Abstract
Alternanthera maritima are used in Brazilian popular medicine for the treatment of inflammatory and infectious diseases. Species of Alternanthera have demonstrated biological activities in previous scientific studies. The aim of this study was to determine whether the ethanol extract of the aerial parts of A. maritima (EEAM) and the isolated compound 2″-O-α-l-rhamnopyranosyl-vitexin inhibit mechanical hyperalgesia and parameters of inflammation in mice. The oral administration of EEAM significantly inhibited carrageenan (Cg)-induced paw edema and reduced leukocyte migration into the pleural cavity. 2″-O-α-l-rhamnopyranosylvitexin significantly inhibited paw edema and reduced both leukocyte migration and the leakage of protein into the pleural cavity. Both EEAM and 2″-O-α-l-rhamnopyranosylvitexin significantly prevented the Cg-induced hyperalgesia. Local administration of 2″-O-α-l-rhamnopyranosylvitexin significantly prevented the Cg- and tumor necrosis factor (TNF)-induced hyperalgesia. In conclusion, this study demonstrated that EEAM is an anti-inflammatory and anti-hyperalgesic agent, and the results suggested that 2″-O-α-l-rhamnopyranosylvitexin is responsible for the effects of EEAM and the mechanism involves the TNF pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Alternanthera maritima belongs to the genus Alternanthera Forkssal consists of 80 species of which 30 are found in Brazil. Many species of Alternanthera are used in folk medicine for the treatment of infection, and scientific studies have demonstrated anti-nociceptive (analgesic), anti-viral, anti-inflammatory, immunomodulatory, and diuretic activities [1–4]. Indeed, products from A. maritima are used in Brazilian popular medicine for the treatment of inflammatory and infectious diseases [5].
Phytochemical studies have identified and led to the isolation of steroids, saponins, flavonoid, lipids, and vitamins [5, 6] from the aerial parts of A. maritima. Some of these substances have potential for the treatment of viral diseases, for modulation of immunomodulatory activity, and for protection against cancer, malaria, and diarrhea [4, 7]. Six flavonoids have been identified in A. maritima including 2″-O-α-l-rhamnopyranosylvitexin [2, 5, 6]. Because extracts of A. maritima have a chemical composition similar to that of Anagallis tenella, these flavonoids have been suggested to be responsible for the effects and pharmacological activities of both species, supporting their use in popular medicine [5, 8, 9].
Pain may be categorized to several different types, which are nociceptive pain, which usually involves inflammation and is a result of tissue damage, neuropathic pain, which is caused by nerve damage, and idiopathic pain, which has no identified cause. Distinguishing between these categories is important because each necessitates a different treatment [10]. For example, chronic pain that is classified as neuropathic pain is generally poorly controlled by non-steroidal anti-inflammatory drugs (NSAIDs) [11]. In contrast, NSAIDs and steroidal anti-inflammatory drugs are very effective in the treatment of inflammatory diseases and nociceptive pain. Pain and inflammation are complex phenomenae that are induced by multifactorial causes and that occur in several diseases. Therefore, the development of new drugs to alleviate the symptoms of pain is important for the treatment of immune and cardiovascular diseases.
Because the presence of flavonoids in the extracts of A. maritima could be associated with popular use of these as an anti-inflammatory agents and for the treatment of infections [3, 12], we investigated the anti-hyperalgesic and anti-inflammatory activities of ethanolic extract of A. maritima (EEAM) and 2″-O-α-l-rhamnopyranosylvitexin (Fig. 1) in mice.
MATERIALS AND METHODS
Plant Material
The aerial parts of A. maritima were collected at Restinga de Maricá, Rio de Janeiro, Brazil, in December 2010 and identified by Prof. Dr. Josafá Carlos de Siqueira, Pontifical Catholic University of Rio de Janeiro (PUC-RJ), Brazil. A voucher specimen (SPFR-4758) was deposited in the herbarium of the Faculty of Philosophy, Sciences and Letters of Ribeirão Preto, University of São Paulo (FFCLRP/USP).
Extraction and Phytochemical Analysis
The air-dried, powdered aerial parts (1.0 Kg) were exhaustively extracted (maceration at room temperature) with ethanol at a 1:2 (w/v) ratio of powder mass plant/solvent. The spent biomass was filtered, and the solvents were removed using a rotatory evaporator at reduced pressure and a temperature below 40 °C, yielding 75 g of crude ethanol extract (EEAM). The ethanol extract (EEAM, 25 g) was submitted to chromatographic fractioning according to [5], resulting in the isolation of 2″-O-α-l-rhamnopyranosylvitexin (250 mg). This compound was identified by analyses of the 1D and 2D NMR and electrospray ionization–mass spectrometry (ESI–MS) through comparison with previously reported data [5, 2]. Moreover, the chemical composition of EEAM was analyzed by (ESI–MS) using a MS system, a Quattro LC triple-stage quadrupole (Micromass, UK), operating in the positive (30 V) and negative (30 V) ion mode. The compounds were identified by comparison of their ESI–MS/MS fragmentation spectra with literature data [5]. In addition, the chemical composition of EEAM was determined by high performance liquid chromatography (HPLC-UV) and comparative analysis using standards (Fig. 2) previously isolated from A. maritima, and the components were identified by comparison of the 1H and 13C NMR spectral data with those of [5] and [2]. HPLC quantitative analysis was performed for 2″-O-α-l-rhamnopyranosylvitexin. Serial concentrations of a standard were prepared in methanol/water (1:1, v/v) at eight concentrations, 0.125–10.00 μg/mL. HPLC analyses were conducted using a RP-18 column (Lichrospher®, 5 μm, 225 × 4.6 mm, Merck KGaA, Darmstadt, Germany). The mobile phase consisted of a linear gradient of solvent A (acetonitrile) and solvent B (water/acetic acid, 99:1, v/v, pH 2.88) as follows: 15 % A (15 min), 15–20 % A (7 min), 20 % A (5 min), 20–40 % A (5 min), 40 % A (5 min), and 40–15 % A (3 min). The analyses were carried out in triplicate at a flow rate of 0.8 mL/min with the UV detector set at λ 330 nm and an injection volume of 20 μL. The calibration graphs showed a linear relationship between the concentration and peak area. The regression equation was found to be y = 0.0601x – 0.0055 (R = 0.9999), where y is the peak area and x is the concentration. Accurately weighed amounts of the EEAM were dissolved in methanol/water (1:1, v/v) and analyzed using the same chromatographic conditions as those for the isolated compound. Identification of the chromatographic peak was based on the retention times of the single compound and confirmed by co-injection with an authentic standard. Under our working conditions, the mean retention time for 2″-O-α-l-rhamnopyranosylvitexin was 13.50 min. The limit of quantification (LOQ) for 2″-O-α-l-rhamnopyranosylvitexin was 0.31 μg/mL. The relative standard deviations (%RSD) were 0.15 %, calculated from the mean of the three replications, whereas the standard deviations of the retention times were less than 1 %.
ESI−MS fingerprints (a) and HPLC-UV/DAD chromatogram (b) of EEAM. 1 Vitexin—ESI–MS: m/z = 431 [M—H]−. 2 2″-O-α-l-rhamnopyranosyl-vitexin—ESI–MS: m/z = 577 [M—H]−; 3 2″-O-β-d-glucopyranosyl-vitexin—ESI–MS: m/z = 593 [M—H]−. 4 Rutin- ESI–MS: m/z = 609 [M—H]−. 5 Ishoramnethin 3-O-α-l-rhamnosyl-(1 → 6)-β-d-galactopyranoside—ESI–MS: m/z = 623 [M—H]−. 6 Ishoramnethin 3-O-α-l-rhamnosyl-(1 → 6)-β-d-glucopyranoside—ESI–MS: m/z = 623 [M—H]−. 7 Acacetin 8-C-[α-l-rhamnopyranosyl-(1 → 2)-β-d-glucopyranoside]—ESI–MS: m/z = 591 [M—H]−. 8 Quercetin-3-O-methyl Esther—ESI–MS: m/z = 315 [M—H]−. 9 Quercetin—ESI-MS: m/z = 301 [M—H]−. 10 Kaempfero l—ESI-MS: m/z = 285 [M—H]−.
Animals
Adult male and female Swiss mice (20–30 g) were provided by the Federal University of Grande Dourados (UFGD) biotherium. The animals were kept in collective cages (5 animals/cage) at a controlled temperature (23 ± 1 °C) and light cycle (12 h light/dark) and allowed water and a commercial diet (10 g/animal/day) ad libitum. The 012/2013 protocol was approved by the Ethics Committee on Animal Use (CEUA/UFGD).
Reagents
λ-Carrageenan (Cg), Bradford reagent, TNF, l-DOPA, and dexamethasone, purchased from Sigma-Aldrich ® Co. LLC (St. Louis, MO, USA), as was phosphate-buffered saline (PBS).
Model of Carrageenan-Induced Paw Edema
Different groups of mice were subjected to increasing oral doses of the crude ethanol extract of A. maritima (EEAM, 30, 100, and 300 mg/Kg) or 2″-O-α-l-rhamnopyranosylvitexin (1, 10, and 20 mg/Kg) dissolved in 0.9 % saline solution. The control was given only 0.9 % saline solution, and the positive control group subcutaneously received 1 mg/Kg of dexamethasone. One hour after dosing, the animals were treated with 50 μL of a 0.9 % saline solution containing 300 μg of carrageenan (λ-Cg, Sigma-Aldrich ®) in their right hind paw. The same volume of saline solution was administered into the left hind paw. Edema was measured with a paw plethysmometer (PANLAB-Havard) and expressed as the differences in the values of the paws at 2 and 4 h after Cg application [13].
Model of Carrageenan-Induced Pleurisy
The EEAM (30, 100, and 300 mg/Kg) and 2″-O-α-l-rhamnopyranosylvitexin (1, 10, and 20 mg/Kg) were administered orally 1 h before Cg pleural injection. The positive control group received 1 mg/Kg of dexamethasone subcutaneously, 30 min before Cg pleural application. Pleurisy was induced by applying 0.25 mL of a suspension containing 300 μg of Cg (diluted in phosphate-buffered saline (PBS), pH = 7.4) to the pleural cavity of the mice. Four hours after the induction of pleurisy, the animals were euthanized, and the pleural inflammatory exudate was collected through pleural lavage with 1 mL of sterile saline. The exudate volume was measured, and an aliquot of 50 μL was diluted with Turk’s solution (1:20). The total leukocytes were counted in a Neubauer chamber, considering four external quadrants, using a light microscope [14]. To each ELISA microwell plate, 10 μL of pleural lavage and 300 μL of Bradford reagent were added [15]. Protein measurement was performed colorimetrically (TP-photometer Reader/Thermo Plate ®).
Carrageenan, TNF, or l-DOPA-Induced Hyperalgesia Model
One day prior to the experiment, basal measurement of all animals was performed. Mice were housed in containment boxes (W × D × H—230 × 200 × 180 mm—Insight ®) under a steel mesh with 1 cm diameter spacing for a period of 30 min. During this time, a digital analgesymeter von Frey (Insight ®—EFF 301—Digital analgesymeter) was used to determine the baseline for the mechanical stimulus in the right hind paw [16, 17].
For the carrageenan-induced hyperalgesia model, the mice were divided into groups (n = 5/group) that were orally treated (gavage) with a 0.9 % saline solution, EEAM (100, 300 and 500 mg/Kg) or 2″-O-α-l-rhamnopyranosylvitexin (1, 10, 20 and 50 mg/Kg), based on the effective doses in inflammation models. Another group received 2″-O-α-l-rhamnopyranosylvitexin (3 μg/paw, 20 μL) through an intraplantar route. After 1 h (for oral treatment) or 15 min (for local treatment), animals received a 300 μg Cg injection subcutaneously in the right hind paw, and each animal was housed in the same containment boxes under the same steel mesh. Mechanical hyperalgesia was measured 3 and 4 h after Cg injection using the digital analgesymeter previously described [18].
The TNF- or l-DOPA-induced hyperalgesia groups (n = 5) received a 0.9 % saline solution or 2″-O-α-l-rhamnopyranosyl-vitexin (3 μg/paw, 20 μL) through an intraplantar route. After 15 min, the animals from the control and treated groups received TNF (1 pg/paw) and l-DOPA (10 mg/paw in a volume of 20 μL) [19, 20] subcutaneously, and each animal was housed in the same containment boxes under the same steel mesh. Mechanical hyperalgesia was measured after 3 h.
Statistical Analyses
The data are presented as the means ± SEM. Differences between two groups were determined by the Student t test, and comparisons of more than two groups were evaluated by analyses of variance (one-way ANOVA) followed by the Newman–Keuls test (the symbols are included in the figures) or the Bonferroni test (the symbols are not included in the figures). The number of animals per group (n = 5) is indicated in the legends, and the total number of animals was 175. Differences were considered to be significant at P < 0.05.
RESULTS
Phytochemical Analysis
The ESI–MS fingerprints of EEAM in the negative mode showed characteristic distributions of the flavonoids, which were identified by comparison of their ESI–MS/MS fragmentation spectra with literature data [5]. These results were confirmed by HPLC-UV analysis using isolated standards, and the structures were unequivocally confirmed by the co-injection of authentic standards and identified from the retention times. Nine flavonoids were identified in EEAM: two aglycones, four C-glycosides, and three glycosides (Fig. 2). The flavone 2″-O-α-l-rhamnopyranosylvitexin (Fig. 1) was isolated from the EEAM, and an HPLC quantitative method was validated for this compound. The content of this compound in the EEAM was 12.2 %.
Effects of EEAM and 2″-O-α-l-rhamnopyranosylvitexin on Carrageenan-Induced Edema Model
Cg injection into the right hind paw of the animals induced edema, peaking between 2 and 4 h. Oral treatment with EEAM at 100 and 300 mg/Kg doses significantly inhibited edema formation at 2 and 4 h; however, the 30 mg/kg doses did not. The maximum inhibitions were 77 % with 300 mg/Kg and 79 % 4 h after Cg injection. The animals treated with dexamethasone, the positive control, showed a significant reduction at all time points (Fig. 3a, b).
Effect of oral administration of EEAM (a, b) and 2″-O-α-l-rhamnopyranosyl-vitexin (c, d) on carrageenan (Cg)-induced paw edema in mice. Animals received the EEAM (30, 100, or 300 mg/Kg, p.o.) or 2″-O-α-l-rhamnopyranosyl-vitexin (1, 10, and 20 mg/Kg, p.o.), dexamethasone (DEXA 1 mg/Kg, s.c.), or vehicle, and after 1 h, an intraplantar injection of Cg (300 μg/paw) was performed. Graphics represented the evaluation of paw edema after 2 (a, c) and 4 (b, d) hours after Cg injection. Each bar represents the mean ± SEM of five animals. Differences between groups were analyzed by analysis of variance (one-way ANOVA) followed by the Newman–Keuls test. And a, b, and c denote statistic values.
The oral administration of 2″-O-α-l-rhamnopyranosylvitexin significantly decreased paw edema in mice at all doses tested compared with the control group (Fig. 3c, d). Therefore, no differences were detected between the groups treated with this compound and those treated with dexamethasone. The maximum inhibitions were 76 % at a dose of 1 mg/Kg, 68 % at a dose of 10 mg/Kg, and 66 % at a dose of 20 mg/Kg.
Anti-inflammatory Activity of EEAM and 2″-O-α-l-rhamnopyranosylvitexin on Carrageenan-Induced Leukocyte Migration and Protein Leakage in the Pleural Cavity
In the pleurisy test, the total leukocyte count decreased in the groups treated with EEAM at doses of 100 and 300 mg/Kg (Fig. 4a) compared to the control group. The maximum inhibitions were 68 and 65 %, respectively. A significant decrease in protein leakage by pleural lavage was not observed in the group treated with 100 mg/Kg of EEAM compared to the control group (Fig. 4b). The anti-inflammatory steroidal group showed decreased leukocyte migration and protein leakage (Fig. 5b).
Effect of oral administration of EEAM (a, b) and 2″-O-α-l-rhamnopyranosyl-vitexin (c, d) at leukocyte migration (a, c) and protein leakage (b, d) on carrageenan (Cg)-induced pleurisy in mice. Mice were treated 1 h before with EEAM (100 and 300 mg/Kg, p.o.), 2″-O-α-l-rhamnopyranosyl-vitexin (1, 10, and 20 mg/Kg, p.o.), dexamethasone (DEXA 1 mg/Kg, s.c.), or saline solution (vehicle). Pleural cavity was washed with PBS/EDTA. Cells were counted and plasma leakage was analyzed. In a and c, it is the number of cells that migrated to pleural cavity, 4 h after Cg injection. In b and d, plasma leakage was measured by Bradford’s reaction. The bars express the mean ± SEM of five animals. Differences between groups were analyzed by analysis of variance (one-way ANOVA) followed by the Newman–Keuls test. And a and b denote statistic values.
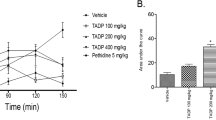
Effect of oral administration of EEAM on mechanical hyperalgesia induced by carrageenan (Cg) in mice. Animals received EEAM (100, 300, and 500 mg/Kg, p.o.) or vehicle, and after 1 h, 300 μg of Cg injection in the right hind paw. Mechanical hyperalgesia was measured at the times 3 and 4 h after Cg injection using the digital analgesymeter. The bars express the mean ± SEM of five animals. Differences between EEAM treated groups and vehicle (Veh—control) were analyzed by analysis of variance (one-way ANOVA) followed by the Newman–Keuls test, and a and b denoted statistic values. The comparison of EEAM treated groups with vehicle (Veh—control) vs basal group was analyzed by test t and the symbol octothorpe denoted the statistical differences.
For the evaluation of the effects of 2″-O-α-l-rhamnopyranosylvitexin on leukocyte migration in the pleural cavity, doses from 1 to 20 mg/Kg and the anti-inflammatory steroid showed significant effects, as both the total leukocyte count and protein extravasation decreased compared with the control group (Fig. 4c, d). The maximum inhibitions measured for leukocyte migration were 61 % (1 mg/kg), 77 % (10 mg/kg), and 59 % (20 mg/kg). For protein leakage, the maximum inhibitions were 40 % (1 mg/kg), 25 % (10 mg/kg), and 56 % (20 mg/kg).
Anti-hyperalgesic Activity of Orally Administered EEAM and 2″-O-α-l-rhamnopyranosylvitexin on Carrageenan-Induced Hyperalgesia
Statistical analysis (symbols not included in the figures) using the Student t test showed that Cg induced hyperalgesia, decreasing the response to mechanical stimulus compared to the basal group (P > 0.001) (Figs. 5 and 6). EEAM doses of 300 and 500 mg/Kg prevented mechanical hyperalgesia (Fig. 5).
Effect of oral (a) and intraplantar (b–d) administration of 2″-O-α-l-rhamnopyranosyl-vitexin on mechanical hyperalgesia induced by carrageenan (a, b), TNF (c), and l-DOPA (d) . Animals received oral (50 mg/kg, p.o.) or intraplantar (0.3, 3, or 300 μg/paw) injection of 2″-O-α-l-rhamnopyranosyl-vitexin, or vehicle, and after 1 h (oral treatment) or 15 min (local treatment), 300 μg of Cg injection or TNF or l-DOPA was injected in the paw. Mechanical hyperalgesia was measured at the times 3 and 4 h after Cg injection or only 3 after l-DOPA or TNF injection. The bars express the mean ± SEM of five animals. Differences between EEAM treated groups and vehicle (Veh—control) were analyzed by analysis of variance (one-way ANOVA) followed by the Newman–Keuls test, and a and b denoted statistic values. The comparison of with EEAM treated groups vehicle (Veh—control) vs Basal group were analyzed by test t and the symbol octothorpe denoted the statistical differences.
Oral and local administration of 2″-O-α-l-rhamnopyranosylvitexin significantly prevented reduction of the sensitivity threshold, as the measured thresholds at a dose of 50 mg/Kg were 76.6 and 90 % at 3 and 4 h after injection of Cg, respectively. In addition, other doses of 2″-O-α-l-rhamnopyranosylvitexin did not induce anti-hyperalgesic effects. Because local administration of 0.3, 3, and 300 μg/paw significantly prevented a reduction in the sensitivity, this compound demonstrates significant anti-hyperalgesic effects. Oral and local administration of 2″-O-α-l-rhamnopyranosylvitexin inhibited the hyperalgesic effects of Cg (Fig. 6a, b).
Anti-hyperalgesic Activity of Locally Administered 2″-O-α-l-rhamnopyranosyl-Vitexin on TNF and l-DOPA-Induced Hyperalsegia
Statistical analysis (symbols not included in the figures) with the Student t test showed that injection with TNF and l-DOPA induced hyperalgesia, decreasing the response to mechanical stimulus compared to the basal group (P > 0.001) (Fig. 6).
Local administration of 2″-O-α-l-rhamnopyranosylvitexin inhibited the hyperalgesic effects of TNF and significantly prevented the decrease in the threshold of sensitivity, showing a threshold of 87.5 % at a dose of 3 μg/paw 3 h after the TNF injection (Fig. 6c). However, local administration of 2″-O-α-l-rhamnopyranosylvitexin did not inhibit the hyperalgesic effects of l-DOPA (Fig. 6d), suggesting the involvement of the inflammatory pathway of TNF in the mechanism of action.
DISCUSSION
In the present study, nine flavonoids were found in the ethanol extract obtained from the aerial parts of the plant A. maritima (EEAM). Among them was 2″-O-α-l-rhamnopyranosylvitexin, which was isolated in approximately 10 % yield and had already been described by [6] and [5]. The flavonoid 2″-O-α-l-rhamnopyranosylvitexin was chosen for this study because, to our knowledge, no analgesic or anti-inflammatory studies with this compound have been conducted, although rare pharmacological activity has been previously attributed to 2″-O-α-l-rhamnopyranosylvitexin.
Flavonoids are widely distributed among plants and exhibit pharmacological effects on the inflammatory process [21]. Oral treatment with EEAM and 2″-O-α-l-rhamnopyranosyl-vitexin reduced the paw edema, leukocyte migration, and protein extravasation induced by Cg and inhibited Cg-induced hyperalgesia, thus demonstrating significant anti-inflammatory and anti-hyperalgesic effects. The present study is the first to show that extracts of A. maritima exhibit anti-inflammatory and anti-hyperalgesic properties and shows results similar to those of previous research studies on other species of Alternanthera sp.
The major compound, 2″-O-α-l-rhamnopyranosyl-vitexin, has also been shown to inhibit the inflammatory parameters at the three doses tested (1, 10, and 20 mg/Kg), while the doses necessary for EEAM to decrease the inflammatory process are 100 and 300 mg/kg. When the results of hyperalgesia were analyzed, the doses of EEAM needed to produce an anti-hyperalgesic effect were higher than those needed to produce anti-inflammatory effects, as significant effects on hyperalgesia were observed with the 300 and 500 mg/kg oral doses. The same phenomenon was observed with 2″-O-α-l-rhamnopyranosylvitexin, as significant effects were observed at a dose of 50 mg/kg. The local injection of 3 μg/paw of 2″-O-α-l-rhamnopyranosylvitexin prevented a Cg-induced decrease in the threshold of mechanical sensitivity, suggesting that the compound directly reduces the sensitization of the nociceptive nerve fibers.
The study of [8] found that the ethanol extract of A. tenella possessed antioxidant/free-radical scavenging properties which seemed to be correlated with its total phenolic content, particularly its 2″-O-α-l-rhamnopyranosylvitexin content. Vitexin, a flavonoid with a similar structure, inhibited Freund’s adjuvant-induced inflammatory pain by inhibiting trpV1, oxidative stress, and cytokines [22]. Our study agreed by showing that 2″-O-α-l-rhamnopyranosylvitexin, a compound with a structure to vitexin, also exhibits anti-hyperalgesic action.
Studies have utilized Cg to research the anti-inflammatory and anti-hyperalgesic properties of A. tenella [3, 9]. Cg-induced nociceptive sensitization to mechanical stimulus in rodents is dependent on mediators such as bradykinin, TNF, IL-1β, keratinocyte-derived chemokine (KC), prostaglandins, and sympathetic amines [19]. Some of these mediators, such as bradykinin, TNF and prostaglandins, are also involved in Cg-induced edema formation in mice and nociceptive sensitization [20]. Therefore, there are multiple sites where 2″-O-α-l-rhamnopyranosylvitexin could be acting to reduce inflammation and nociceptive sensitization. In this study, we demonstrated that 2″-O-α-l-rhamnopyranosylvitexin inhibits nociception induced by TNF but not nociception induced by l-DOPA.
TNF is produced by phagocytes and is responsible for immune and physiological responses as well as the induction of other inflammatory cytokines [23]. Although there is no clear evidence that neurons in the spinal cord synthesize and release TNF, this mediator has been proposed to be one of the substances for which there are receptors in the spinal cord [24]. Therefore, TNF may be involved in both edema formation and hyperalgesia, which would explain the effects of 2″-O-α-l-rhamnopyranosylvitexin observed in the animal models. The other pathway for Cg-induced hyperalgesia involves sympathetic amines, and l-DOPA injection activates this pathway. However, 2″-O-α-l-rhamnopyranosylvitexin did not interfere with activation of this pathway, demonstrating that it is not important for the anti-hyperalgesic effects of 2″-O-α-l-rhamnopyranosyl-vitexin. These results suggest that the main mechanism responsible for the anti-hyperalgesic actions of EEAM and 2″-O-α-l-rhamnopyranosyl-vitexin most likely involves the TNF pathway.
CONCLUSION
The present study showed that the ethanol extract from leaves of A. maritima (EEAM) has anti-inflammatory and anti-hyperalgesic effects in the edema, pleurisy, and hyperalgesia induced by carrageenan in mice. 2″-O-α-l-rhamnopyranosylvitexin, a flavonoid found in A. maritima, seems t responsible for the effects of the EEAM. When tested in doses based on the extract yield, both orally and locally, it was able to reduce the edema, leukocyte migration, protein leakage, and hyperalgesia that were induced by Cg. TNF seems to be involved in its mechanism of action; however, other potential mechanisms should be investigated.
References
Siqueira, J.C. 1987. Importância alimentícia e medicinal das Amaranthaceaes do Brasil. Acta Biologica Leopoldensia 9: 5–22.
Salvador, M.J., P.S. Pereira, S.C. França, R.C. Candido, I.Y. Ito, and D.A. Dias. 2004. Comparative study of antibacterial and antifugal activity of callus culture and adult plants extracts from Alternanthera maritima (Amaranthaceae). Brazilian Journal of Microbiology 35(1–2): 131–136.
Guerra, R.N.M., H.-A.W. Pereira, L.M.S. Silveira, and R.S.G. Olea. 2003. Immunomodulatory properties of Alternanthera tenella Colla aqueous extracts in mice. Brazilian Journal of Medical and Biological Research 36: 1215–1219.
Sekar, K.C. 2012. Invasive alien plants of Indian Himalayan region—diversity and implication. American Journal of Plant Sciences 3(2): 177–184.
Souza, J.G., R.R. Tomei, A. Kanashiro, L.M. Kabeya, A.E.C.S. Azzolini, D.A. Dias, M.J. Salvador, and Y.M. Lucisano-Valin. 2007. Ethanolic crude extract and flavonoids isolated from Alternanthera maritima: neutrophil chemiluminescence inhibition and free radical scavenging activity. Zeitschrift fur Naturforschung C: Journal of Biosciences 62(5–6): 339–347.
Salvador, M.J., and D.A. Dias. 2004. Flavone C-glycosides from Alternanthera maritima (Mart.) St. Hil. (Amaranthaceae). Biochemical Systematics and Ecology 32(1): 107–110.
Hundiwale, Jogendra C., V. Patil Avinash, V. Kulkarni Mohan, D.A. Patil, and G. Mali Ravindra. 2012. A current update on phytopharmacology of the genus Alternanthera. Journal of Pharmacy Research 5(4): 1924–1929.
Salvador, M.J., E.O. Ferreira, S.U. Mertens-Talcott, W.D. Castro, V. Butterweck, H. Derendorf, and D.A. Dias. 2006. Isolation and HPLC quantitative analysis of antioxidant flavonoids from Alternanthera tenella Colla. Zeitschrift fur Naturforschung C: Journal of Biosciences 61(1–2): 19–25.
Biella, C.A., M.J. Salvador, D.A. Dias, M. Dias-Baruffi, and L.S. Pereira-Crotti. 2008. Evaluation of immunomodulatory and anti-inflammatory effects and phytochemical screening of Alternanthera tenella Colla (Amaranthaceae) aqueous extracts. Memórias do Instituto Oswaldo Cruz 103(6): 569–577.
Thakur, M., A.H. Dickenson, and R. Baron. 2014. Osteoarthritis pain: nociceptive or neuropathic? Nature Reviews. Rheumatology 10(6): 374–380.
Treede, R.D., T.S. Jensen, J.N. Campbell, G. Cruccu, J.O. Dostrovsky, J.W. Griffin, P. Hansson, R. Hughes, T. Nurmikko, and J. Serra. 2008. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 70(18): 1630–1635.
Moraes, V.L., L.F. Santos, S.B. Castro, L.H. Loureiro, O.A. Lima, M.L. Sousa, L.M. Yien, B. Rossi-Bergmann, and S.S. Costa. 1994. Inhibition of lymphocyte activation by extracts and fractions of Kalanchoe, Alternanthera, Paullinia and Mikania species. Phytomedicine 1(3): 199–204.
Henriques, M.G., P.M. Silva, M.A. Martins, C.A. Flores, F.Q. Cunha, J. Assreuy-Filho, and R.S. Cordeiro. 1987. Mouse paw edema. A new model for inflammation? Brazilian Journal of Medical and Biological Research 20(2): 243–249.
Vinegar, R., J.F. Truax, and J.L. Selph. 1973. Some quantitative temporal characteristics of carrageenin-induced pleurisy in the rat. Proceedings of the Society for Experimental Biology and Medicine 143(3): 711–714.
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72(1–2): 248–254.
Chaplan, S.R., F.W. Bach, J.W. Pogrel, J.M. Chung, and T.L. Yaksh. 1994. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods 53(1): 55–63.
Mori, L.S., S. Boller, C.A.L. Kassuya, M.É.A. Stefanello, and A.R. Zampronio. 2011. Analgesic effects of the ethanolic extract from Magnolia ovata (Magnoliaceae) trunk bark and of N-acetylxylopine, a semi-synthetic analogue of xylopine. Phytomedicine 18(2–3): 143–147.
Piccinelli A.C., J.A. Santos, E.C. Konkiewitz, S.A. Oesterreich, A.S. Formagio, J. Croda, E.B. Ziff, C.A.L. Kassuya. 2014. Antihyperalgesic and antidepressive actions of (R)-(+)-limonene, α-phellandrene, and essential oil from Schinus terebinthifolius fruits in a neuropathic pain model. Nutritional Neuroscience http://dx.doi.org/10.1179/1476830514Y.0000000119.
Cunha, T.M., W.A. Verri Jr., J.S. Silva, S. Poole, F.Q. Cunha, and S.H. Ferreira. 2005. A cascade of cytokines mediates mechanical inflammatory hypernociceptionin mice. Proceedings of the National Academy of Sciences of the United States of America 102(5): 1755–1760.
Cunha, T.M., W.A. Verri Jr., I.R. Schivo, M.H. Napimoga, C.A. Parada, S. Poole, M.M. Teixeira, S.H. Ferreira, and F.Q. Cunha. 2008. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. Journal of Leukocyte Biology 83(4): 824–832.
Coutinho, M.A.S., M.F. Muzitano, and S. Costa. 2009. Flavonóides: potenciais agentes terapêuticos para o processo inflamatório. Revista Virtual de Química 1(3): 241–256.
Borghi, S.M., T.T. Carvalho, L. Staurengo-Ferrari, M.S. Hohmann, P. Pinge-Filho, R. Casagrande, and W.A. Verri Jr. 2013. Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. Journal of Natural Products 76(6): 1141–1149.
Ma, Y., Y. Li, X. Li, and Y. Wu. 2013. Anti-inflammatory effects of 4-methylcyclopentadecanone on edema models in mice. International Journal of Molecular Sciences 14: 23980–23992.
Gomes, R.P., E. Bressan, T.M.D. Silva, S.C. Domenech, and C.R. Tonussi. 2013. Evidence that a physical activity protocol can reduce synovial leukocyte count in arthritic rats. Revista Brasileira de Medicina do Esporte 19(1): 70–73.
ACKNOWLEDGMENTS
The authors are thankful do CAPES, CNPq, FUNDECT, and FAPESP for the financial support.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Santana Aquino, D.F., Piccinelli, A.C., Soares, F.L.P. et al. Anti-hyperalgesic and Anti-inflammatory Activity of Alternanthera Maritima Extract and 2″-O-α-l-rhamnopyranosylvitexin in Mice. Inflammation 38, 2057–2066 (2015). https://doi.org/10.1007/s10753-015-0187-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0187-0