Abstract
Bioactive phytocompounds are studied by several bioactivities demonstrated, as their cytotoxic effects. The aim of this work was to evaluate the phytochemical profile, the toxic effect using the Drosophila melanogaster animal model and the anti-inflammatory and antimicrobial effect of the Alternanthera brasiliana (EEAB) ethanol extract. The phytochemical profile was performed using HPLC. The cytotoxic effect was evaluated in vivo using D. melanogaster. The anti-inflammatory effect was determined by neurogenic and antiedematogenic assays, and the antimicrobial activity was assayed using a microdilution method to determine the minimum inhibitory concentration (MIC) of the EEAB alone and in association with antibiotics. The main compound identified on the EEAB was luteolin (1.93%). Its cytotoxic effect was demonstrated after 24 h in the concentrations of 10, 20 and 40 mg/mL. The extract demonstrated an antiedematogenic effect, with a reduction of the edema between 35.57 and 64.17%. The MIC of the extract was ≥1.024 μg/mL, thus being considered clinically irrelevant. However, when the EEAB was associated with gentamicin, a synergism against all bacterial strains assayed was observed: Staphylococcus aureus (SA10), Escherichia coli (EC06) and Pseudomonas aeruginosa (PA24). Due to these results, the EEAB demonstrated a low toxicity in vivo and anti-inflammatory and synergistic activities. These are promising results, mainly against microbial pathogens, and the compounds identified can be a source of carbon backbones for the discovery and creation of new drugs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The capacity of an organism to feel pain is due to a signal from the central nervous system informing the organism that an aggressive process which poses a risk to its physical integrity exists (Usunoff et al. 2006). A collection of reactions aimed at the adaptation of the psychophysiological, autonomic, motor and objective mechanisms are triggered to separate the organism from the causative agent of pain. Pain perception is also known as nociception, and is therefore an individual perception alert or sensation of pain (Tracey and Mantyh 2007). Pain is directly linked to inflammatory processes, and it is important to highlight that inflammatory processes can be associated with bacterial infections (Yoshikai 2001; Chiu et al. 2013).
In the last years, the production of efficient drugs for the control of bacterial infections revolutionized medical treatment reducing mortality caused by microbial infections (Gafter-Gvili et al. 2005). However, the dissemination of the use of antibiotics has also prompted bacteria to develop diverse defence mechanisms against commercially available antibacterial agents, leading to the development of bacterial resistance (Okeke et al. 2005). The bacterial resistance phenomenon is linked to diverse antibiotics and chemotherapeutic agents, imposing serious limitations to the treatment of bacterial infections thus representing a great public health problem (Levy and Marshall 2004; Zankari et al. 2012). Various microorganisms can cause infectious processes in humans, and become resistant to drugs currently available in the market, which can be highlighted as Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus (De Kraker et al. 2011).
Many scientific studies are associated with ethnopharmacological research aimed at proving the therapeutic properties of many popularly used medicinal plants, as antibacterial and anti-inflammatory (Souza et al. 2014; De Carvalho Nilo Bitu et al. 2015; Ferreira Júnior et al. 2016). Therefore, these serve as a starting point for the development of future medicines to be released on the market (Heinrich 2013; David et al. 2015).
In popular medicine, the therapeutic use of natural products and isolating compounds for various diseases has been demonstrated, and some properties are present in the genus Alternanthera, such as analgesic and anti-inflammatory (de Santana Aquino et al. 2015; Rodrigues et al. 2016); in the treatment of infections (Ogundare and Oladejo 2014; Trapp et al. 2015); in disorders of the gastrointestinal tract (Garín-Aguilar et al. 2014) or antihyperglycaemic metabolic disease (Hossain et al. 2014); anticancer (Firdhouse and Lalitha 2013), antiasthma and antidiarrhoea properties (Kumar and Sanjib 2013; Fathima et al. 2016); for the treatment of skin injuries (Barua et al. 2013b) and other uses present in folk medicine (Hundiwale Jogendra et al. 2012).
Within medicinal plants, we can highlight those of the genus Alternanthera; these cover a wide diversity of species which possess widely distributed representatives throughout the world (Iamonico and Sánchez-Del Pino 2016). Some species belonging to this genus are used for therapeutic ends presenting diverse biological activities that have already been proven and previously cited. However, among the species of this genus, the species Alternanthera brasiliana, a herbaceous plant, stands out (Duarte and Debur 2004), known as “penicillin” and “terramycin”, “Doril”, “Perpétua” and “Perpétua do mato” (Silva et al. 2015). This plant presents importance in popular medicine as therapeutic potential used for diverse diseases as wound healing (Barua et al. 2012, 2013b), antiviral (Lagrota et al. 1994), analgesic and antinociceptive (De Souza et al. 1998; Barua et al. 2009), anxiolytic and anticonvulsant (Barua et al. 2013a), antimicrobial (Pereira et al. 2007; Silva et al. 2010), antitumoral (Samudrala et al. 2014), anti-inflammatory (Kumar et al. 2014; Shivashankar et al. 2017) and others.
In this manner, the present study aims to characterize the phytochemical profile of the ethanolic extract from A. brasiliana leaves (EEAB) and to evaluate its antinociceptive and antiedematogenic action in mice as well as its antibacterial activity and its antibiotic modulatory activity against multiresistant bacterial strains and evaluate the toxicity of the extract in a Drosophila melanogaster model.
Materials and methods
Alternanthera brasiliana (L.) Kuntze botanical material
The A. brasiliana (L.) Kuntze botanical material was collected at 09:00 ± 00:30 h from the Botanical Garden of the Research Laboratory of Natural Products—LPPN, Regional University of Cariri—URCA, Crato, CE, Brazil (coordinates: 07° 14′ 19.2″ S latitude and 39° 24′ 52.8″ longitude W. of Greenwich). The botanical identification of exsiccates from A. brasiliana (L.) Kuntze, Amaranthaceae family, was performed by Profa. Dra. Maria Arlene Pessoa da Silva, and a voucher was deposited at the Caririense Dárdano de Andrade-Lima Herbarium of the Regional University of Cariri—URCA under the number 10.936.
Preparation of the ethanolic extract
To prepare the extract, leaves were collected and perforated in pieces of approximately 1 cm2, which were submerged in ethanol for 72 h at room temperature; after that period, these were filtered and concentrated in a rotary evaporator (model Q-344B-Quimis, Brazil) for evaporation of the ethanol. The dehydration was carried out in a water bath (model Q214M2—Quimis, Brazil); once concentrated and frozen at −20 °C, the extract was lyophilized and conditioned in an amber glass and stored in a freezer. The ethanolic extract yield was 0.95%.
Identification of the constituents through HPLC-DAD
All chemicals were of analytical grade. Methanol, acetic acid, gallic acid, caffeic acid and chlorogenic acid were purchased from Merck (Darmstadt, Germany). Orientin, quercetin, vitexin, luteolin and apigenin were acquired from Sigma Chemical Co. (St. Louis, MO, USA). High-performance liquid chromatography (HPLC-DAD) was performed with a Shimadzu Prominence Autosampler (SIL-20A) HPLC system (Shimadzu, Kyoto, Japan), equipped with Shimadzu LC-20AT reciprocating pumps connected to a DGU 20A5 degasser with a CBM 20A integrator, SPD-M20A diode array detector and LC solution 1.22 SP1 software.
Reverse phase chromatographic analyses were carried out under gradient conditions using C18 column (4.6 mm × 250 mm) packed with 5-μm-diameter particles; the mobile phase was water containing 2% acetic acid (A) and methanol (B), and the composition gradient was 5% (B) for 2 min; 25% (B) until 10 min and 40, 50, 60, 70 and 80% (B) every 10 min, following the method described by Barbosa Filho et al. (2014) with slight modifications. Rifocina ethanolic extract and mobile phase were filtered through a 0.45-μm membrane filter (Millipore) and then degassed by ultrasonic bath prior to use; the extract was analysed at a concentration of 10 mg/mL. The flow rate was 0.6 mL/min, and the injection volume was 50 μL. The sample and mobile phase were filtered through a 0.45-μm membrane filter (Millipore) and then degassed by ultrasonic bath prior to use. Stock solutions of standard references were prepared in the HPLC mobile phase at a concentration range of 0.025–0.300 mg/mL for orientin, quercetin, vitexin, luteolin and apigenin, and 0.035–0.300 mg/mL for gallic, caffeic and chlorogenic acids.
Quantification was carried out by integration of the peaks using the external standard method, at 271 nm for gallic acid, 325 nm for chlorogenic and caffeic acids, 337 nm for vitexin and 366 for quercetin, orientin, luteolin and apigenin. The chromatography peaks were confirmed by comparing its retention time with those of reference standards and by DAD spectra (200 to 600 nm). Calibration curve for gallic acid was Y = 12,708x + 1345.9 (r = 0.9999), for caffeic acid Y = 11,867x + 1274.6 (r = 0.9995), for chlorogenic acid Y = 13,095x + 1351.9 (r = 0.9998), for vitexin Y = 11,968x + 1193.5 (r = 0.9999), for quercetin Y = 12,756x + 1178.6 (r = 0.9997), for orientin Y = 13,256x + 1275.2 (r = 0.9996), for luteolin Y = 11,528x + 1345.8 (r = 0.9999) and for apigenin Y = 12,509x + 1185.5 (r = 0.9998). All chromatography operations were carried out at ambient temperature and in triplicate. LOD and LOQ were calculated based on the standard deviation of the responses and the slope using three independent analytical curves, as defined by Boligon et al. (2013). LOD and LOQ were calculated as 3.3 and 10 σ/S, respectively, where σ is the standard deviation of the response and S is the slope of the calibration curve.
Drosophila stock and culture
D. melanogaster (Harwich strain) was obtained from the National Species Stock Center, Bowling Green, OH. The flies were grown in 2.5 × 6.5-cm2 glass bottles containing 5 mL of standard medium (1% w/v brewer’s yeast; 2% w/v sucrose; 1% w/v powdered milk; 1% w/v agar; 0.08% v/w Nipagin) at constant temperature and humidity (25 ± 1 °C and 60% relative humidity, respectively). All experiments were performed with the same strain.
A. brasiliana ethanolic extract exposure and D. melanogaster assays
Exposure of flies to the ethanolic extract was performed as described: 20 adult flies (males and females) were placed in 330-cm3 flasks containing a filter paper soaked in 1% sucrose in distilled water at the bottom. The flasks received the following treatments: 1% sucrose (control) and 10, 20 and 40 μg mL−1 ethanol extract. Survival readings of the flies were made at 3, 6, 12, 24 and 48 h. The results are presented as percentage (%) of live flies (mean ± SEM) obtained from three independent experiments.
Locomotor assay
The locomotor capacity was evaluated by following the negative geotaxis behaviour as described by Coulom and Birman (24) with some modifications. Twenty adult flies (1–4 days old; both genders) were exposed to the ethanolic extract as detailed above. After completion of the treatment, flies were immobilized on ice for 1–2 min and placed separately in vertical glass columns (length, 25 cm; diameter, 1.5 cm). After 30 min recovery, flies were gently tapped to the bottom of the column and the number of flies that reached 8 cm of the column (top) and flies that remained below this mark (bottom) were registered. The assays were repeated five times for each fly. Results are presented as number of flies on top (mean ± SEM) obtained from three independent experiments.
Animals used in the experiment
The present study was submitted to the Animal Experimentation and Use Committee (CEUA) of the Regional University of Cariri (URCA) and approved under the protocol number 15/2012. During the in vivo tests, mice of the Mus musculus strain of the Swiss line of both sexes, with an average age of 3 months, weighing between 25 and 30 g were used. Fifteen mice were housed per cage and kept in a vivarium at URCA, in an environment with a temperature of 22 and 23 °C in a 12-h light/dark cycle, with free access to potable water and food ad libitum.
Antinociceptive and antiedematogenic activity: formalin test
The mice were divided in treatment groups with the extract at the doses of 25, 50 and 100 mg/kg, 0.9% saline solution and 10 mg/kg indomethacin, all administered orally. The mice were then injected with 20 μL of 2.5% formalin in the sub-plantar region of the right paw, then placed individually under an inverted glass funnel for subsequent observation. The time elapsed in seconds of paw licking during the initial (0–5 min) and late (15–30 min) phases after induction of the phlogistic agent was registered (Hunskaar and Hole 1987; Tjølsen et al. 1992).
Bacterial strains
The MDR strains used were E. coli 06, P. aeruginosa 24 and S. aureus 10. The strains were maintained on blood agar base (Difco Laboratories Ltd., Brazil) slants, and prior to use, the cells were grown overnight at 37 °C in Heart Infusion Agar (HIA) slants (Difco) (Table 1).
Drugs
The antibiotic was dissolved in sterile water (concentration of 1024 μg/mL). Gentamicin was used as the antibiotic, obtained from Sigma Chemical Co., St. Louis, USA. Stock solutions were prepared in 1 mL of 10 mg/mL DMSO, after which they were diluted to 1024 μg/mL in distilled water. The antibiotics were prepared according to the CLSI et al. (2008) guidelines.
Antibacterial activity test by minimal inhibitory concentration
The minimal inhibitory concentration (MIC) of the extract was determined in a microdilution assay utilizing a 100 μL inoculum suspended in saline solution, 0.5 of the McFarland scale, which was added to brain heart infusion (BHI) in Eppendorf. Afterwards, these were transferred to 96-well microtiter plates and serial dilution of each substance was performed with concentrations ranging from 0.5 to 512 μg/mL (1:1). The plates were incubated at 37 °C for 24 h, and bacterial growth was assessed by the use of resazurin. The MIC was defined as the lowest concentration in which no growth can be observed, according to (NCCLS 2015). Antibacterial assays were performed in triplicates, and results were expressed as an average of replicates.
Evaluation of the modification of antibiotic activity
To verify the potentiation of the action of the antibiotics against multiresistant strains, a sub-inhibitory concentration of the vegetal extract was used. A 100-μL sample of a solution containing inoculums, suspended in saline solution, 0.5 of the McFarland scale, was added to brain heart infusion (BHI) in Eppendorf. Afterwards, these were transferred to 96-well microtiter plates and 100 μL of antimicrobial drug serial dilutions were performed (1:1). The plates were incubated at 37 °C for 24 h, and bacterial growth was assessed by the use of resazurin. The MIC was defined with antibiotic concentrations ranging between 0.6 and 2500 μg/mL. The controls were performed using the MIC of antibiotics alone. Antibacterial assays were performed in triplicates, and the results were expressed as an average of replicates.
Statistical analysis of microbiological results
For microbiological assay, the results of the tests were analysed in triplicates and expressed as the geometric mean. Others assay the results of the analyses which were based on the mean and its corresponding mean ± error standard of the mean (S.E.M.). All statistical analysis was applied using one-way ANOVA or two-way ANOVA followed by Tukey test or Bonferroni’s post hoc test, respectively, using the software GraphPad Prism 5.0., and p values <0.05 were considered significant differences.
Results
HPLC analysis
The A. brasiliana ethanolic extract HPLC profile was also acquired, where the analysis is shown in Fig. 1. The A. brasiliana sample extracts contain other minor compounds in addition to gallic acid (retention time (tR) 10.85 min, peak 1), chlorogenic acid (tR = 22.07 min, peak 2), caffeic acid (tR = 24.98 min, peak 3), orientin (tR = 35.46 min, peak 4), vitexin (tR = 39.95 min, peak 5), quercetin (tR = 48.17 min, peak 6), luteolin (tR = 54.83 min, peak 7) and apigenin (tR = 59.74 min, peak 8) (Table 2).
Drosophila melanogaster toxicological assays
Results of the in vivo toxicity test in D. melanogaster demonstrated that mortality differed significantly from the control only after 24 h of exposure without a dose-dependent response. However, this exposure up to 48 h was not sufficient to kill more than 50% of the flies (Fig. 2).
Antinociceptive and antiedematogenic activity
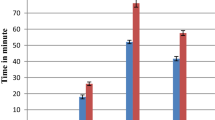
In the first phase of the experimental evaluation, which represents the neurogenic phase of the edematogenic process, the extract was not significantly effective in any of the tested concentration doses, in comparison with the control group. In the second phase of the experiment, characteristic of the inflammatory phase, the extract, at the doses of 25, 50 and 100 mg/kg, was capable of significantly reducing the edematogenic process by 35.57, 64.67 and 64.17%, respectively (Fig. 3).
EEAB effect at the doses of 25, 50 and 100 mg/kg on the paw licking time during the first phase of the formalin test. The values represent the mean ± S.E.M (standard error of the mean) for six animals. a2p < 0.01; a4p < 0.0001 vs saline. One-way ANOVA followed by Bonferroni’s test. ns = no significant
Antibacterial activity and antibiotic modifying activity
Regarding the antibacterial activity of the ethanolic extract from A. brasiliana leaves, the minimum inhibitory concentration was verified as ≥1024 μg/mL. This is being considered clinically irrelevant. However, in the antibiotic modulatory activity against gentamicin, a synergistic effect was verified, with the association reducing the MIC in all the tested strains when compared to the control (Fig. 4).
Effect of ethanolic extract of Alternanthera brasiliana (L.) Kuntze, associated with gentamicin against strain of a Staphylococcus aureus, b Escherichia coli, and c Pseudomonas aeruginosa. Values represent the geometric mean ± standard error of the mean (S.E.M). t test. a1p < 0.05 vs gentamicin. a3p < 0.001 vs gentamicin. ns no significant
Discussion
The A. brasiliana extract presents diverse biological activities which have been proven, among which are analgesic activity (De Souza et al. 1998; Hossain et al. 2014), anti-inflammatory (Kumar et al. 2014; Shivashankar et al. 2017) and antiviral activity (Lagrota et al. 1994), and the application of ointments made from the A. brasiliana ethanolic extract which provided effective wound healing (Barua et al. 2012, 2013b) can be cited. The previously cited results corroborate with the results obtained in this study as both show the pharmacological potential of this plant, and because they mainly point to an anti-inflammatory potential that this plant possesses.
D. melanogaster has been used for toxicity assays, as an alternative method to the use of animals, and it has become an excellent alternative model for several assays (Rand 2010; Tiwari et al. 2011). The results showed low toxicity of this extract and demonstrates a potential in pharmacological application with evidence of dual therapeutic benefit with anti-inflammatory effect and antibiotic modulatory activity.
The formalin test employed in this study is a behavioural experiment used to evaluate the efficacy of the antinociceptive and anti-inflammatory action of the tested agent. The first phase of the experiment corresponds to the neurogenic phase which is related to the direct stimulation of C-type afferent nociceptive fibres and the release of nitric oxide, substance P and excitatory amino acids. The second phase corresponds to the 15- to 30-min period after the induction of the phlogistic agent and is characterized with pain of an inflammatory origin which is involved with the stimulation and release of pro-inflammatory mediators, such as bradykinin, histamine, prostaglandins and serotonin (Tjølsen et al. 1992; Coderre et al. 2013).
The chemical constitution of the extract pointed to flavonoids luteolin, apigenin, orientin and vitexin, as the main compounds present. Luteolin is a known compound that has proven action in the reduction of nitric oxide production, in addition to increasing the expression of nitric oxide-inhibiting enzymes. This compound can promote the suppression of pro-inflammatory cytokine expression and inhibition of the NFκB pathway, Akt and the mitogen-activated protein kinase (MAPK) pathway. (Ziyan et al. 2007; Seelinger et al. 2008). The flavone apigenin demonstrated inflammatory actions by suppressing COX-2 and NFκB pathway and significantly inhibited liberation of inflammatory cytokines, such as interleukin-6 (IL-6), IL-1β and tumour necrosis factor-α (TNF-α) through modulating multiple intracellular signalling pathways (Wang et al. 2014; Zhang et al. 2014). Orientin suppressed LPS-induced membrane disruption, migration of monocytes, expression of cell adhesion molecules (CAMs) and inhibition of cytokines as TNF-α level and IL-6 (Lam et al. 2016). Anti-inflammatory effects of vitexin may also be related to the inactivation of transduction ways such as p38, ERK1/2 and JNK signalling pathways and inhibition of cytokines (He et al. 2016). Thus, the interaction of these compounds with each other may be acting in a modulatory manner in relation to the anti-inflammatory action described in the present work, validating the potential anti-inflammatory already elucidated in other experiments.
It is known that inflammation can be due to infectious processes caused by various microorganisms, especially bacteria. The bacteria E. coli, P. aeruginosa and S. aureus are the cause of many inflammatory processes in humans, mainly when individuals acquire doors which allow the entry of this organism in previously sterile locations in the organism (Medzhitov 2008). In addition, the problem becomes bigger if infections that generate inflammation come from bacteria that are multiresistant to various antibiotics (Sartor 2008).
A group of antibiotics for which several bacteria acquire resistance are the aminoglycosides. These kill the bacteria by inhibiting the synthesis of proteins that bind to RNA16S and alter the integrity of the bacterial cell membrane in sensitive strains. Before entering the cell, the aminoglycosides bind electrostatically to negatively charged residues on the outer membrane of Gram-negative bacteria in a passive transport in the non-energy-dependent process. After its diffusion through pore channels in the external membrane, these move towards the periplasmic space. Now, the transport across the cytoplasmic membrane requires metabolic energy from the electron transport system of an oxygen-dependent process (Shakil et al. 2008).
Therefore, in general term there are four mechanisms of action by which bacteria acquire resistance to aminoglycosides: (a) deactivation of aminoglycosides by N-acetylation, adenylation or O-phosphorylation; (b) reduction of the aminoglycoside intracellular concentration through changes in the outer membrane permeability or decreased internal membrane transport or active efflux; (c) targeted mutations in the 30S ribosomal subunit and (d) methylation of the aminoglycoside-binding site. In the case of aminoglycoside deactivation, the action is caused by phosphotransferase, acetyltransferase and nucleotidyltransferase (Shakil et al. 2008). All the strains used in this study are resistant to a diverse group of antibiotics, including aminoglycosides. It is possible that multiple secondary metabolites of the extract have acted in one/some of the steps of the aminoglycoside action mentioned above.
The A. brasiliana extract presents in its composition luteolin, apigenin and orientin as major phenolic compounds which can interfere in the mechanisms of resistance previously mentioned. However, antibacterial mechanisms of action of various flavonoids can be variant actions as inhibition of nucleic acid synthesis, DNA gyrase, was inhibited by apigenin. It was also was found that quercetin binds to the GyrB subunit of the E. coli DNAgyrase and inhibits the enzyme’s ATPase activity (Ohemeng et al. 1993). For example, the B ring of flavonoids may play a role in the intercalation or hydrogen bonding of the stacking of nucleic acid bases and this may explain the inhibitory action on DNA and RNA synthesis (Mori et al. 1987). Other mechanisms of action are inhibition of cytoplasmic membrane function; numerous observations were obtained in complex experimental settings indirectly indicating interactions with efflux transporters, as ABC transporters, with flavonoids (Morris and Zhang 2006). Flavonoids may also be interfering with energy metabolism in a similar way to respiratory-inhibiting antibiotics (Haraguchi et al. 1998).
Several studies conclude that the extract from A. brasiliana leaves possess activity against bacteria (Pereira et al. 2007; Silva et al. 2010; Trapp et al. 2015). The study by Pereira et al. (2007) shows the action of the ethanol extract and dichloromethane, ethyl acetate and butane fractions, against various strains such as P. aeruginosa, E. coli and S. aureus. This study was carried out by the broth microdilution method. In all of the assays, the MIC was greater than 1000 μg/mL; similar to our results, this did not show clinical relevance. With respect to the synergistic activity verified in the association with antibiotics, this is the first study that performs this type of association of the extract from A. brasiliana leaves with antibiotics, and as mentioned earlier, the results were significant. However, there are several studies on extracts with the same polarity of the solvent and similar chemical constituents, which showed their capacity to increase the action of antibiotics.
Conclusion
The A. brasiliana (L.) Kuntze ethanolic extract demonstrated low toxicity in the D. melanogaster model. An antinociceptive activity of central origin was not found; however, a pronounced antiedematogenic activity was demonstrated, suggesting a possible anti-inflammatory activity to be elucidated by other studies. With regards to the antibiotic modifying activity, the results have shown to be promising in view of the synergistic effect presented against multiresistant strains; however, new in vivo studies are needed to better evidence the mechanisms of action.
References
Barbosa Filho VM, Waczuk EP, Kamdem JP, Abolaji AO, Lacerda SR, Costa JGM, Menezes IRA, Boligon AA, Athayde ML, Rocha JBT, Posser T (2014) Phytochemical constituents, antioxidant activity, cytotoxicity andosmotic fragility effects of Caju (Anacardium microcarpum). Ind Crops Prod 55:280–288
Barua CC, Talukdar A, Begum SA et al (2009) Antinociceptive activity of methanolic extract of leaves of Alternanthera brasiliana Kuntz. In animal models of nociception. Pharmacol Online 3:49
Barua CC, Ara Begum S, Talukdar A et al (2012) Influence of Alternanthera brasiliana (L.) Kuntze on altered antioxidant enzyme profile during cutaneous wound healing in immunocompromised rats
Barua CC, Begum SA, Barua AG et al (2013a) Anxiolytic and anticonvulsant activity of methanol extract of leaves of Alternanthera brasiliana (L.) Kuntze (Amaranthaceae) in laboratory animals
Barua CC, Begum SA, Pathak DC, Bora RS (2013b) Wound healing activity of Alternanthera brasiliana Kuntze and its anti oxidant profiles in experimentally induced diabetic rats. J Appl Pharm Sci 3:161
Boligon AA, Kubiça TF, Mario DN, Brum TF, Piana M, Weiblen R, Lovato L, Alves SH, Santos RCV, Alves CFS, Athayde ML (2013) Antimicrobial and antiviral activity-guided fractionation from Scutia buxifolia Reissek extracts. Acta Physiol Plant 35: 2229–2239
Chiu IM, Heesters BA, Ghasemlou N et al (2013) Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501:52–57
CLSI Clinical and Laboratory Standards Institute (2008) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard, Tenth Edit. NCCLS, Wayne, Pennsylvania. pp. 120–126
Coderre TJ, Abbott FV, Sawynok J (2013) Formalin test. In: Encyclopedia of pain. Springer, pp 1303–1308
David B, Wolfender J-L, Dias DA (2015) The pharmaceutical industry and natural products: historical status and new trends. Phytochem Rev 14:299–315
De Carvalho Nilo Bitu V, De Carvalho Nilo Bitu V, Matias EFF et al (2015) Ethnopharmacological study of plants sold for therapeutic purposes in public markets in Northeast Brazil. J Ethnopharmacol 172:265–272. doi:10.1016/j.jep.2015.06.022
De Kraker MEA, Davey PG, Grundmann H, Group BS (2011) Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med 8:e1001104
de Santana Aquino DF, Piccinelli AC, Soares FLP et al (2015) Anti-hyperalgesic and anti-inflammatory activity of Alternanthera maritima extract and 2 ″-O-α-l-rhamnopyranosylvitexin in mice. Inflammation 38:2057–2066
De Souza MM, Kern P, Floriani AEO, Cechinel-Filho V (1998) Analgesic properties of a hydroalcoholic extract obtained from Alternanthera brasiliana. Phyther Res 12:279–281
Duarte M d R, Debur M d C (2004) Characters of the leaf and stem morpho-anatomy of Alternanthera brasiliana (L.) O. Kuntze, Amaranthaceae. Rev Bras Ciências Farm 40:85–92
Fathima SN, Salwa A, Anusha S, Fatima S (2016) Study of antiasthmatic activity of ethanolic extract of Alternanthera sessilis leaves. Int J Pharma Res Heal Sci 4:1478–1482
Ferreira Júnior WS, Da Silva TG, Alencar Menezes IR, Albuquerque UP (2016) The role of local disease perception in the selection of medicinal plants: a study of the structure of local medical systems. J Ethnopharmacol 181:146–157. doi:10.1016/j.jep.2016.01.038
Firdhouse MJ, Lalitha P (2013) Biosynthesis of silver nanoparticles using the extract of Alternanthera sessilis—antiproliferative effect against prostate cancer cells. Cancer Nanotechnol 4:137
Gafter-Gvili A, Fraser A, Paul M, Leibovici L (2005) Meta-analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med 142:979–995
Garín-Aguilar ME, Benavides-Catalán D, Segura Cobos D et al (2014) Spasmolytic effect of Alternanthera repens on isolated rat ileum. Pharm Biol 52:479–485
Haraguchi H, Tanimoto K, Tamura Y et al (1998) Mode of antibacterial action of retrochalcones from Glycyrrhiza inflata. Phytochemistry 48:125–129
He M, Min J-W, Kong W-L et al (2016) A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 115:74–85
Heinrich M (2013) Ethnopharmacology and drug discovery. Compr Nat Prod II Chem Biol dev Modif Bioactivity 3:351–381
Hossain AI, Faisal M, Rahman S et al (2014) A preliminary evaluation of antihyperglycemic and analgesic activity of Alternanthera sessilis aerial parts. BMC Complement Altern Med 14:169
Hundiwale Jogendra C, Patil Avinash V, Kulkarni Mohan V et al (2012) A current update on phytopharmacology of the genus Alternanthera. J Pharm Res 5:1924–1929
Hunskaar S, Hole K (1987) The formalin test in mice: dissociation between inflammatory and noninflammatory pain. Pain 30: 103–114
Iamonico D, Sánchez-Del Pino I (2016) Taxonomic revision of the genus Alternanthera (Amaranthaceae) in Italy. Plant Biosyst Int J Deal with all Asp Plant Biol 150:333–342
Kumar YS, Sanjib D (2013) Evaluation of anti-diarrhoeal property of crude aqueous extract of Alternanthera sessilis Linn. Int J Pharm Inov 3:213–217
Kumar S, Barua C, Das S (2014) Evaluation of anti-inflammatory activity OF Alternanthera brasiliana leaves. Int J Pharma Bio Sci 5:33–41
Lagrota MHC, Wigg MD, Santos MMG et al (1994) Inhibitory activity of extracts of Alternanthera brasiliana (Amaranthaceae) against the herpes simplex virus. Phyther Res 8:358–361
Lam KY, Ling APK, Koh RY et al (2016) A review on medicinal properties of orientin
Levy SB, Marshall B (2004) Antibacterial resistance worldwide: causes, challenges and responses. Nat Med 10:S122–S129
Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454:428–435
Mori A, Nishino C, Enoki N, Tawata S (1987) Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry 26:2231–2234
Morris ME, Zhang S (2006) Flavonoid–drug interactions: effects of flavonoids on ABC transporters. Life Sci 78:2116–2130
NCCLS (2015) NCCLS document M7-A10. In: NCCLS (ed) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard, Tenth Edit. NCCLS, Wayne, Pennsylvania, pp M7-A10
Ogundare AO, Oladejo BO (2014) Antibacterial activities of the plant extract of Alternanthera repens. Eur J Bot Plant Sci Phytol 1:1–7
Ohemeng KA, Schwender CF, Fu KP, Barrett JF (1993) DNA gyrase inhibitory and antibacterial activity of some flavones (1). Bioorg Med Chem Lett 3:225–230
Okeke IN, Laxminarayan R, Bhutta ZA et al (2005) Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis 5:481–493
Pereira DF, dos SANTOS M, Pozzatti P et al (2007) Antimicrobial activity of a crude extract and fractions from Alternanthera brasiliana (L.) O. Kuntze leaves. Lat. Am J Pharm 26:893
Rand MD (2010) Drosophotoxicology: the growing potential for Drosophila in neurotoxicology. Neurotoxicol Teratol 32:74–83
Rodrigues LB, Martins AOBPB, Cesário FRAS et al (2016) Anti-inflammatory and antiedematogenic activity of the Ocimum basilicum essential oil and its main compound estragole: in vivo mouse models. Chem Biol Interact 257:14–25
Samudrala PK, Augustine BB, Kasala ER et al (2014) Evaluation of antitumor activity and antioxidant status of Alternanthera brasiliana against Ehrlich ascites carcinoma in Swiss albino mice. Pharm Res 7:66–73
Sartor RB (2008) Microbial influences in inflammatory bowel diseases. Gastroenterology 134:577–594
Seelinger G, Merfort I, Schempp CM (2008) Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med 74:1667–1677
Shakil S, Khan R, Zarrilli R, Khan AU (2008) Aminoglycosides versus bacteria—a description of the action, resistance mechanism, and nosocomial battleground. J Biomed Sci 15:5–14
Shivashankar P, Purushotham K, Lahkar M (2017) Effect of Alternanthera brasiliana in experimentally induced inflammatory bowel disease in albino rats. Int J Basic Clin Pharmacol 5:1809–1815
Silva LC d, Pegoraro KA, Pereira AV et al (2010) Antimicrobial activity of Alternanthera brasiliana Kuntze (Amaranthaceae): a biomonitored study. Lat am J Pharm 30:147–153
Silva IV, Aalves SK, Araújo CF et al (2015) Planta Medicinal: Por Dentro de Alternanthera brasiliana (L.) O. Kuntze (Amaranthaceae) Cultivada em Quintais (Alta Floresta-MT)
Souza RKD, da Silva MAP, de Menezes IRA et al (2014) Ethnopharmacology of medicinal plants of carrasco, northeastern Brazil. J Ethnopharmacol 157:99–104. doi:10.1016/j.jep.2014.09.001
Tiwari AK, Pragya P, Ram KR, Chowdhuri DK (2011) Environmental chemical mediated male reproductive toxicity: Drosophila melanogaster as an alternate animal model. Theriogenology 76:197–216
Tjølsen A, Berge O-G, Hunskaar S et al (1992) The formalin test: an evaluation of the method. Pain 51:5–17
Tracey I, Mantyh PW (2007) The cerebral signature for pain perception and its modulation. Neuron 55:377–391
Trapp MA, Kai M, Mithöfer A, Rodrigues-Filho E (2015) Antibiotic oxylipins from Alternanthera brasiliana and its endophytic bacteria. Phytochemistry 110:72–82
Usunoff KG, Popratiloff A, Schmitt O, Wree A (2006) Functional neuroanatomy of the pain system. Springer
Wang J, Liu Y-T, Xiao L et al (2014) Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway. Inflammation 37:2085–2090
Yoshikai Y (2001) Roles of prostaglandins and leukotrienes in acute inflammation caused by bacterial infection. Curr Opin Infect Dis 14:257–263
Zankari E, Hasman H, Cosentino S et al (2012) Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644
Zhang X, Wang G, Gurley EC, Zhou H (2014) Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS One 9:e107072
Ziyan L, Yongmei Z, Nan Z et al (2007) Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta Med 73:221–226
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The present study was submitted to the Animal Experimentation and Use Committee (CEUA) of the Regional University of Cariri (URCA) and approved under the protocol number 15/2012.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Coutinho, H.D.M., de Morais Oliveira-Tintino, C.D., Tintino, S.R. et al. Toxicity against Drosophila melanogaster and antiedematogenic and antimicrobial activities of Alternanthera brasiliana (L.) Kuntze (Amaranthaceae). Environ Sci Pollut Res 25, 10353–10361 (2018). https://doi.org/10.1007/s11356-017-9366-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9366-x