Abstract
The study of the effects of the insecticide cypermethrin (CY) technical grade and its Sherpa® commercial formulation on Hypsiboas pulchellus tadpoles assessing lethality, behavior, growth, and abnormalities under standardized laboratory conditions is reported. Observed behaviors were identified and categorized by means of a ranking system according to the loss of mobility. Results of acute lethal effects indicate higher potency for Sherpa® at this level of assessment. All effects on behavior showed an increasing degree of injury as insecticide concentration increased. Organisms exposed to technical grade CY showed lower body length with respect to controls from 3.44 µg CY/L to higher concentrations, whereas those exposed to Sherpa® exhibited lower growth from 0.83 µg CY/L. Both forms of the tested insecticide caused abnormalities between 0.34 and 4.18 µg CY/L, but 100% of malformed individuals was detected from 34.4 µg CY/L for those exposed to the technical grade CY, and from 8.36 µg CY/L for those exposed to Sherpa®. This study proposes the use of easily identifiable and distinguishable sublethal end-points. The high input loads of CY in natural environments, the detected concentrations in the field, in addition to the low levels of this insecticide required to induce sublethal effects (which could eventually lead to death), allow for the conclusion that the insecticide is a risk factor for amphibians inhabiting agroecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The production of soybean has dramatically increased in Latin America in the last 10 years and will continue to grow in the future (Bindraban et al. 2009). Direct seeded soybean has become the main crop of Argentina, at present reaching 18 million hectares. The increase of agricultural activity has created an increase in pesticide consumption, mostly cypermethrin (CY), presently one of the most extensively used insecticides, with application rates between 60 and 280 g of active ingredient (a.i.) per hectare (CASAFE 2007).
Cypermethrin is an α-cyano pyrethroid, whose primary target site in the vertebrate nervous system is the sodium channel of the nerve membrane (WHO 1992). Several studies have indicated that pyrethroids are highly toxic to a number of non-target organisms (Carriquiriborde et al. 2007; Crossland 1982; Edwards et al. 1986; Ronco et al. 2008; Saha and Kaviraj 2008). Sublethal effects of pyrethroids on tadpoles have been detected. Salibián (1992) reports neurotoxicity caused by deltamethrin in Rhinella arenarum, and Campana et al. (2003) the induction of micronuclei caused by λ-cyhalothrin exposure in Lithobates catesbeianus. Also, induction of micronuclei was observed in Odontophrynus americanus exposed to CY (Cabagna et al. 2006). Particularly, data on the lethal effects of CY on anuran species are scarce. The 48-h LC50 for Rana temporaria was 6.5 μg CY/L (Paulov 1990), and the 96-h LC50 for Duttaphrynus melanostictus was 9 μg CY/L (Saha and Kaviraj 2008). The previous references correspond to studies with either technical grade or formulations of pesticides, but do not include comparisons between them. In this sense it is important to highlight that the information on the effects of toxicants from databanks mostly refer to pure compounds, though pesticides are generally introduced in the environment as formulations. This fact was pointed by Mann et al. (2009) indicating the lack of knowledge on the effects of a.i. and formulations of pesticides on anurans.
Extinctions and decreases in the number of amphibian populations have been reported on a global scale (Blaunstein and Kiesecker 2002; Houlahan et al. 2000; Pechman and Wake 1997; Sparling et al. 2001; Stuart et al. 2004). Different factors have been proposed as possible causes of this problem, environmental pollution by agrochemicals used in agriculture being one of them (Beebee and Griffiths 2005; Mann et al. 2009; Relyea 2009).
The aim of this study was to evaluate lethal and sublethal effects of technical grade CY alone and in the Sherpa® commercial formulation on Hypsiboas pulchellus tadpoles by means of lethal and sublethal (behavior, growth, and abnormalities) end points with toxicity tests under standardized laboratory conditions.
Materials and methods
Test species
Hypsiboas pulchellus is a very wide-spread and abundant species in the Pampas region (Cei 1980) of Argentina, where 72% of the surface is dedicated to biotech crop production. Frequent reproduction, large numbers of eggs in nests, and easiness in maintenance under laboratory conditions make this organism an interesting candidate for toxicity testing (Agostini et al. 2009; Natale et al. 2000, 2006).
Adult frogs were collected from an unpolluted (Natale 2006) permanent pond located near the La Plata province of Buenos Aires (35°01′ S, 57°51′ W), under authorization of the Ministry of Agriculture, Office for Protected Areas and Biodiversity Conservation of the Buenos Aires Province (Resol. #15). Mating pairs were captured during amplexus and placed in 2.8 L glass jars containing water and vegetation from the site. The jars, each containing a pair, were placed in the natural habitat until egg nests were observed. The eggs were acclimatized and maintained in the laboratory (temperature: 25 ± 1°C; photoperiod: 16:8) in testing water (dechlorinated tap water; pH 7.7–8.3; hardness: 180–250 mg CaCO3/L) until the individuals reached stage 25 (Gosner 1960), at which point they were deposited randomly in the trial recipients according to the experimental design.
Exposure to CY
Toxicity tests were performed using technical grade CY (Gleba Chemical Company, 95% purity, mixture of four diastereoisomers) and the commercial formulation Sherpa® (25% a.i., by Bayer®) using the following concentrations: 0.1; 0.5; 1; 5; 10; 50; 100; 250; 500; 750; 1,000; 2,500; 5,000; 7,500 and 10,000 μg CY a.i./L. Preliminary tests allowed us to find a wide concentration range within which to test lethal and sublethal effects. Test dilutions were prepared from stock solutions containing 1,000 mg of CY/L. The CY technical grade stock solution was dissolved previously in analytical grade ethanol (99% purity). The concentration of CY in each stock solution was analyzed by chromatographic methods using a GC-ECD with an HP 1.30 m × 0.53 mm column, N2 as a carrier, and a temperature ramp between 180 and 220°C, with a limit of detection of 0.01 mg/L (injected simple extract) (Marino and Ronco 2005).
Tests were performed in 1 L glass jars with lids, with five organisms per jar and 500 ml of corresponding test solution. Tests were done using four replications per dilution and two control groups. Dilution water was dechlorinated by aeration for 48 h. Acute effects were studied during 96 h exposure with no feeding or aeration, and with media renewal every 24 h before end-point assessment. After 96 h, when all parameters were recorded, all individuals were identified and fixed in 10% v/v formaldehyde for further evaluation of abnormalities.
Mortality
Mortality was quantified every 24 h during the exposure time. Each individual was considered dead if no movement was detected after gentle prodding.
Sublethal end-points
Registration of behavior every 24 h was done after gently swirling the water five times with a glass rod and observing for 1 min the swimming activity of each organism. Observed behaviors were identified previously and categorized as regular swimming, delayed response (regular swimming delayed after stimulation), slow swimming (due to tail torsion), or immobility (no movement, or trembling).
Growth was assessed by measuring body length (BL) with a digital caliper of 0.01 mm after 96 h exposure. Measurements were taken on live organisms. Abnormalities were described according to the categories proposed by Bantle et al. (1996). The 96-h-exposed fixed organisms were observed under a binocular stereoscopic microscope (Zeiss Stemi V11) and photographed with an Olympus C-4000 digital camera. Dead organisms were excluded from this type of analysis.
Data analysis
Probit analysis (Finney 1971) was used on lethality and behavioral end points for LC/EC50 estimations from concentration response curves with their 95% confidence limits for technical grade CY and CY as the a.i. in Sherpa®. Regression (a and b) and correlation (r) coefficients were calculated for each concentration response curve. Tests of the significance of the regression and correlation coefficients and comparison of two linear regression equations (technical grade CY and Sherpa®) were performed following Zar (2010). Toxicological parameters and statistical assumptions from the Probit method were corroborated using the Probit Analysis Program, version 1.5 (USEPA 1999). The increment in the level of effect with the concentration of toxicant was assessed by means of a ranking score from 1 point (delayed response) through 3 (immobility) and 4 (mortality). With this ranking we performed a regression and lineal correlation between the logarithm of the concentration and level of effect.
Growth was assessed on organisms exposed to technical grade CY and Sherpa® by one-way ANOVA with the Dunnett′s test (Zar 2010), taking CY concentration as the independent variable and BL as the response variable.
Two types of tests were used for the analysis of abnormalities (dependency between concentration and type of abnormality) according to Bantle et al. (1996), with two contingency tables (technical grade CY and CY as the a.i. in Sherpa®). To determine the existence of a relationship between concentration and abnormality proportion (with no rank distinction), a regression and correlation of arcsine-transformed variable data were performed (Zar 2010).
Results
Chemical analysis of stock solutions of CY (nominal concentration: 1,000 mg a.i. CY/L) showed 344 mg/L in the technical grade CY and 836 mg/L in Sherpa®. All the given data were calculated taking into account the measured concentrations in stocks solutions.
Mortality
Results from the Probit analysis for technical grade CY and Sherpa® are given in Table 1. The comparison of the concentration response curves for both treatments indicates no significant differences (p = 0.15) between the slopes and significant differences (p < 0.05) in the elevations, showing higher potency for Sherpa®.
Behavior
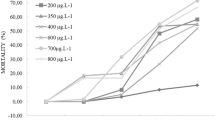
All of the behavioral tested end points after 96 h exposure showed significant differences (p < 0.05) between concentration response curves for both of the tested solutions (technical grade CY and Sherpa®). All effects on behavior showed an increasing degree of injury as insecticide concentration increased (Fig. 1). The regression and correlation analysis to test gradual effects indicated significant lineal correlation (p < 0.05) between the concentration of the insecticide and assigned scores of effects for technical grade CY and Sherpa® (technical grade CY: r = 0.9828, a = −0.6699, and b = 1.5098; CY as a.i. in Sherpa®: r = 0.9977, a = 0.9729, and b = 1.1316).
Growth
Tests with technical grade CY showed significant differences with BL (p < 0.05) from concentrations of 3.44 µg CY/L and higher, whereas for the case of Sherpa® significant differences (p < 0.05) in growth were detected from 0.83 µg CY/L.
Abnormalities
Detected abnormalities after 96 h of exposure to the insecticide can be seen in Fig. 2. The same types of abnormalities were associated with both technical grade CY and Sherpa®. These included head and axial, eye, gut, and face abnormalities (Bantle et al. 1996). Regression and correlation analyses show a lineal and significant correlation (p < 0.05) between the concentration and proportion of abnormalities (with no distinction of type). Contingency analysis shows significant independence (p < 0.05) between the concentrations and the type of abnormalities that appear. Both forms of the tested insecticide caused abnormalities between 0.34 and 4.18 µg CY/L, but 100% of individuals were abnormal when exposed to 34.4 µg CY/L of the technical grade CY, or 8.36 µg CY/L of Sherpa®.
Abnormalities in H. pulchellus tadpoles exposed to CY. A Different type of axial abnormalities for individuals exposed between 10 and 100 μg CY(a.i.)/L in Sherpa. ( a) Normal individual in control group. B Eye abnormalities in individuals exposed at 2500 μg CY(a.i.)/L in Sherpa, (b ) normal eye in controls. C Gut abnormalities in individuals exposed at 5000 μg CY/L technical grade, (c) normal development of intestines in controls. D Different type of head and face abnormalities in individuals exposed between 100 and 2,500 μg CY(a.i.)/L in Sherpa
Discussion
The CY commercial formulation Sherpa® exhibits higher effects than the technical grade CY for equivalent a.i. concentrations, as most of pesticides do by the action of coadjuvants (Mann and Bidwell 1999; Relyea 2009), due to both a maximization of the active ingredient activity and additional toxicity from excipients. Results for toxicity of Sherpa® obtained in this study are in agreement with those reported for Physalaemus biligonigerus (Izaguirre et al. 2000) and H. pulchellus tadpoles (Agostini et al. 2009). With respect to technical grade CY toxicity values, results differ from other data in the literature. Paulov (1990) reports a 48 h LC50 for R. temporaria of 6.5 μg CY/L, and Greulich and Pflugmacher (2003) observed 100% mortality at concentrations above 10 μg CY/L. A similar behavior has been observed for other pyrethroids. Materna et al. (1995) reported an 96 h LC50 of 7.29 μg/L for esfenvalerate in Rana spp., and Jolly and Avault (1978) an 96 h LC50 of 7.03 μg/L for permethrin in L. cataesbianus, while other research do not register acute lethal effects exposing R. temporaria at equivalent concentrations of esfenvalerate (Johansson et al. 2006). Furthermore, Berril et al. (1993) did not detect effects of permethrin at concentrations up to 2000 μg/L on six amphibian species. Differences could be associated with experimental conditions due to the physicochemical properties of this compound (low water solubility, sorption to container materials), hence reducing its bioavailability.
Cypermethrin is a highly toxic insecticide to aquatic arthropods, fish, and bees (WHO 1992), fish being the most sensitive of the vertebrates (Edwards et al. 1986). Previous studies have shown a range of LC50 values between 0.93 and 21.4 μg CY/L for several fish species (Carriquiriborde et al. 2007; Polat et al. 2002; Stephenson 1982). If we consider the reported LC50 values for fish and tadpoles, H. pulchellus is a species with low sensitivity to this insecticide. The reported sublethal effects on amphibians include cellular apoptosis (Cabagna et al. 2006), micronuclei formation (Campana et al. 2003), inhibition of cholinesterase activity (Khan et al. 2003), decrease in hatching success, delays in metamorphosis, reduction in body growth, and abnormalities (Greulich and Pflugmacher 2003) within a concentration range of 0.1 and 10 μg CY/L. These results are in agreement with behavioral effects seen in this study at concentrations of 3 μg CY/L, the inhibition of body size at 3.44 μg CY/L, and the presence of abnormalities at 0.34 μg CY/L. These lethal and sublethal effects were observed under exposure to technical grade CY or the formulation, and related to CY action. Treated organisms exhibit higher effects with Sherpa® when compared to the technical grade CY.
The effects of CY on behavior such as twisting, writhing, and noncoordinated swimming are typical signs of cyano pyrethroid poisoning, and were reported by Berril et al. (1993) and Greulich and Pflugmacher (2003) as the expression of neurotoxicity observed for pyrethroids (Gammon et al. 1981; Vijverberg and Van Der Bercken 1982). Different levels of narcosis induced by the NCS present as alterations in movement, an effect easily assessed without sophisticated equipment.
This study has proposed the use of easily identifiable and distinguishable end points and demonstrated the existence of a positive correlation between CY concentration and level of effect. The sequential appearance of effects after 96 h of exposure occurred in the following order: delayed response (level 1) at concentrations of 17.2 and 4.1 μg CY/L; slow swimming (level 2) at 34.4 and 8.36 μg CY/L; immobility (level 3) at 172 and 41.8 μg CY/L for technical grade CY and Sherpa®, respectively (see Fig. 1). This suggests that behavioral effects could be considered early indicators of CY exposure.
If we take into account observations by Materna et al. (1995) on Rana spp. tadpoles exposed to the pyrethroid insecticide esfenvalerate, which exhibited convulsions and eventually died, and that concentrations inducing acute sublethal effects could lead to lethality under chronic exposure (Bridges 2000), we could assume that the sublethal effects described here are irreversible. Once effects are induced, they will worsen, leading to death of organisms, regardless of the persistence of CY exposure.
Willbur and Collins (1973) proposed a model to predict the timing of amphibian metamorphosis. They considered that there is a minimum body size level to initiate the process of metamorphosis. H. pulchellus reproduce in temporary ponds that dry in the summer (usually the time of pesticide spray). If larvae do not reach the size required for metamorphosis, this would lead to mortality for lack of water in the environments where they develop. Hence, the observed growth inhibition would be a disadvantage for the species. The phenotypic plasticity allowing tadpoles to reach a high developmental rate of growth if environmental conditions are unfavorable (Alford and Harris 1988; Harris 1999; Hensley 1993) will not provide a solution compensating for CY damage to growth, since even though they may reach a minimum size to initiate metamorphosis (accelerating the growth rate), the small size could decrease terrestrial performance (Emerson 1986; Pough and Kamel 1984; Taigen and Pough 1985).
The minimum recommended doses in Argentina for CY application are within 60 and 150 cm3/ha (depending on the culture and pest to be treated). Reports of concentrations found in streams of water near agroecosystems ranged from 0.2 to 150 μg CY/L (Jergentz et al. 2005; Marino and Ronco 2005). These concentrations would induce the sublethal effects demonstrated in this study and also reported for H. pulchellus and Rhinella fernandezae in field experiments (Agostini et al. 2009) carried out in the same sampling sites.
The high input loads of CY and associated concentrations in natural environments, in addition to the low levels required to induce sublethal effects (which could eventually lead to death), allow for the conclusion that the insecticide is a risk factor for amphibians inhabiting agroecosystems.
References
Agostini MG, Natale GS, Ronco AE (2009) Impact of endosulphan and cypermethrin mixture on amphibians under field use forbiotech soya bean production. Int J Environ Health 3(4):379–389
Alford RA, Harris RN (1988) Effects of larval growth history on anuran metamorphosis. Am Nat 131:91–106
Bantle JA, Dumont JN, Finch RA, Linder G, Fort DJ (1996) Atlas of abnormalities. A guide for the performance of FETAX, 2nd edn. Oklahoma State University, Stillwater
Beebee TJC, Griffiths RA (2005) The amphibian decline crisis: a watershed for conservation biology? Biol Conserv 125:271–285
Berril M, Bertram S, Wilson A, Louis S, Brigham D, Stromberg C (1993) Lethal and sublethal impact of pyrethroid insecticides on amphibian embryos and tadpoles. Environ Toxicol Chem 12:525–539
Bindraban PS, Franke AC, Ferraro DO, Ghersa CM, Lotz LAP, Nepomuceno A, Smulders MJM, van de Wiel CCM (2009) GM-related sustainability: agro-ecological impacts, risks and opportunities of soy production in Argentina and Brazil. Plant Research International B.V, Wageningen
Blaunstein AR, Kiesecker JM (2002) Complexity in conservation: lessons from the global decline of amphibian populations. Ecol Lett 57:21–43
Bridges CM (2000) Long-term effects of pesticide exposure al various life stages of the southern leopard frog (Rana sphenocephala). Arch Environ Contam Toxicol 39:91–96
Cabagna M, Lajmanovich RC, Peltzer PM, Attademo M, Ale E (2006) Induction of micronucleus in tadpoles of Odontophrynus americanus (Amphibia: Leptodactylidae) by the pyrethroid insecticide cypermethrin. Toxicol Environ Chem 88:729–737
Campana MA, Panzeri AM, Moreno VJ, Dulout FN (2003) Micronuclei induction in Rana catesbeiana tadpoles by the pyrethroid insecticide lambda-cyhalothrin. Genet Mol Biol 26:99–103
Carriquiriborde P, Diaz J, Mugni H, Bonetto C, Ronco EA (2007) Impact of cypermethrin on stream fish populations under field-use in biotech-soybean production. Chemosphere 68:613–621
CASAFE (2007) Guía de productos fitosanitarios. Cámara de Sanidad Agropecuaria y Fertilizantes, Buenos Aires
Cei JM (1980) Amphibians of Argentina. Monit Zool Italiano, Ital J Zool Monogr 2
Crossland NO (1982) Aquatic toxicology of cypermethrin. II. Fate and biological effects in pond experiments. Aquat Toxicol 2:205–222
Edwards R, Millburn P, Hutson DH (1986) Comparative toxicity of cis-cypermetrhrin in Rainbow trout, frog, mouse and quail. Toxicol Appl Pharmacol 84:512–522
Emerson SB (1986) Heterochony and frogs: the relationship of a life history trait to morphological form. Am Nat 127:167–183
Finney DJ (1971) Probit analysis. Cambridge University Press, London
Gammon DW, Brown MA, Casida JE (1981) Two classes of pyrethroid action in the cockroach. Pestic Biochem Physiol 15:181–191
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetology 16:183–190
Greulich K, Pflugmacher S (2003) Differences in susceptibility of various life stages on amphibians to pesticide exposure. Aquat Toxicol 65:329–336
Harris RN (1999) The biology of anuran larvae. In: McDiarmid RW, Altig R (eds) Tadpoles. The University of Chicago Press, Chicago, pp 279–294
Hensley FR (1993) Ontogenetic loss of phenotypic plasticity of age at metamorphosis in tadpoles. Ecology 74:2405–2412
Houlahan JE, Findlay CS, Schmidt BR, Meyers AH, Kusmin SL (2000) Quantitative evidence for global amphibians population declines. Environ Pollution 82:277–288
Izaguirre MF, Lajmanovich RC, Peltzer PM, Soler AP, Casco VH (2000) Cypermethrin-induced apoptosis in the telencephalon of Physalaemus biligonigerus tadpoles (Anura: Leptodactylidae). Bull Environ Contam Toxicol 65:501–507
Jergentz S, Mugni H, Bonetto C, Schulz R (2005) Assessment of insecticide contamination in runoff and stream water of small agricultural streams in the main soybean area of Argentina. Chemosphere 61:817–826
Johansson M, Piha H, Kylin H, Merilä J (2006) Chemistry toxicity of six pesticides to common frog (Rana temporaria) tadpoles. Environ Toxicol Chem 25:3164–3170
Jolly AL, Avault JW (1978) Acute toxicity of permethrin to several aquatic animals. Trans Am Fish Soc 107:825–827
Khan MZ, Tabassum R, Naqvi SNH, Shah EZ, Tabassum F, Ahmad I, Fatima F (2003) Effect of cypermethrin and permethrin on cholinesterase activity and protein contents in Rana tigrina (Amphibia). Turk J Zool 27:243–246
Mann RM, Bidwell JR (1999) The toxicity of glyphosate and several glyphosate formulations to four species of southwestern Australian frogs. Arch Environ Contam Toxicol 36:193–199
Mann RM, Hyne RV, Choung CB, Wilson SP (2009) Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ Pollut 157(11):2903–2927
Marino D, Ronco EA (2005) Cypermethrin and chlorpyrifos concentration levels in surface water bodies of the Pampa Ondulada, Argentina. Bull Environ Contam Toxicol 75:820–826
Materna EJ, Rabeni CF, LaPoint TW (1995) Effect of the synthetic pyrethroid insecticide esfenvalerate on larval leopard frog (Rana spp.). Environ Toxicol Chem 14:613–622
Natale GS (2006) Análisis Ecotoxicológico de una Comunidad de Anuros de la Región Pampeana: Efecto del Cr(VI) Sobre Embriones y Larvas de Distintas Especies de una Taxocomunidad, PhD Thesis, Universidad Nacional de La Plata, Buenos Aires
Natale GS, Basso NG, Ronco AE (2000) Effect of Cr(VI) on early life stages of three species of hylid frogs (Amphibia, Anura) from South America. Environ Toxicol 15:509–512
Natale GS, Ammassari LL, Basso NG, Ronco AE (2006) Acute and chronic effects of Cr(VI) on Hypsiboas pulchellus embryos and tadpoles. Dis Aquat Org 72:261–267
Paulov S (1990) Potential impact of pyrethroids (cypermethrin) on the model amphibians (Rana temporaria). Biologia (Bratislava) 45:133–139
Pechman JH, Wake DB (1997) Declines and disappearances of amphibian populations. In: Meffe GK, Carrol CR (eds) Principles of conservation biology, 2nd edn. Sinauer, Sunderland, pp 135–137
Polat H, Erkoç FÜ, Viran R, Koçak O (2002) Investigation of acute toxicity of beta-cypermethrin on guppies Poecilia reticulata. Chemosphere 49:39–44
Pough FH, Kamel F (1984) Post-metamorphic change in activity metabolism of anurans in relation to life history. Oecologia 65:138–144
Relyea RA (2009) A cocktail of contaminants: how mixtures of pesticides at low concentrations affect aquatic communities. Oecologia 159:363–376
Ronco EA, Carriquiriborde P, Natale GS, Martin ML, Mugni H, Bonetto C (2008) Integrated approach for the assessment of biotech soybean pesticides impact on low order stream ecosystems of the Pampasic Region. In: Chen J, Guo C (eds) Ecosystem ecology research trends. Nova Science, Hauppauge, pp 209–239
Saha S, Kaviraj A (2008) Acute toxicity of synthetic pyrethroid cypermethrin to some freshwater organisms. Bull Environ Contam Toxicol 80:49–52
Salibián A (1992) Effects of deltamethrin on the South American toad, Bufo arenarum, tadpoles. Bull Environ Contam Toxicol 48:616–621
Sparling DW, Fellers GM, McConnell L (2001) Pesticides and amphibian population declines in California, USA. Environ Toxicol Chem 20:1591–1595
Stephenson RR (1982) Aquatic toxicology of cypermethrin. I. Acute toxicology to some freshwater fish and invertebrates in laboratory tests. Aquat Toxicol 2:175–185
Stuart SN, Chanson JS, Cox NA, Young BE, Rodriguez ASL, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinction worldwide. Science 306:1591–1595
Taigen TL, Pough FH (1985) Metabolic correlates of anuran behavior. Am Zool 25:987–997
United States Environmental Protection Agency (USEPA) (1999) http://www.epa.gov/scripoly/sap/1999/february/pyreth.pdf. Accessed 28 December 2009
Vijverberg HPM, Van Der Bercken J (1982) Action of pyrethroid insecticides on the vertebrate nervous system. Neuropathol Appl Neurobiol 8:421–440
Willbur HM, Collins JP (1973) Ecological aspect of amphibian metamorphosis. Science 182:1305–1314
Word Health Organization (WHO) (1992) Alpha-cypermethrin. Environmental Health Criteria
Zar JH (2010) Biostatistical analysis. Prentice Hall, Englewood Cliffs
Acknowledgments
The authors wish to thank Dr. N. G. Basso for allowing use of instrumentation, Dr. D. Marino for chemical analysis of samples, CIMA-Grupo Anfibio for assistance in the experiments, and C. Knipp for editorial suggestions. This study was funded by the ANPCyT PICT project 38350.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agostini, M.G., Natale, G.S. & Ronco, A.E. Lethal and sublethal effects of cypermethrin to Hypsiboas pulchellus tadpoles. Ecotoxicology 19, 1545–1550 (2010). https://doi.org/10.1007/s10646-010-0539-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-010-0539-3