < 0.01, significant from 6.0 mg L−1, Tukey test, p < 0.01). The exposure time was significant for mortality in embryos (F 3,24 = 5.83; p < 0.01, significant for 96 h of exposure, Tukey test, p < 0.01) but not for larvae (F 3,16 = 1.57; p = 0.23).
Deltamethrin
The tested commercial formulation of deltamethrin insecticide presented greater toxicity to larvae than that for embryos (Table 2). The LC50 96h observed for embryos was 3.04 mg L−1 (2.65–3.48). This value was 0.5 mg L−1 (0.45–0.55). For embryos (Fig. 1c), the highest mortality occurred within the last 24 h of the test.
There was no difference in the number of deaths among embryos and larvae exposed to deltamethrin (F 1,37 = 1.41, p = 0.24). The concentration was significant for mortality in embryos (F 7,40 = 24.51, p < 0.01, significant from 3.5 mg L−1, Tukey test, p < 0.01) and larvae (F 9,50 = 101.18, p < 0.01, significant from 0.6 mg L−1, Tukey test, p < 0.01). There was no influence of exposure time on the mortality of embryos (F 3,19 = 2.15, p = 0.12) but there was for larvae (F 3.12 = 3.63, p = 0.04, significant for 24 h of exposure, Tukey test, p < 0.01). For larvae, a short-term pesticide exposure is sufficient to cause mortality (Fig. 1d).
Mobility
Embryos exposed to cypermethrin (all tested concentrations) showed mobility lower than the control after 48 h of exposure. At 5.0 mg L−1, the majority of exposed individuals showed a change in mobility (66.6%), with spasmodic contractions in 60% of those exposed at the end of the test. The concentration of cypermethrin significantly affected the change in mobility (embryos with lower than control and spasmodic contractions) of the exposed individuals (F 8,63 = 2.43, p = 0.02, significant for all concentrations compared to the control, Dunnet test, p < 0.05). The length of exposure was significant for changes in mobility (F = 4,75 = 6.51, p < 0.01, significant from 48 h, Dunnet test, p < 0.05).
Embryos exposed to deltamethrin (2.5 and 3.0 mg L−1) presented mobility lower than the control within 24 h of testing (33.3% embryos exposed). After 96 h of exposure (1.5 and 3.0 mg L−1), individuals presented spasmodic contractions (37.9% embryos exposed). The concentration of deltamethrin affected mobility (F = 6,49 = 27.23, p < 0.01, significant from 1.5 mg L−1, Dunnet test, p < 0.05), but the length of exposure was not significant (F = 4,51 = 1.55, p = 0.20).
Ecological risk assessment
Acute risk evaluation analysis for embryos and larvae exposed to cypermethrin and deltamethrin was performed using the acute hazard quotients (AHQ = EEC/LC50 of lethality). The EEC was 0.194 mg L−1 for cypermethrin (Marino and Ronco 2005) and 0.05 mg L−1 for deltamethrin (Moraes et al. 2003). We estimated AHQs of 0.0015 (embryos) and 0.036 (larvae) for P. gracilis exposed to cypermethrin and 0.0014 (embryos) and 0.0108 (larvae) for P. gracilis exposed to deltamethrin. The AHQ is lower than the level of concern (LOC = 0.5).
Discussion
We found that the toxicity of both tested pyrethroids was greater for larvae than for embryos of P. gracilis. In the early stages, the pellicle protects the embryos from various environmental disturbances (Biga and Blaustein 2013) and works as a protective agent against certain chemicals (Edginton et al. 2006). Therefore, it is understandable that embryos were less affected than larvae. The protective action of the gelatinous pellicle to embryos is also clear when observing the mortality time. Mortality was higher in the last 24 h of testing, exactly when the embryos were at stages 19:20 and leaving the pellicle (McDiarmid and Altig 1999). As they reach the larval stage, the external gill filaments appear, and cardiovascular activity begins (Sá 2015). In the larvae, where the gill breathing was full, mortality was higher in the first 24 h. In this case, the contact of the gills with insecticides was immediate. During breathing, water enters the mouth of the amphibian larvae, reaching the internal gills where gas exchange takes place; then, the water is expelled through the spiracle (Alonso 2003). Therefore, the gills can increase the uptake of pesticides, which explains the survival decrease of P. gracilis larvae for the two insecticides.
To compensate for the toxic effects of some components, amphibians spend energy trying to restore their physiological balance. This energy expense can lead to mobility and physiological fitness reduction (Greulich and Pflugmacher 2004). In the present study, we tested the initial mobility of the amphibians and compared this to different concentrations of both insecticides. When compared with the control, the exposed individuals were slower and presented spasms during the last test hours. The spasm occurrence in embryos exposed to cypermethrin and deltamethrin may indicate neurological effects since pyrethroids can cause neurotoxic effects in vertebrates (Montanha and Pimpão 2012). In the cypermethrin case, the spasmodic contractions have been associated with the occurrence of apoptosis in the central nervous system (Izaguirre et al. 2000, 2006). Aquatic-phase amphibians are critically important to amphibian populations (Johnson et al. 2017) and the high sensitivity of amphibian to pesticides could lead to declining populations and multiple species.
The calculated acute risk evaluation analysis for embryos and larvae exposed to cypermethrin and deltamethrin indicated minimal risks for acute toxicity. This indicates a high resistance of this species to the commercial formulation of the studied pesticides and highlights the need for further studies on ecological risk using sublethal effects. Despite the low acute risk, the geographic range of the P. gracilis includes much agricultural area (and this species is adapted to heavily disturbed and polluted areas; Lavilla et al. 2010). Therefore, P. gracilis is expected to be in constant contact with pesticides. Brazil has no legislation on the permitted value of cypermethrin and deltamethrin in water. Only the state of Rio Grande do Sul sets a limit on cypermethrin in water for human consumption (0.3 mg L−1; BRASIL 2014).
It is important for conservation efforts to know the susceptibility of non-target species to pesticides. P. gracilis is clearly more resistant to insecticides than other studied species, except for larvae of the Rhinella arenarum for cypermethrin (LC50 of 28.07 and 11.20 mg L−1 (Svartz and Pérez-Coll 2013) and 6.43 mg L−1 (Svartz et al. 2016)). For embryos of R. arenarum, the LC50 was lower than that for P. gracilis 0.11 mg L−1 (Izaguirre et al. 2006). The acute toxicity of cypermethrin has been reported as 0.129 mg L−1 for larvae of Physalaemus biligonigerus (Izaguirre et al. 2000), 11.2 and 0.00334 mg L−1 for Duttaphrynus melanostictus (David et al. 2012), and 0.1752 mg L−1 for Hypsiboas pulchellus (Agostini et al. 2010).
For deltamethrin, P. gracilis was also more resistant than other species, including Bufo bufo (LC50 0.00093 mg L−1; L’Hotellier and Vincent 1986), Xenopus laevis (LC50 0.00626 mg L−1; Aydin-Sinan et al. 2012), and R. arenarum (LC50 0.00000436 mg L−1; Salibian 1992). Pesticide resistance is expected in non-target species that live in altered locations exposed to these chemicals; if they were not resistant, they would no longer exist. Nevertheless, it is essential to know the toxicity of each pesticide, especially those in commercial formulations.
References
Agostini MG, Natale GS, Ronco AE (2010) Lethal and sublethal effects of cypermethrin to Hypsiboas pulchellus tadpoles. Ecotoxicology 19:1545–1550. doi:10.1007/s10646-010-0539-3
Alonso DG (2003) Desarrollo y diferenciación de las branquias externas e internas em embriones y larvas de Bufo arenarum: análisis descriptivo y experimental. Tese, Universidad de Buenos Aires (in spanish)
Aydin-Sinan H, Güngördü A, Ozmen M (2012) Toxic effects of deltamethrin and λ-cyhalothrin on Xenopus laevis tadpoles. J Environ Sci Health B 47:397–402. doi:10.1080/03601234.2012.648545
Biga LM, Blaustein AR (2013) Variations in lethal and sublethal effects of cypermethrin among aquatic stages and species of anuran amphibians. Environ Toxicol Chem 32:2855–2860. doi:10.1002/etc.2379
Borges-Martins M, Colombo P, Zank C, Becker FG, Melo MTQ (2007) Anfíbios. http://www.researchgate.net/profile/Fernando_Becker2/publication/255992592_Anfbios/links/00b49521505c2c6599000000.pdf. Accessed 03 November 2015 (in Portuguese)
Brandão FP, Marques S, Rodrigues S, Santos B, Travasso R, Venâncio C, Pereira R, Ortiz-Santaliestra M, Soares AMVM, Gonçalves F, Lopes I (2011) Influência da temperatura na toxicidade de cobre em girinos de rã verde Pelophylax perezi. Captar 3:66–77 (in portuguese)
BRASIL (2014) Secretaria da Saúde do Estado do Rio Grande do Sul-Portaria N° 320 de 28/01/2014 (in portuguese)
David M, Marigoudar SR, Patil VK, Halappa R (2012) Behavioral, morphological deformities and biomarkers of oxidative damage as indicators of sublethal cypermethrin intoxication on the tadpoles of D. melanostictus (Schneider, 1799). Pestic Biochem Physiol 103:127–134. doi:10.1016/j.pestbp.2012.04.009
Edginton AN, Rouleau C, Stephenson GR, Boermans HJ (2006) 2, 4-D Butoxyethyl ester kinetics in embryos of Xenopus laevis: the role of the embryonic jelly coat in reducing chemical absorption. Arch Environ Contam Toxicol 52:113–120. doi:10.1007/s00244-005-0215-4
Frost DR (2017) Amphibian species of the world: an online reference. Version 6.0. American Museum of Natural History http://research.Amnh.Org/herpetology/amphibia/index.Html. Accessed 23 may 2017
Gonçalves MW, Carvalho WF, Pereira RR, Silva DM, Bastos RP, Cruz AD (2014) Avaliação de danos genômicos em anfíbios anuros do cerrado goiano. Estudos 41:89–104 (in portuguese)
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Greulich K, Pflugmacher S (2004) Uptake and effects on detoxication enzymes of cypermethrin in embryos and tadpoles of amphibians. Arch Environ Contam Toxicol 47:489–495. doi:10.1007/s00244-004-2302-3
Izaguirre MF, Lajmanovich RC, Peltzer PM, Soler AP, Casco VH (2000) Cypermethrin-induced apoptosis in the telencephalon of Physalaemus biligonigerus tadpoles (Anura: Leptodactylidae). Bull Environ Contam Tox 65:501–507. doi:10.1007/s001280000152
Izaguirre MF, Marín L, Vergara MN, Lajmanovich RC, Peltzer P, Casco VH (2006) Modelos experimentales de anuros para estudiar los efectos de piretroides. Cien Docen Tec 32:181–206 (in spanish)
Johnson MS, Aubee C, Salice CJ, Leigh KB, Liu E, Pott U, Pillard D (2017) A review of ecological risk assessment methods for amphibians: comparative assessment of testing methodologies and available data. Integr Environ Assess Manag. doi:10.1002/ieam.1881
L’Hotellier M, Vincent P (1986) Assessment of the impact of deltamethrin on aquatic species. British Crop Protection Conference-Pests and Diseases, British Crop Protection Enterprises, UK, 1109–1117
Lavilla E, Kwet A, Segalla MV, Langone J, Baldo D (2010) Physalaemus gracilis. In the IUCN red list of threatened species. The IUCN red list of threatened species. Version 2017-1. www.Iucnredlist.Org. Accessed on 23 may 2017
Marino D, Ronco EA (2005) Cypermethrin and chlorpyrifos concentration levels in surface water bodies of the Pampa Ondulada, Argentina. Bull Environ Contam Toxicol 75:820–826
McDiarmid RW, Altig R (1999) Tadpoles: the biology of anuran larvae. The University of Chicago Press, Chicago and London
Montanha FP, Pimpão CT (2012) Efeitos toxicológicos de piretróides (cipermetrina e deltametrina) em peixes - Revisão. Rev Cien Elet Med Vet 18:1–58 (in portuguese)
Moraes R, Elfvendahl S, Kylin H, Molander S (2003) Pesticide residues in rivers of a Brazilian rain forest reserve: assessing potential concern for effects on aquatic life and human health. Ambio 32:258–263. doi:10.1579/0044-7447-32.4.258
Romero-Carvajal A, Sáenz-Ponce N, Venegas-Ferrín M, Almeida-Reinoso D, Lee C, Bond J, Ryan MJ, Wallingford JB, Del Pino EM (2009) Embryogenesis and laboratory maintenance of the foam-nesting túngara frogs, genus Engystomops (= Physalaemus). Dev Dyn 238:1444–1454. doi:10.1002/dvdy.21952
Sá JFOF (2015) Efeito da poluição do igarapé do Quarenta sobre a expressão gênica e da contaminação por cobre sobre o comportamento e o desenvolvimento larvário, dos anuros Rhinella granulosa e Scinax ruber. Tesis, Instituto Nacional de Pesquisas da Amazônia (in Portuguese)
Salibian A (1992) Effects of deltamethrin on the south American toad, Bufo arenarum, tadpoles. Bull Environ Contam Toxicol 48:616–621
Solomon K, Giddings J, Maund S (2001) Probabilistic risk assessment of cotton pyrethroids: I. Distributional analyses of laboratory aquatic toxicity data. Environ Toxicol Chem 20:652–659
Svartz G, Meijide F, Coll CP (2016) Effects of a fungicide formulation on embryo-larval development, metamorphosis, and gonadogenesis of the south American toad Rhinella arenarum. Environ Toxicol Pharmacol 45:1–7. doi:10.1016/j.etap.2016.05.008
Svartz GV, Pérez-Coll CS (2013) Comparative toxicity of cypermethrin and a commercial formulation on Rhinella arenarum larval development (Anura: Bufonidae). Int J Environ Health 6:320–320. doi:10.1504/IJENVH.2013.056973
Zank C, Anés A C, Colombo P, Borges-Martins M (2014) Anfíbios https://www.researchgate.net/publication/269697878_Anfibios. Accessed 16 June 2016 (in Portuguese)
Acknowledgments
Valuable help in laboratorial work was provided by Jessica Herek, Jéssica Slaviero, and Gregori B. Bieniek. We are grateful to the Federal University of Fronteira Sul—UFFS for providing logistical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Macagnan, N., Rutkoski, C.F., Kolcenti, C. et al. Toxicity of cypermethrin and deltamethrin insecticides on embryos and larvae of Physalaemus gracilis (Anura: Leptodactylidae). Environ Sci Pollut Res 24, 20699–20704 (2017). https://doi.org/10.1007/s11356-017-9727-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9727-5