Abstract
The present study was designed to investigate different phytochemical groups present in ethanolic extract of Argemone mexicana aerial parts (EAMA) as well as to assess the analgesic, antidiarrheal, antibacterial, anthelmintic and cytotoxic activities of EAMA. Moreover, peripheral and central analgesic activities were evaluated by acetic acid-induced writhing test, formalin-induced paw licking test, tail immersion test and hot plate test. In vivo castor oil-induced diarrheal model and magnesium sulphate induced diarrheal model in mice were utilized for the assessment of antidiarrheal activity. Again, antibacterial activity was evaluated using disc diffusion assay. Anthelmintic activity was carried out on Paramphistomum cervi (Trematoda). Cytotoxic activity was assessed through brine shrimp lethality bioassay. The extract demonstrated the presence of alkaloids, flavonoids, phenolic compounds, tannins, glycosides and gums in phytochemical screening. In case of acetic acid-induced writhing and formalin induced paw licking test, both lower and higher doses of EAMA showed significant percentage inhibition of writhing as well as paw licking respectively (*P < 0.05, vs. control). EAMA at the doses of 200 mg/kg and 400 mg/kg revealed significant latency response (*P < 0.05, vs. control) in delayed phase in both tail immersion as well as hot plate test. Moreover, significant percentage inhibition (*P < 0.05, vs. control) of diarrhea was exposed by both doses of EAMA in in vivo diarrheal models. In disc diffusion assay, EAMA showed antibacterial activities against both gram positive and gram negative bacterial strains. Again, the extract exhibited anthelmintic and cytotoxic activity in a dose dependent manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exploration of new drugs having maximum therapeutic effects as well as minimal side effects is a keen interest of the researchers. The most promising origin of lead compounds are medicinal plants that plays a vital role in the discovery of noble drugs. The medicinal plants derived bioactive compounds are considered as the raw materials for the discovery of new drugs (Hasan et al. 2017). Various phytochemical groups including flavonoids, tannins, volatile oils, alkaloids, glycosides, etc., possess potential pharmacological activities which are obtained from medicinal plants (Islam et al. 2013). Nociception or pain refers to the unpleasant emotional as well as sensory events associated with tissue damage which is either definite or probable. The mechanical, thermal, chemical instigations as well as presence of pathological procedure including inflammation, nerve damage, muscle spasm and tumor are responsible for tissue damage. (Moushome et al. 2016). At present, analgesic drugs namely corticosteroids (e.g., hydrocortisone), narcotics (e.g., opioids) and non-narcotics (e.g., salicylates) are prescribed for the alleviation of pain as well as inflammatory condition (Ganguly et al. 2016). Most of the opioid analgesics primarily exert their action through MORs (Gi/o-coupled receptors). The presence of DORs, MORs along with KORs (δ-, μ-, and κ-opioid receptors respectively) at the peripheral, spinal as well as supraspinal levels play significant role for the termination of pain. The activation of these receptors causes potential analgesic effect. The unwanted side effects of traditional opioid drugs are mainly initiated by the MOR receptor. For the eradication of these undesirable side effects researchers are looking for novel analgesics with their constant effort and it has been a burning issue in the treatment of pain (Carneiro et al. 2017). Diarrheal disease has been a primary problem in health care among the children particularly in the developing countries that is the prominent cause of morbidity as well as mortality. In human, diarrhea is mainly caused by Escherichia coli, Shigella flexneri, Staphylococcus aureus, and Salmonella typhi. Humans also suffer from diarrhea and the causative agent is Candida albicans (Umer et al. 2013). Not only for the treatment but also for the management of diarrhea, a program on diarrhea disease control has been founded by the World Health Organization (WHO) which deals with the practice of traditional medicine accompanied by health education as well as prevention approaches and is established on herbal products. So, in Bangladesh, a developing country, where enormous people involving children are suffering from diarrhea, the prime interest of the researcher is to explore medicinal plants having antidiarrheal activities (Hasan et al. 2017). Drug resistance of the microorganisms towards the available antimicrobials has been the prime cause for the successive enhancement of outbreak of the infectious disease along with complications originated from microorganisms. From the late 20th century, one of the key difficulties of humanity is the antibiotic resistance. The necessity of new antimicrobials has horribly magnified due to their combating power of the resistant microbes. The quick resistance of the antimicrobials has made the traditional approaches unsuccessful to search new drugs. Therefore, discovery of antimicrobial compounds through new approaches is a demand of time and medicinal plants may be the most potential sources among all of the natural sources. Antimicrobial activity is exerted by the secondary metabolites obtained from the plant (Ginovyan et al. 2017). The mechanism by which plant derived compounds exhibit antimicrobial activity is different from currently used antibiotics and hence may be clinically significant in the treatment of resistant microbial strains. (Rafshanjani et al. 2014). Helminthes are considered to the key problem to the production of livestock all over the tropics. Helminthes causes the diseases; naturally most of them are either chronic or debilitating. Moreover, helminthes exposes higher degree of mobidity rate and economic and social deprivation than any individual class of parasites among the humans as well as animals. Various species of stomach as well as intestinal worms comprising of mixed infection cause parasitic gastroenteritis which lead to weakness, reduced weight gain, loss appetite, decreased productivity and decreased feed efficiency (Balamurugan and Selvarajan 2009). Moreover, it has been proposed to screen medicinal plants having anthelmintic activity due to enhancement of problems associated with resistance in helminthes towards anthelmintic (Lateef et al. 2003). The detection of bioactive compounds can be confirmed in the plant extracts by using brine shrimp lethality bioassay. According to National Cancer Institute (NCI, USA), there is a significant correlation between in vitro growth inhibition of solid tumor cell lines in human with brine shrimp bioassay because it is regarded as a pre-screening tool for the research of antitumor drugs (Rafshanjani et al. 2014).
Argemone mexicana, belonging to the family papaverace possesses prickly stems along with leaves and capsules (Apu et al. 2012). It is medium in size with a height of 60–90 cm containing yellow latex. The leaves are not only simple but also spiny as well as sessile. Moreover, flowers are large and bright yellow whereas fruits are prickly capsules (Jain et al. 2012). In Mexico, this medicinal plant is usually known as ‘Mexican Poppy’ in the rural areas (Apu et al. 2012). It is widely distributed in the United States, Ethiopia, India and Bangladesh (Prabhakaran et al. 2016). Various isoquinoline alkaloids and phenolic compounds have been isolated from this plant such as cheilanthifoline, thalifoline, scoulerine, berberine, muramine, coptisine, stylopine, cryptopine, sanguinarine, protopine and argemexitin, 5,7-dihydroxychromone-7-neohesperidoside(I), eriodictyol respectively (Dash and Murthy 2011; Jaliwala et al. 2011). Generally the plant is well known for many traditional uses. It is used as analgesic, diuretic, anti-inflammatory, antibacterial, antimalarial, antispasmodic, sedative, narcotic, purgative, an antidote to various poisons and also effective against worms, itching, various skin diseases (Dash and Murthy 2011; Apu et al. 2012). Seeds are beneficial for the treatment of asthma and cough (Bhalke et al. 2009). The milky and fresh yellow seed extract having protein dissolving substances have been found to be useful for the treatment of cutaneous infections, jaundice, skin diseases, cold sores, itches, warts and dropsy (Singh et al. 2009). The roots are effective in helminthiasis. Traditionally leaves of A. mexicana are known as antiasthamatic (Bhalke et al. 2009). Reviewing the literature of this plant it was found that different parts of the plant revealed some pharmacological activities including analgesic, anti-cancer, anti-fungal, anthelmintic, anti-oxidant, anti-inflammatory, anti-microbial, anti-mutagenic, anxiolytic, cytotoxicity, hypoglycaemic, neuropharmacological and thrombolytic activities (Apu et al. 2012; Jain et al. 2012; Jaliwala et al. 2011; Sourabie et al. 2012; Araujo et al. 2015; More and Kharat 2016; Martinez et al. 2016; Duhan et al. 2011; Rout et al. 2011; Anarthe and Chaudhari 2011).
So the current study was carried out to identify phytochemical groups as well as to evaluate analgesic, antidiarrheal, antibacterial, anthelmintic and cytotoxic activities of the ethanolic extract of A. mexicana Linn. aerial parts (EAMA).
Materials and methods
Chemicals and reagents
Acetic acid, formalin, ethanol, dimethyl Sulfoxide (DMSO) and magnesium sulphate were obtained from Merck, Germany. Castor oil and Tween-80 were provided by Loba Chemie Pvt Ltd., India. Moreover, standard drug diclofenac sodium, Loperamide HCl and Albendazole were purchased from Bangladeshi Manufacturer namely Square Pharmaceuticals Ltd. Tramadol and vincristine sulphate were obtained from Acme Laboratories Ltd. and Beacon Pharmaceuticals Ltd., Bangladesh respectively. The solvents used in the experiments were of analytical grade.
Experimental animals
To conduct the present in vivo pharmacological activities the young Swiss albino mice of either male or female having a average age of 6–7 weeks as well as 25–30 g weight were purchased from the Department of Pharmacy, Jahangirnagar University, Savar, Dhaka, Bangladesh. Under suitable housing condition these experimental animals were kept where the relative humidity was 55–65% along with 12:12 h (light: dark) cycle and the temperature also maintained at (27.0 ± 1.0) °C. Rodent foods as well as water ad libitum were provided to the animals. A period of 1 week was considered for the adaptation of the experimental animals with the laboratory conditions. Prior to the study, the design of the experiments involving mice were approved by the Ethical Review Committee, Faculty of Biological Science and Technology, Jessore University of Science and Technology and all the experiments were conducted according to their guidelines.
Test pathogens
Both gram positive and gram negative bacteria were collected from ICCDR, B including Staphylococcus epidermidis, Staphylococcus pyogens, Staphylococcus aureus, Streptococcus agalactiae, Enterococcus faecalis as well as Pseudomonus spp., Shigella boydigus, Shigella sonnei, Escherichia coli, Shigella flexineri respectively and were preserved in microbiology Lab, department of pharmacy, Jessore university of science and technology, Jessore-7408 for the evaluation of antibacterial activity.
Collection of the parasites
Paramphistomum cervi parasites were collected from freshly slaughtered cattle at local abattoirs. After cleaning, parasites were stored in 0.9% phosphate buffered saline (pH = 7.4).
Collection of the brine shrimp
The eggs of the brine shrimp were collected from an aquarium shop (Khulna, Bangladesh) and hatched in artificial seawater (3.8% NaCl solution) for 48 h to get mature shrimp called Nauplii.
Collection and identification of the plant
A. mexicana Linn. aerial parts were collected from Nowapara (23°1′52″N, 89°23′57″E), Jessore, Bangladesh, in June, 2013. The identification of the species was verified by Sarder Nasir Uddin (Principal Scientific Officer, Bangladesh National Herbarium) and the accession no. was (DACB-45000). A dried specimen was preserved in the Herbarium for future reference.
Preparation and extraction of the plant material
Fresh aerial parts of A. mexicana were rinsed successively with running water for 3–4 times as well as once with sterile distilled water. Moreover, drying of the aerial parts was completed by the application of shade drying for a period of 7 days, which were then grinded into coarse powder by using a laboratory grinding mill (MACSALAB 200 Cross Beater, Eriez, Erie, Pennsylvania, USA) and also passed through a 40-mesh sieve to obtain fine powder. Ethanolic extraction of the powdered plant material was carried out following cold extraction procedure. 250 g powdered material was submerged in 800 ml ethanol in a glass container for 10 days along with continuous shaking and stirring. Then filtration was performed on the extract with clear cotton plug to separate the plant debris. Again, the residue was submerged in 450 ml ethanol for a period of 3 days and also filtered by Whatman No. 1 filter papers to eradicate the plant debris. Evaporation of the filtrate was completed by a rotary vacuum evaporator (Bibby RE200, Sterilin Ltd., UK). Then dried extract was kept in an air-tight container and stored in refrigerator at 4 °C to prevent fungal attack as well as to conduct further studies. The percentage yield of the extract was 6% (w/w).
Phytochemical screening
Various qualitative tests were carried out on freshly prepared EAMA to identify the presence of phytochemical groups such as carbohydrates, alkaloids, phenolic compounds, saponins, tannins, flavonoids, anthraquinones, glycosides, acidic compounds and gums through distinctive color changes according to Patil et al. (2014) and Moushome et al. (2016).
Acute toxicity study
According to the method of Islam et al. (2013), EAMA was subjected for acute toxicity test for the assessment of possible toxicity as well as safe doses. Acute toxicity deals with the adverse effects due to the exposure of either single or multiple exposure of a given substance. Usually it takes short time e.g. less than 24 h to elicit acute toxicity. The guidelines provided by the Organization of Economic Cooperation and Development (OECD) were followed to determine half lethal dose (LD50) of the experimental sample through acute toxicity study. Generally two groups namely control group and test group (EAMA) were made and number of mice in each group was five. Different concentrations of the experimental sample such as 100, 250, 500, 1000, 2000, 3000 and 4000 mg/kg body weight were administered to the respective group of the mice by oral route. After an observation period of every 1 h for next 5–6 h, some parameters including mortality, salivation, injury, diarrhea, convulsion, noisy breathing, changes in locomotor activity, pain, weakness, food or water refusal, coma, aggressiveness, discharge from eyes and ears or any signs of toxicity were checked in the respective group of mice (Moushome et al. 2016).
Analgesic activity study
Acetic acid-induced writhing test
According to the method of Sarkar et al. (2013), the acetic acid induced writhing model in mice was employed for the evaluation of the analgesic activity of test sample (EAMA). Twenty mice were selected randomly and divided into four groups including control group, positive control group and test groups (EAMA 200 mg/kg and 400 mg/kg body weight, respectively) and each group contained five mice. The animals were fasted for 16 h with water ad libitum before the experiment. The mice of control group and positive control group were administered distilled water at the dose of 10 ml/kg body weight and diclofenac sodium at the dose of 25 mg/kg body weight, respectively through oral route using feeding needle whereas mice of the third group and fourth group were subjected for oral administration of EAMA 200 mg/kg and EAMA 400 mg/kg respectively. After the 30 min of oral administration, the mice of the each group were given intraperitoneal injection of acetic acid (0.6% v/v) at the dose of 10 ml/kg body weight. Then for a period of 10 min the number of writhes of each mouse was counted after an interval of 5 min of the intraperitoneal injection. The number of writhes of mice in positive control groups as well as test groups was taken into consideration to compare to those of control group and hence, the percentage of inhibition of writhing was calculated using following formula:
where W1 and W0 represent the mean writhing of the control and standard or sample groups respectively.
Formalin-induced paw licking test
Formalin induced paw licking test was performed following the method of Moushome et al. (2016) and Bukhari et al. (2016) to assess the analgesic activity of EAMA. Before the experiment, the animals were fasted for 16 h with water ad libitum. Grouping of the mice as well as sample administration were completed as like as aforementioned test. Diclofenac sodium (25 mg/kg body weight) and distilled water (10 ml/kg body weight) were used as positive control and control respectively. After an interval of 1 h, formalin (2.7% v/v)) at the dose of 20 µL was injected subcutaneously into the left hind paw (dorsal surface) of the each mouse in the respective groups and then placed into the transferrent box quickly for observation. The time (in second) spent in licking response of the injected paw was counted for each mouse. After formalin injection, the response of the mice was observed for a period of 5 min in two phases including acute phase (0–5 min) and delayed phase (20–25 min) respectively. Hence, the percentage of inhibition of licking in both phases was calculated using following formula:
where T1 and T0 represent the mean licking time of the control and standard or sample groups respectively.
Tail immersion test
The method of Moushome et al. (2016) and Khatun et al. (2015) was followed to carry out this test. Generally the mechanism of centrally acting analgesics is evaluated through this method. This method is established on the principal of the prolongation of time required for the tail withdrawal of mice from hot water having morphine like drugs. Here the thermal stimulus produced painful reactions in animals when the tips of the tail were placed in hot water maintained at (55 ± 1) °C. Mice were grouped as well as administered as discussed above. It should be noticed that, a cutoff point (latency period of 15 s) was considered to avoid accidental damage of the tail of the mice. Mice of the third and fourth groups were pretreated with extracts (EAMA 200 mg/kg and 400 mg/kg body weight, respectively). Moreover, Tramadol (10 mg/kg) was supplied to mice of the positive control group and mice of the control group were treated with distilled water (10 ml/kg). 30 min prior to the treatment of the respective group, the basal reaction time (in second) i.e. the time required to withdraw it from hot water was calculated through the immersion of the tail tips (last 1–2 cm) of mice in hot water (55 ± 1) °C. After the respective treatment of each group, the latent period of tail flick response was also recorded at an interval of 30 min, 60 min, 120 min and 180 min consecutively. The results of the latency periods were compared to the control group.
Hot plate test
Hot plate test was carried out following the method of Moushome et al. (2016). The purpose of using hot plate method is to evaluate the mechanism of centrally acting analgesics (Mannan et al. 2017). Prior to the experiment, mice were placed on the hot plate maintained at (55 ± 1) °C and selected for the test on the basis of their initial reaction time or latency response (15 s or less). It should be noticed that, a cutoff point of 15 s was considered to avoid accidental damage of the paw of the mice. The experimental animals were fasted for 16 h before the experiment with water ad libitum. Grouping of the mice as well as sample administration were completed as like as aforementioned test. The mice of the control group were supplied distilled water at the dose of 10 ml/kg body weight and Tramadol (10 mg/kg) was used as positive control. The response latencies of the mice of the respective group (control group, positive control, EAMA 200 mg/kg and EAMA 400 mg/kg) were recorded before 30 min and at an interval of 30 min, 60 min, 120 min and 180 min after respective treatment of each group through the observations of some parameters like jumping, licking or removal of the paw from the hot plate.
Antidiarrheal activity study
Castor oil induced diarrheal test
The method of Shoba and Thomas (2001) was utilized to perform Castor oil induced diarrheal test. The selection of the mice for the experiment was completed by observing the diarrhea started upon administration of 0.5 ml of castor oil. The selected twenty mice were divided into four groups named control, positive control, EAMA 200 mg/kg and EAMA 400 mg/kg containing five mice in each group. The mice of first and second groups received distilled water and Loperamide HCl at the dose of 10 ml/kg and 3 mg/kg body weight respectively. The mice of the third and fourth groups were treated with EAMA at a dose of 200 mg/kg as well as 400 mg/kg body weight respectively. The test samples were supplied orally to the mice. Before the experiment, the animals were fasted with water ad libitum for 16 h. After an interval of 30 min, the treated mice of the respective group were also supplied with 0.5 ml castor oil through oral route for commencing diarrhea. Then, each mouse was kept in the isolated cage having blotting paper at the base. The number of diarrheal feces was counted at every hour for a period of 4 h and blotting papers were changed every hour. Therefore, Percent inhibition of defecation of the respective group was determined as well as compared. The following formula was used to estimate percent inhibition of defecation.
% inhibition of defecation = (1 − D0/D1) × 100, where D0 is the number of defecation of control group, and D1 is the number of defecation of test or standard group.
Magnesium sulphate induced diarrheal test
The antidiarrheal activity of EAMA was evaluated using MgSO4 induced diarrheal model described by Saha et al. (2013). The selection of the mice for the experiment was completed by observing the diarrhea started upon oral administration of 2 g/kg of MgSO4. The selected twenty mice were divided into four groups named control, positive control, EAMA 200 mg/kg and EAMA 400 mg/kg containing five mice in each group. Here, Loperamide HCl (3 mg/kg) and distilled water (10 ml/kg) were used as positive control or standard and control group respectively. The each mouse of the respective group was supplied with test sample as like as Castor oil induced diarrheal test. The experimental animals were fasted for 16 h with water ad libitum before the experiment. After an interval of 30 min of the pretreatments, diarrhea was started in the mice by supplying magnesium sulphate orally at the dose of 2 g/kg body weight. An isolated cage containing blotting paper at the base was taken for the placement of each mouse. The observation period was 4 h and the number of diarrheal feces was counted at every hour. It should be noted that, blotting papers were changed every hour. Therefore, percent inhibition of defecation of the respective group was calculated following the same formula used in Castor oil induced diarrheal test.
Antibacterial activity test
Disc diffusion assay
Evaluation of the antibacterial activity of EAMA was completed according to disc diffusion assay (Bauer et al. 1966). Desired concentrations of the extract were prepared by using DMSO. Moreover, Kanamycin (30 μg/disc) was used as positive control whereas Sterile blank discs (BBL, Cocksville, USA) containing DMSO as control. The blank discs were impregnated with test extract (EAMA) at the concentrations of 250 and 500 μg/disc using micropipette. Then discs were dried. Sample discs (EAMA 250 and 500 μg/disc), Kanamycin (30 μg/disc) and blank disc were applied on the petridishes containing nutrient agar medium seeded with bacteria which were accompanied by sterile forceps. Therefore, petridishes were subjected for incubation for a period of 16 h at 37 °C. After incubation period, all experiments were performed in triplicates and the zone of inhibition surrounding each disc was recorded through digital slide calipers.
Anthelmintic activity test
According to the method of Islam et al. (2015), anthelmintic activity of EAMA was performed on Paramphistomum cervi (Trematoda) because of their availability as well as resemblance to the intestinal worms. The collected parasites were rinsed with distilled water for the prevention of fecal matters and divided into four groups namely control (0.1% tween-80 in PBS), positive control (Albendazole 15 mg/ml), EAMA 25 mg/ml and EAMA 50 mg/ml containing four parasites in each group. Then standard along with test samples were applied on the petridishes for the determination of the time taken for paralysis as well as death in case of selected parasites. Time for paralysis was recorded when no movement until shaken the parasites robustly and time for death also noted when it was observed that the parasites neither moved after vigorous shaking nor dipping into warm water (50 °C).
Cytotoxic activity test
Brine shrimp lethality bioassay
The method of Firdaus et al. (2013) was utilized to carry out cytotoxic activity of EAMA following brine shrimp lethality bioassay. In order to get Nauplii (Larvae), the eggs of brine shrimp (Artemia salina) were kept in 1 L of sea water accompanied by aeration and were subjected to hatching for a period of 16 h at 37 °C. Different concentrations including 320 µg/ml, 160 µg/ml, 80 µg/ml, 40 µg/ml, 20 µg/ml, 10 µg/ml, 5 µg/ml and 5 µg/ml, 2.5 µg/ml, 1.25 µg/ml, 0.625 µg/ml, 0.312 µg/ml were prepared using simulated sea water along with DMSO (Dimethylsulfoxide) for EAMA and standard respectively. Then the diluted solutions were added to the 5 ml of sea water containing 10 Nauplii. After 24 h, the mortality of the brine shrimp for the respective treatments was measured. The LC50 values (concentration of sample/standard required to kill 50% of brine shrimp) were calculated by plotting concentration versus percentage of mortality (at the confidence interval level of 5%) through Microsoft Excel® 2007.
Statistical analysis
All the results were expressed as mean ± SE (standard error). One-way ANOVA following Dunnet’s test (P <0.05, vs. control) was utilized for the analysis of all test results. In case of tail immersion and hot plate test, repeated measure ANOVA (RM-ANOVA) was performed for the analysis of the results. Moreover, comparison of the mean values among different groups (except control) was also carried out by One-way ANOVA following Post-hoc Bonferroni test (P <0.05, vs. standard/extract) which was considered statistically significant. SPSS software (version 20; IBM Corporation, New York, USA) was used for the analysis of all data.
Results
Phytochemical screening
Phytochemical screening of the EAMA demonstrated the presence of some phytochemical groups, which are summarized in Table 1. Alkaloids, flavonoids, phenolic compounds, tannins, glycosides and gums were present in EAMA whereas tests for carbohydrates, saponins, acidic compounds, and anthraquinones showed the negative results. In addition to, tests for flavonoids and glycosides demonstrated inconsistent results. Flavonoid contents as well as glycoside contents were observed by alkaline test and Molisch’s test respectively but lead acetate test indicated for flavonoids and concentrated sulphuric acid test for glycoside did not exhibit positive results.
Acute toxicity study
During the observation period of 14 days, it was noticed that the doses of EAMA supplied to the mice (even the maximum dose of 4000 mg/kg body weight) did not produce any mortality or signs of toxicity or behavioral changes. The control group exhibited the same result. This indicates that the minimum lethal dose of EAMA required to generate acute oral toxicity is more than 4000 mg/kg.
Acetic acid-induced writhing test
In acetic acid-induced writhing test, significant percentage inhibition of writhing was shown by all groups (*P < 0.05, vs. control). Both the lower and higher doses of EAMA (200 mg/kg and 400 mg/kg body weight) revealed the significant (*P < 0.05, vs. control) percentage inhibition of writhing (36.75 ± 2.36% and 59.58 ± 2.23% respectively). Moreover, diclofenac sodium showed 84.53 ± 2.11% inhibition of writhing at the dose of 25 mg/kg as compared to the control. So, the analgesic activity of the extract (EAMA) was comparable to the standard diclofenac sodium (Table 2).
Formalin-induced paw licking test
The significant highest percentage of inhibition (70.43 ± 1.95%) of paw licking in mice was demonstrated by EAMA 400 mg/kg body weight during the delayed phase of formalin injection (*P < 0.05, vs. control). Moreover, diclofenac sodium 25 mg/kg exhibited significant percentage inhibition (*P < 0.05, vs. control) of paw licking not only in acute phase but also in delayed phase of formalin injection. A gradual increase in percentage inhibition of paw licking was revealed by EAMA 400 mg/kg from acute phase to delayed phase. But EAMA 200 mg/kg showed decreased percentage inhibition of paw licking during late phase of formalin injection as compared to the acute phase (Table 3).
Tail immersion test
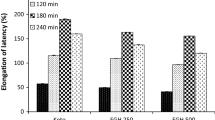
After an interval of 60 min of the administration of Tramadol, EAMA 200 mg/kg and EAMA 400 mg/kg, they exhibited significant increase in response latency (*P < 0.05, vs. control). Moreover, significant latency was shown by Tramadol at 60 min, 120 min and 180 min (*P < 0.05, vs. control). EAMA 200 mg/kg demonstrated significant latency only at 60 min (*P < 0.05, vs. control). In addition to, EAMA 400 mg/kg showed significant latency at 30 min, 60 min and 120 min (*P < 0.05, vs. control). Gradual increase in response latency from 2nd observation to 4th observation period was revealed by Tramadol and EAMA 400 mg/kg respectively. Both Tramadol and higher dose of EAMA (EAMA 400 mg/kg) exhibited maximum effect at 120 min (Table 4).
Tests of within-subjects effects conducted by repeated measure analysis of variance reveal that for the factor “time” calculated = 51.304 for all methods and P value = 0.000 in every case. So time is highly significant at any level of significance.
Hot plate test
After the administration of Tramadol, EAMA 200 mg/kg and EAMA 400 mg/kg, the significant latency was elicited by Tramadol and EAMA 400 mg/kg at 60 min, 120 min and 60 min, 180 min respectively (*P < 0.05, vs. control). Again, Tramadol showed gradual increase in response latency from 2nd observation to 4th observation period and it revealed maximum effect at 120 min. Moreover, fluctuations in response latency was noticed by EAMA 200 mg/kg during different observation periods and it didn’t show any significant latency (*P < 0.05, vs. control). Gradual increase in response latency was elicited by EAMA 400 mg/kg except 4th observation. In addition to, both the lower and higher doses of EAMA (EAMA 200 mg/kg and 400 mg/kg respectively) revealed maximum effect at 60 min (Table 5).
Tests of within-subjects effects conducted by repeated measure analysis of variance reveal that for the factor “time” calculated F = 16.599 for all methods and P value is not 0.000 in every case. So time is not highly significant at any level of significance.
Castor oil induced diarrheal test
In castor oil induced diarrheal model, antidiarrheal effects were exhibited by Loperamide HCl, EAMA 200 mg/kg and EAMA 400 mg/kg in mice. Both the lower and higher doses of EAMA (EAMA 200 mg/kg and 400 mg/kg) as well as Loperamide HCl significantly reduced the total number of diarrheal feces after 4 h (*P < 0.05, vs. control). Moreover, EAMA 400 mg/kg exhibited the highest and significant (*P < 0.05, vs. control) percentage inhibition of diarrhea (59.77 ± 4.00%). So, EAMA showed percentage inhibition of diarrhea comparable to that of Loperamide HCl (88.24 ± 2.27%) (Table 6).
MgSO4 induced diarrheal test
In MgSO4 induced diarrheal test, Loperamide HCl, EAMA 200 mg/kg and EAMA 400 mg/kg showed antidiarrheal effects in mice. After an observation period of 4 h, significant reduction in total number of diarrheal feces was revealed by both the lower and higher doses of EAMA (EAMA 200 mg/kg and 400 mg/kg) as well as Loperamide HCl (*P < 0.05, vs. control). Moreover, EAMA 400 mg/kg exhibited the highest and significant (*P < 0.05, vs. control) percentage inhibition of diarrhea (69.15 ± 4.09%). So, EAMA showed percentage inhibition of diarrhea comparable to that of Loperamide HCl (84.86 ± 2.26%) (Table 7).
Antibacterial activity test
Disc diffusion assay
In disc diffusion assay, the efficacy of antibacterial activity of both lower and higher doses of EAMA (250 µg/disc and 500 µg/disc respectively) has been depicted in the Table 8. From Table 8, it is noticeable that EAMA 250 µg/disc and EAMA 500 µg/disc showed zone of inhibition against different bacterial strains (both gram positive and gram negative) ranging from 5.56 ± 0.239 mm to 10.24 ± 0.101 mm and 6.41 ± 0.231 mm to 12.58 ± 0.147 mm respectively. Again, highest antibacterial activity was demonstrated by both doses (250 µg/disc and EAMA 500 µg/disc) against gram positive bacteria namely Staphylococcus pyogens. But EAMA 250 µg/disc exhibited lowest zone of inhibition (5.56 ± 0.239 mm) against Escherichia coli and in case of higher dose it was 6.41 ± 0.231 mm against Shigella flexineri. In addition to, Kanamycin (30 µg/disc) revealed zone of inhibition ranging from 16.31 ± 0.173 mm to 34.21 ± 0.193 mm against different strains. So, the EAMA showed significant antibacterial activity comparable to that of standard.
Anthelmintic activity test
The test for Anthelmintic activity of EAMA showed varying degree of activity including paralysis as well as death of the intestinal worms at various concentrations (25 mg/ml and 50 mg/ml). EAMA at the concentration of 25 mg/ml showed paralysis and death at 52.06 ± 1.091 min and 76.07 ± 2.400 min respectively. Again, time taken for paralysis and death was 41.69 ± 1.341 min as well as 57.56 ± 1.520 min respectively at the concentration of EAMA 50 mg/ml. Moreover, for Albendazole 15 mg/ml exhibition of paralysis time was 18.01 ± 1.226 min whereas death time was 26.12 ± 1.111 min. So, EAMA (25 mg/ml and 50 mg/ml) showed dose dependant (short time taken for paralysis and death at 50 mg/ml) anthelmintic activity comparable to that of standard (15 mg/ml) (Fig. 1).
Cytotoxic activity test
Brine shrimp lethality bioassay
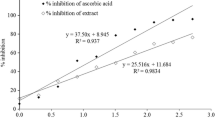
In brine shrimp lethality bioassay, a plot between % of mortality and concentrations of EAMA as well as vincristine sulphate made an approximate linear correlation between them respectively. The rate of percentage of mortality increased with the enhancement of concentration. The EAMA showed LC50 value of 51.0105 µg/ml and LC50 value of vincristine sulphate was 0.364 µg/ml Fig. 2.
Discussion
Medicinal herbs exert diversified activities due to the presence of bioactive compounds in the phytochemical groups of the plant extract (Alabri et al. 2014). Most of the plants species contain primary metabolites including monosaccharides, polysaccharides, amino acids, proteins, nucleic acid, and lipids. On the other hand, there are few plants in which secondary metabolites exist. All of these compounds are responsible for the resistance against herbivores as well as pathogens. They also provide mechanical support, absorb detrimental ultraviolet radiation, attract pollinators and fruit dispersers and decrease the growth of nearby competing plants. Medicinal properties might be possessed by some secondary metabolites like alkaloids, glycosides, phenolics, fatty acids, terpenoids, waxes and their derivatives. The identification of the bioactive compounds through preliminary screening of secondary metabolites play vital role in drug discovery as well as development (Aziz 2015).
In traditional system of medicine, a large number of products obtained from medicinal plants are used for their medicinal value but a few of them have been subjected for toxicological studies. So, it is important to carry out toxicological studies up on them. Moreover, there is a limited number of toxicity data for numerous plants. Again, preliminary toxicological data of the plant derived compound may be found up on its single administration in acute toxicity. Therefore, it is crucial to conduct acute oral toxicity study for the estimation of therapeutic range of the doses regarding successive usage as well as recognition of the adverse effects of the compounds. The gradual increment of the dose of EAMA up to 4000 mg/kg didn’t produce any mortality in the experimental animals. Hence, LD50 of EAMA couldn’t be determined. Therefore, the plant extract was considered to be safe having a wide range of therapeutic response and two doses of EAMA (200 mg/kg and 400 mg/kg) were utilized in in vivo experiments (Aziz 2015; Moushome et al. 2016).
Acetic acid–induced writhing test has been considered as a tool for the evaluation of central as well as peripheral antinociceptive activity (Mannan et al. 2017). Moreover, liberation of endogenous mediators of pain including prostaglandins, kinins, etc. is involved in the establishment of writhing response as a model visceral pain. Significant dose dependent antinociceptive effect was demonstrated by EAMA in acetic acid-induced writhing test after oral administration of EAMA (Table 2). An increment in cyclooxygenase (COX), prostaglandins (PGs), serotonin, lipoxygenase (LOX), substance P, histamine, bradykinin, IL-1β, IL-8, TNF-α is provoked in the peripheral tissue fluid due to administration of acetic acid through intraperitoneal route. Then entrance of the primary afferent nociceptors into dorsal horn of the central nervous system is caused due to their incitement through elevated level of the aforementioned mediators. Enlargement of the blood brain barrier (BBB) permeabilization or disruption might be occurred due to the release of these inflammatory mediators. Again, intraperitoneal injection of acetic acid (pain inducing agent) is also involved in the elevation of vasodilation as well as vascular fluid permeability (Imam and Moniruzzaman 2014). All of these events were blocked by plant extract (EAMA). Therefore, it was obvious in our study that, EAMA revealed not only peripheral antinociceptive effect but also central effect through the inhibition of writhing response. These antinociceptive effects may be either through inhibition of release of endogenous nociceptive mediators or inhibition of permeability at both BBB and vascular level (Imam and Moniruzzaman 2014).
The formalin induced paw licking test is a suitable method to explore discrimination between the central and peripheral antinociceptive action. This method consists of two phases of nociceptive response namely first phase and second phase. In case of initial phase, nociceptive neurons are prompted directly those activate C-fiber and produces neurogenic pain. Again, inflammation of the local tissue as well as functional changes in the dorsal horn of the spinal cord generates inflammatory pain in second phase. It is noticeable that, injection of formalin instantly (0–5 min after injection) causes initial phase whereas post-injection of formalin (20–30 min after injection of formalin) elicits the second phase. Moreover, neurogenic pain of the phase is revealed through the participation of substance P and bradykinin. Inflammatory pain in second phase is generated by the mediators such as histamine, prostaglandin, serotonin and bradykinin (Hossain et al. 2015). Both of the phases including first phase and second phase are arrested by the central analgesics such as opioids while only the second phase is inhibited by the peripheral analgesics including acetylsalicylic acid. So, antinociceptive effects of both doses of EAMA (EAMA 200 mg/kg and 400 mg/kg) in both phases indicates the central along with the peripheral effects which might be due to the inhibition of different pain mediators including substance P, histamine, prostaglandin, serotonin and bradykinin (Table 3). However, further studies are necessary to identify the exact antinociceptive mechanism of plant extract (Moushome et al. 2016).
Tail immersion model is well known as one of the significant acute pain models. Generally, centrally acting analgesics (opioids) are distinctively involved with the tail-withdrawal response and peripherally acting drugs are insensitive at heat-induced pain response. Tail immersion is related to the spinal reflex which is exerted through μ2- and δ-opioid receptors. Moreover, centrally acting analgesics (opioids) elicits antinociceptive activity not only in early phase but also in late phase. Table 4 shows that, both doses of EAMA (200 mg/kg and 400 mg/kg body weight) demonstrated significant latency (*P < 0.05, vs. control) at 60 min and 30 min, 60 min and 120 min respectively which might be due to the involvement of μ-opioid receptors (Imam and Moniruzzaman 2014; Moushome et al. 2016).
The hot-plate test is conducted for the evaluation of mechanism of centrally acting analgesics. In this test μ1 and μ2 opioid receptors are responsible for supraspinal reflex (Imam and Moniruzzaman 2014). Latency response of the mice against thermal stimuli is measured through hot plate test. Tramadol showed gradual increase in response latency from 2nd observation to 4th observation period whereas gradual increase in response latency was elicited by EAMA 400 mg/kg except 4th observation (Table 5). This plant extract might produce central antinociceptive effect by releasing endogenous peptides including endorphin or enkephalin through the stimulation of periaqueductal gray matter (PAG). These endogenous peptides descend the spinal cord and inhibits pain impulse transmission at the synapse in the dorsal horn (Imam and Sumi 2014).
Phytochemical screening of the EAMA demonstrated the presence of alkaloids, flavonoids, phenolic compounds, tannins, glycosides and gums which might be responsible for the elicitation of antinociceptive activities in these animal models. Flavonoids may exhibit analgesic activity by subduing the rising level of intracellular Ca2+ ion along with liberation of pro-inflammatory mediators including TNF-α. Again, the interference of flavonoids in the central mechanism of analgesics may be occurred through the elevated amount of endogenous serotonin by flavonoids as well as binding of it with 5-HT2 and 5-HT-3 receptors. It has been reported that flavonoids also exert analgesic activity due to its antioxidant properties characterized by targeting prostaglandins. The production of the terminal products of cyclooxygenase as well as lipoxygenase pathways named ecosanoids including prostaglandins is inhibited by flavonoids. Besides, these prostaglandins are responsible for several immunological responses. Tannins are also involved in antinociceptive activity. So, it can be concluded that, antioxidant activity together with cyclooxygenase as well as lipoxygenase inhibitory pathways may be responsible for the reduced production of prostaglandins along with its precursor arachidonic acids and finally alleviate the pain sensation (Imam and Moniruzzaman 2014).
Castor oil induced diarrheal model is a widely used method for the assessment of antidiarrheal activity that is involved in production of secretory as well as motility diarrhea (Yacob et al. 2016). Ricinoleic acid is the active metabolite of castor oil that is released through the action of lipases in the upper part of the small intestine and subsequently causes diarrhea (Degu et al. 2016). After binding with EP3 prostanoid receptors on smooth muscle cells it exerts its action (Tadesse et al. 2017). Ricinoleic acid plays important role for the formation of ricinoleate salts with Na+ as well as K+ in the lumen of the intestine. This ricinoleate is responsible for the exhibition of anti-absorptive effect on the mucosa. Moreover, the activity of the enzyme Na+–K+ ATPase is blocked by ricinoleate and it also accelerate the permeability of the intestinal epithelium. So, extract may exhibit antidiarrheal activity against castor oil induced diarrheal by antielectrolyte permeability action. Again, Ricinoleic acid is responsible for local irritation along with inflammation of the intestinal mucosa which initiates to the liberation of prostaglandins (PGs) (Yacob et al. 2016). These prostaglandins are involved in amplifying vasodilatation, contraction of the smooth muscle along with mucus secretion and increment of secretion of water and electrolytes into the small intestine. The Prostaglandins (E series) are well known as potent diarrheogenic agents not only in experimental animals but also in human beings. Therefore, delay in castor oil induced diarrhea is caused by the inhibitors of prostaglandins biosynthesis (Umer et al. 2013). Flavonoids and phytosterols are the two important secondary metabolites which are responsible for the modified production of cyclooxygenase 1 and 2 (COX-1, COX-2) and lipooxygenase (LOX) and eventually blockage of prostaglandins synthesis. Protein tannates generated through the precipitation of proteins in intestinal mucosa by tannins are related to the exhibition of resistance of the intestinal mucosa towards chemical alteration and sequentially decrease the peristaltic movements as well as intestinal secretion (Tadesse et al. 2017). From Table 6, it is clear that, EAMA revealed significant and dose dependent antidiarrheal activity in mice due to the presence of bioactive compounds named flavonoids and tannins. Most of the antidiarrheal agents exert action through reduction of secretion as well as dirigible movement of GI smooth muscles. So, EAMA was also subjected for MgSO4 induced diarrheal test to explore the exact mechanism of antidiarrheal agents.
Magnesium sulphate induced diarrheal model is another useful method for the evaluation of antidiarrheal activity. In this case, oral administration of magnesium sulphate causes accumulation of fluid in the intestinal lumen along with its movement (from proximal to distal intestine). Again, it also liberates nitric acid as well as cholecystokinin from duodenal mucosa which initiates two concurrent events. Firstly, increment in the secretion and motility of small intestine and secondly, inhibition of water and NaCl reabsorption (Moushome et al. 2016). Moreover, the crude extract EAMA showed significant antidiarrheal activity in the dose dependent manner because of the enhancement in water and NaCl reabsorption (Table 7).
The evaluation of antibacterial activity against pathogenic bacteria is performed by the flexible, economical as well as simple method called Disk diffusion (Saha et al. 2013). The crude plant extract exerts antibacterial properties extensively due to various groups of active secondary metabolite including saponins, tannins, phenols and alkaloids present in the extract. The antimicrobial properties may be broadly obtained either from individual or combined modes of action of compounds of the secondary metabolites. Microbial growth is hindered by the alkaloids because of its sticking capacity with the components of DNA molecules and successively hindrance of DNA synthesis characterized by topoisomerase inhibition. In addition to, complexation of proteins with tannins occurs through different non-specific forces including hydrogen, hydrophobic and covalent bindings. Thus, the complexing capacity of tannins with enzymes as well as other membranous proteins present in the microorganisms leads to the inhibition of growth of microorganisms. Again, phenolic compounds exhibit bacteriostatic action either through iron deprivation or hydrogen binding with vital proteins (microbial enzymes). These bioactive compounds may elicit their antimicrobial activities after entering into the inner portion of the microorganisms through specific surface receptors (Njateng et al. 2017). The Table 8 showed that, EAMA (250 µg/disc and 500 µg/disc) elicited antibacterial activity against gram positive and gram negative bacteria, which may be due to the presence of alkaloids, phenolic compounds and tannins in EAMA. Moreover, both doses of EAMA revealed maximum zone of inhibition against gram positive bacteria Staphylococcus pyogens. It may be due to several reasons like degree of resistance of gram positive bacteria to antibiotics is less than that of gram negative bacteria (Paz et al. 1995; Chowdhury and Islam 2004). Besides, cell wall of gram negative bacteria is composed of multilayer structure and gram positive bacteria contain single layer in their cell wall (Khodaie et al. 2012). Again, cell wall of gram negative bacteria contains thick murine layer that retards the inhibitor’s entrance (Evans et al. 1986). Kanamycin (30 µg/disc) showed potent antibacterial activity against Pseudomonus spp. (gram negative) because of its broad spectrum of activity. Antibacterial activity of EAMA was shown against both gram positive and gram negative bacteria but disk diffusion assay is associated with some drawbacks. Polar compounds can easily diffuse in the culture medium whereas it is difficult for the less polar compounds due to the involvement of water in agar media and thus diffuse slowly as well as poorly. Therefore, exact estimation of antibacterial activity exerted by the non polar compounds is quite impossible in disk diffusion assay (Saha et al. 2013). So it is necessary to carry out other antibacterial tests up on EAMA.
The previous studies reported that, crude stems extracts of A. mexicana including n-hexane, chloroform, ethyl acetate and ethanol showed antibacterial activity against different bacterial strains (gram positive and gram negative respectively) at a concentration of 10 μL and the zones of inhibition were in the range of 10.1–21.4 mm along with MIC value of 62.5–500 μg/mL (Brahmachari et al. 2013). Again, dry extract of the roots of A. mexicana showed antibacterial activity against gram positive bacteria namely Bacillus subtilis, Staphylococcus aureus and Staphylococcus epidermidis and the zones of inhibition were in the range of 10.3 nm to 12.7 nm but not against Gram negative bacteria including Escherichia coli and Salmonella typhimurium (Mas et al. 2018). Moreover, different parts of A. mexicana exhibited antibacterial activity against a number of food borne gram positive as well as gram negative bacteria (Rekha and Vidyasagar 2014). So, EAMA showed different antibacterial activities as compared to previous reports because majority of the bacterial strains were different and the extract was fractionated into different solvent systems.
Paralysis or loss of movement along with death or complete destruction of live parasites was considered as an indication for the evaluation of in vitro anthelmintic activity of the EAMA (Goto et al. 1990). Several phytochemical groups are mainly responsible for the generation of potential anthelmintic activity such as tannins, alkaloids, phenols etc. (Aziz et al. 2014). From Fig. 1, it is noticeable that EAMA (25 mg/ml and 50 mg/ml) exhibited significant and dose dependent (*P < 0.05, vs. control) anthelmintic activity which may be due to presence of phytochemical groups such as tannins, alkaloids, phenols in EAMA. Tannins and phenolic compounds exert anthelmintic activity because of their ability to interfere with energy generation in helminthes through uncoupling oxidative phosphorylation or binding ability to the glycoprotein on the cuticle of parasite and cause death (Lakshmi et al. 2012). It was also reported that, alkaloids cause paralysis of the worms due to their action in the central nervous system (Roy et al. 2010). Albendazole demonstrated anthelmintic activity through flaccid paralysis of the worms characterized by increased chloride ion conductance leading to hyper polarization and reduced excitability. In addition to, it acts as an anthelmintic by reducing the production of ATP (adenosine triphosphate) through degenerative changes in endoplasmic reticulum and mitochondria required for the survival of the worms (Lakshmi et al. 2012; Martin 1985).
In case of anthelmintic activity, previously studied articles report that, anthelmintic activity against Indian earthworm Pheritima posthuma was shown by aqueous plant extracts of A. mexicana. Again, alcohol and aqueous extracts of leaves of A. mexicana exhibited dose dependent anthelmintic activity at the concentrations of 6.25, 12.5, 25, 50, 100 mg/mL against P. posthuma and Ascardia galli (Brahmachari et al. 2013). A. mexicana extracts also showed anthelmintic activity against Indian earthworm, Pheritima posthuma as well as Ascardia galli (Rekha and Vidyasagar 2014). So, EAMA also showed dose dependent anthelmintic activity in comparison to previous reports although the worms and concentrations were different.
Brine shrimp lethality bioassay is broadly used for the evaluation of cytotoxic action of crude extracts upon the brine shrimp. It is used as an indication of toxicity of the plant extracts on the test materials. This method has been used for the identification and isolation of plant derived antitumor and pesticidal bioactive products (Sahgal et al. 2010). Figure 2 indicated that, different concentrations of EAMA showed varying degree of percent rate of mortality. However, cytotoxic activity of EAMA may be due to some bioactive compounds like phenolic compounds, flavonoids, tannins etc. which are regarded as normal cell differentiation promoter, free radical scavenger, hydrogen donor, detoxification inducer, antioxidant enzymes activator, apoptosis inducer, tumor production and proliferation cell inhibitor (Firdaus et al. 2013; Kumar et al. 2011).
The previous studies reported that, cytotoxic activity was shown by methanol extract of A. mexicana leaves against healthy mouse fibroblasts (NIH3T3) and three human cancer-cell lines (AGS, HT-29 and MDA-MB-435S) using the MTT [3-(4,5dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide] assay (Brahmachari et al. 2013). Moreover, A. mexicana extracts also exhibited cytotoxic activity against healthy mouse fibroblasts and three human cancer-cell lines, Human nasopharyngeal carcinoma and human gastric cancer cell-lines (Rekha and Vidyasagar 2014). So, EAMA showed different cytotoxic activity due to the different methods and models.
Conclusion
The results of the current study demonstrate that ethanolic extract of A. mexicana Linn. aerial parts might possess analgesic, antidiarrheal, antibacterial, anthelmintic and cytotoxic activities. Yet, isolation of pure bioactive compounds and elucidation of their structures along with specific mechanism of action are required through further investigational studies to understand the exact causes of the aforementioned bioactivities. Moreover, biological testing for the specific compound is being investigated extremely expected to be involved in the exhibition of these bioactivities.
References
Alabri THA, Musalami AHSA, Hossain MA, Al-Riyami AMWQ (2014) Comparative study of phytochemical screening, antioxidant and antimicrobial capacities of fresh and dry leaves crude plant extracts of Datura metel L. J King Saud Univ Sci 26:237–243
Anarthe S, Chaudhari S (2011) Neuropharmacological study of Argemone mexicana Linn. J Appl Pharm Sci 1(4):121–126
Apu AS, Baizyd AHA, Ara F, Bhuyan SH, Matin M, Hossain MF (2012) Phytochemical analysis and bioactivities of Argemone mexicana Linn. leaves. PhOL 3:16–23
Araujo MGS, Silva ALL, Silva-Junior EF, Santos Junior PFS, Santos MS, Bernardo THL, Bastos MLA, Alexandre-Moreira MS, Araujo-Junior JX, Verissimo RCSS (2015) Evaluation of antimicrobial and cytotoxic potential of Argemone mexicana L. J Chem Pharm Res 7(12):482–489
Aziz MA (2015) Qualitative phytochemical screening and evaluation of anti-inflammatory, analgesic and antipyretic activities of Microcos peniculata barks and fruits. J Integr Med 13(3):173–184
Aziz A, Raju GS, Das A, Ahmed J, Moghal MMR (2014) Evaluation of in vitro anthelmintic activity, total phenolic content and cytotoxic activity of Crinum latifolium L. (Family: Amaryllidaceae). Adv Pharm Bull 4(1):15–19
Balamurugan G, Selvarajan S (2009) Preliminary phytochemical screening and anthelmintic activity of Indigofera tinctoria Linn. Int J Drug Dev Res 1(1):157–160
Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol 45:493–496
Bhalke RD, Mandole YP, Mali NB (2009) Phytochemical investigation and effect of various extracts of Argemone mexicana (Papaveraceae) leaves on clonidine and haloperidol-induced catalepsy in mice. J Pharm Res 2(4):765–767
Brahmachari G, Gorai D, Roy R (2013) Argemone mexicana: chemical and pharmacological aspects#. Rev Bras Farmacogn Braz J Pharmacogn 23(3):559–575
Bukhari IA, Gilani AH, Meo SA, Saeed A (2016) Analgesic, anti-inflammatory and antiplatelet activities of Buddleja crispa. BMC Complement Altern Med. https://doi.org/10.1186/s12906-016-1021-4
Carneiro LU, Gomes da Silva I, Alves de Souza ME, Wellington Côrtes, da Silva Geraldo, de Carvalho M, Marinho BG (2017) Antinociceptive and anti-inflammatory activities of leaf extracts from Annona tomentosa R.E.Fr. J Integr Med 15(5):379–387. https://doi.org/10.1016/S2095-4964(17)60349-2
Chowdhury AA, Islam MS (2004) Antibacterial activity of Trema orientalis. Dhaka Univ J Pharam Sci 3(1–2):115–117
Dash GK, Murthy PN (2011) Evaluation of Argemone mexicana Linn. Leaves for wound healing activity. J Nat Prod Plant Resour 1(1):46–56
Degu A, Engidawork E, Shibeshi W (2016) Evaluation of the anti-diarrheal activity of the leaf extract of Croton macrostachyus Hocsht. ex Del. (Euphorbiaceae) in mice model. BMC Complement Altern Med 16:379. https://doi.org/10.1186/s12906-016-1357-9
Duhan JS, Bhardwaj M, Surekha S (2011) Free radical-scavenging and antimutagenic potential of acetone, chloroform and methanol extracts of leaf of Argemone mexicana. IJPBS 2(1):455–464
Evans JS, Pattison E, Moris P (1986) Antimicrobial agents from plant cell culture. In: Moris PA, Scraggs A, Stafford A, Flower M (eds) Secondary metabolites in plant cell culture. Cambridge University, London
Firdaus M, Prihanto AA, Nurdiani R, Widodo N (2013) Antioxidant and cytotoxic activity of Acanthus ilicifolius flower. Asian Pac J Trop Biomed 3(1):17–21
Ganguly A, Al Mahmud Z, Saha SK, Rahman SMA (2016) Evaluation of antinociceptive and antidiarrhoeal properties of Manilkara zapota leaves in Swiss albino mice. Pharm. Biol. https://doi.org/10.3109/13880209.2015.1103757
Ginovyan M, Petrosyan M, Trchounian A (2017) Antimicrobial activity of some plant materials used in Armenian traditional medicine. BMC Complement Altern Med 17:50. https://doi.org/10.1186/s12906-017-1573-y
Goto C, Kasuya S, Koga K, Ohtomo H, Kagel N (1990) Lethal efficacy of extract from zingiber officinale (traditional Chinese medicine) or [6]-shogaol and [6]-gingerol in anisakis larvae in vitro. Parasitol Res 76:653–656
Hasan MM, Hossain A, Shamim A, Rahman MM (2017) Phytochemical and pharmacological evaluation of ethanolic extract of Lepisanthes rubiginosa L. leaves. BMC Complement Altern Med 17:496. https://doi.org/10.1186/s12906-017-2010-y
Hossain CF, Al-Amin M, Sayem ASM, Siragee IH, Tunan AM, Hassan F, Kabir MM, Sultana GNN (2015) Antinociceptive principle from Curcuma aeruginosa. BMC Complement Altern Med 15:191. https://doi.org/10.1186/s12906-015-0720-6
Imam MZ, Moniruzzaman M (2014) Antinociceptive effect of ethanol extract of leaves of Lannea coromandelica. J Ethnopharmacol 154:109–115. https://doi.org/10.1016/j.jep.2014.03.032
Imam MZ, Sumi CD (2014) Evaluation of antinociceptive activity of hydromethanol extract of Cyperus rotundus in mice. BMC Complement Altern Med 14:83
Islam MK, Mahmud I, Saha S, Sarker AB, Mondal H, Monjur-Al-Hossain ASM, Anisuzzman M (2013) Preliminary pharmacological evaluation of Alocasia indica Schott tuber. J Integr Med 11(5):343–351. https://doi.org/10.3736/jintegrmed2013045
Islam MK, Siraj MA, Sarker AB, Saha S, Mahmud I, Rahman MM (2015) In-vitro anthelmintic activity of three Bangladeshi plants against Paramphistomum cervi and Haemonchus contortus. J Complement Integr Med 10:10. https://doi.org/10.1515/jcim-2014-0059
Jain RA, Agarwal RC, Dubey D, Verma R, Jain R (2012) Evaluation of antibacterial and antioxidant activity of fruits extract of Argemone mexicana Linn. IJPI 2(1):45–51
Jaliwala YA, Panda PK, Chourasia N, Bhatt NK, Pandit A, Mohanty PK (2011) In vitro anthelmintic activity of aerial parts of Argemone mexicana Linn. J Pharm Res 4(9):3173–3174
Khatun A, Imam MZ, Rana MS (2015) Antinociceptive effect of methanol extract of leaves of Persicaria hydropiper in mice. BMC Complement Altern Med 15:63. https://doi.org/10.1186/s12906-015-0558-y
Khodaie L, Delazar A, Lotfipour F, Nazemiyeh H (2012) Antioxidant and Antimicrobial activity of Pedicularis sibthorpii Boiss. and Pedicularis wilhelmsiana Fisch ex. Adv Pharm Bull 2(1):89–92
Kumar S, Kumar V, Chandrashekhar MS (2011) Cytotoxic activity of isolated fractions from methanolic extract of Asystasia dalzelliana leaves by brine shrimp lethality bioassay. Int J Pharm Pharm Sci 3(3):133–134
Lakshmi VK, Triveni KB, Anitha S, Shashidhara S (2012) In Vitro anthelmintic activity of Rotula Aquatica Lour Bark. Pharma Sci Monit 3(4–1):2332–2339
Lateef M, Iqbal Z, Khan MN, Akhtar MS, Jabbar A (2003) Anthelmintic activity of Adhatoda vesica roots. Int J Agric Biol 5(1):86–90. http://www.ijab.org
Mannan MA, Khatun A, Khan MFH (2017) Antinociceptive effect of methanol extract of Dalbergia sissoo leaves in mice. BMC Complement Altern Med 17:72. https://doi.org/10.1186/s12906-017-1565-y
Martin RJ (1985) Gamma-Amino butyric acid and piperazine activated single channel current from Ascaris suum body muscle. Br J Pharmacol 84(2):445–461
Martinez AIA, Muniz ODM, Ortiz MAD, Velez MVS, Hernandez MV, Lopez MGA (2016) Anxiolytic-like effect of ethanolic extract of Argemone mexicana and its alkaloids in Wistar rats. Avicenna J Phytomed 6(4):476–488
Mas D, Martinez Y, Bullain M, Betancur C, Ruiz C (2018) Secondary metabolites and in vitro antimicrobial activity of roots of Cuban Argemone mexicana Linn. WJPMR 4(6):46–51
More NV, Kharat AS (2016) Antifungal and anticancer potential of Argemone mexicana L. Medicines 3(28):1–10. https://doi.org/10.3390/medicines3040028
Moushome RA, Akter MI, Aziz MA (2016) Phytochemical screening and antinociceptive and antidiarrheal activities of hydromethanol and petroleum benzene extract of Microcos paniculata barks. BioMed Res Int 2016, Article ID 3167085. https://doi.org/10.1155/2016/3167085
Njateng GSS, Du Z, Gatsing D, Mouokeu RS, Liu Y, Zang Hong-Xia GuJ, Luo X, Jules-Roger Kuiate (2017) Antibacterial and antioxidant properties of crude extract, fractions and compounds from the stem bark of Polyscias fulva Hiern (Araliaceae). BMC Complement Altern Med 17:99. https://doi.org/10.1186/s12906-017-1572-z
Patil A, Vadera K, Patil D, Phatak A, Juvekar A, Chandra N (2014) In vitro anticancer activity of Argemone mexicana L. seeds and Alstonia Scholaris (L.) R. Br. Bark on different Human cancer cell lines. WJPPS 3(11):706–722
Paz EA, Lacy RN, Bakhtiar M (1995) The betalactum antibiotics penicillin and Cephalosporin. Prespective Hodder Stongton, London, p 227
Prabhakaran D, Rajeshkanna A, Senthamilselvi MM (2016) Antimicrobial activity of Argemone mexicana linn (flowers). Indo Am J Pharm Sci 3(5):450–455
Rafshanjani MAS, Parvin S, Kader MA (2014) In vitro antibacterial activities and brine shrimp lethality bioassay of ethanolic extract from Moringa oleifera Lam. Leaves. Int Res J Pharm 5(11):856–860
Rekha S, Vidyasagar GM (2014) plant profile, phytochemistry and pharmacology of Argemone mexicana Linn. A review. Int J Pharm Pharm Sci 6(7):45–53
Rout SP, Kar DM, Mandal PK (2011) Hypoglycaemic activity of aerial parts of Argemone mexicana L. in experimental rat models. Int J Pharm Pharm Sci 3(5):533–540
Roy H, Chakraborty A, Bhanja S, Nayak BS, Mishra SR, Ellaiah P (2010) Preliminary phytochemical investigation and anthelmintic activity of Acanthospermum hispidum DC. J Pharm Sci Technol 2(5):217–221
Saha S, Hossain F, Anisuzzman M, Islam MK (2013) Pharmacological evaluation of Musa seminifera Lour. fruit. J Integr Med 11(4):253–261. https://doi.org/10.3736/jintegrmed2013025
Sahgal G, Ramanathan S, Sasidharan S, Mordi MN, Ismail S, Mansor SM (2010) Brine shrimp lethality and acute oral toxicity studies on Swietenia mahagoni (Linn.) Jacq. seed methanolic extract. Pharmacogn Res 2(4):215–220
Sarkar KK, Hossain ML, Hossin A, Mondal H, Monjur-Al-Hossain ASM, Ahmed MI, Sadhu SK (2013) Pharmacological studies on Glycosmis Pentaphylla (Corr.) whole plant. PhOL 1:230–236
Shoba FG, Thomas M (2001) Study of antidiarrheal activity of four medicinal plants in castor oil induced diarrhea. J Ethnopharmacol 76(1):73–76
Singh SK, Pandey VD, Singh A, Singh C (2009) Antibacterial activity of seed extracts of Argemone mexicana L. on some pathogenic bacterial strains. Afr J Biotechnol 8(24):7077–7081
Sourabie TS, Ouedraogo N, Sawadogo WR, Nikiema JB, Guissou IP, Nacoulma OG (2012) Biological evaluation of anti-inflammatory and analgesic activities of Argemone mexicana Linn. (Papaveraceae) aqueous leaf extract. IJPSR 3(9):451–458
Tadesse E, Engidawork E, Nedi T, Mengistu G (2017) Evaluation of the anti-diarrheal activity of the aqueous stem extract of Lantana camara Linn (Verbenaceae) in mice. BMC Complement Altern Med 17:190. https://doi.org/10.1186/s12906-017-1696-1
Umer S, Tekewe A, Kebede N (2013) Antidiarrhoeal and antimicrobial activity of Calpurnia aurea leaf extract. BMC Complement Altern Med 13:21. http://www.biomedcentral.com/1472-6882/13/21
Yacob T, Shibeshi W, Nedi T (2016) Antidiarrheal activity of 80% methanol extract of the aerial part of Ajuga remota Benth (Lamiaceae) in mice. BMC Complement Altern Med 16:303. https://doi.org/10.1186/s12906-016-1277-8
Acknowledgements
The authors are grateful to the Department of Pharmacy, Jessore University of Science and Technology for providing all of the laboratory facilities, instrumental support as well as chemicals and reagents required to carry out this research work. Again, we would like to thank to the Department of Pharmacy, Jahangirnagar University, Savar, Dhaka, Bangladesh and International Centre for Diarrheal Disease and Research, Bangladesh (ICDDR, B) for supplying experimental mice and bacterial strains respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
The design of the experiments involving mice were approved by the Ethical Review Committee of Faculty of Biological Science and Technology, Jessore University of Science and Technology, Jessore-7408 [Ref: ERC/FBS/JUST/2018-4(A)].
Conflict of interest
This manuscript described has not been published before; not under consideration for publication anywhere else; and has been approved by all co-authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliation.
Rights and permissions
About this article
Cite this article
Sarkar, K.K., Mitra, T., Acharyya, R.N. et al. Phytochemical screening and evaluation of the pharmacological activities of ethanolic extract of Argemone mexicana Linn. aerial parts. Orient Pharm Exp Med 19, 91–106 (2019). https://doi.org/10.1007/s13596-018-0357-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-018-0357-3