Abstract
Plutonium associated with higher molecular weight molecules is presumed to be poorly mobile and hardly plant available. In our present study, we investigate the uptake and effects of Pu treatments on Solanum tuberosum plants in amended Hoagland medium at concentrations of [242Pu] = 100 and 500 nm, respectively. We found a direct proof of oxidative stress in the plants caused by these rather low concentrations. For the confirmation of oxidative stress, we explored the production of nitric oxide (NO) and hydrogen peroxide (H2O2) by epifluorescence microscopy. Oxidative stress markers like lipid peroxidation and superoxide radicals (O2 •−) are monitored through histochemical analysis. The biochemical parameters i.e. chlorophyll and carotenoids are measured as an indicator of cellular damage in the tested plants including the enzymatic parameters such as catalase and glutathione reductase. From our work, we conclude that Pu in low concentration has no significant effects on the uptake of many trace and macroelements. In contrast, the content of O2 •−, malondialdehyde (MDA), and H2O2 increases with increasing Pu concentration in the solution, while the opposite effects was found for NO, catalase, and glutathione reductase. These findings prove that even low concentration of Pu regulates ROS production and generate oxidative stress in S. tuberosum L.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plutonium (Pu) was released into the environment by a number of severe nuclear accidents (Salbu et al. 1994; Entwistle et al. 2003; Kashparov et al. 2009, 2012; Bisinger et al. 2010; Zheng et al. 2012), testing of nuclear weapons (Hamilton et al. 2009; Lachner et al. 2010), and operation of nuclear reprocessing plants (Kershaw et al. 1999). Pu migration strongly depends on speciation (Poinssot and Geckeis 2012). While colloidal transport was found to be rather effective (Kersting et al. 1999; Novikov et al. 2006; Zheng and Yamada 2006), tetravalent Pu strongly sorbs to mineral surfaces (Kirsch et al. 2011), causing high retention and low migration rates in soil. Interaction with organic substances is another important factor (Francis et al. 2006). Pu becomes plant available mainly after oxidation to higher oxidation states [e.g., following formation of PuO2+x as proposed by Haschke et al. (2000)]. Another important mobilization mechanism is the interaction with microbes (Francis and Dodge 2015).
Pu is considerably less mobile and volatile than other relevant radionuclides released by nuclear activities such as e.g., 90Sr (Bunzl et al. 1992; Steinhauser et al. 2015). Chelation by naturally occurring soil organic matter (hydroxamate siderophores, organic acids, and amino acids) was proposed as the most probable tool for increasing Pu mobility and subsequent uptake by plants (Bondietti et al. 1976; Francis 1973). Complexation by low molecular weight ligands (e.g., citrate and oxalate) makes Pu more mobile and readily available for plant uptake (Livens et al. 1987). Pu coordinated to higher molecular weight molecules in some cases was less mobile and non-available to plants (Livens et al. 1987). Pu contamination in plants can also occur due to aerial release and wind transport followed by deposition to plant shoots by fallout (dry deposition) or washout (wet deposition) (Federov et al. 1986; Little et al. 1980; Pinder et al. 1990), and very few information are available concerning Pu root uptake from soil (Pinder et al. 1990). Nishita and Hamilton (1980) reported that solubility and extractability of Pu(IV) was affected by carbonate concentration in the solution and only approximately 3% of the total Pu present in the soil was readily transferable. The amount of Pu found in the organic fraction of soil varies strongly (2–13%, depending on pH) while the major fraction (73–88%) was found in the reductant soluble fraction (Fe-Mn oxides) and the rest (approx. 19%) was incorporated into the mineral matrix (Muller 1978). Lipton and Goldin (1976) reported that Pu uptake increases by up to three orders of magnitude due to chelation in plants. It has been shown in plants that the assimilation rates are constant irrespective of the form of 238Pu contamination used (Brown and McFarlane 1978).
To our knowledge, there is no report on Pu’s effect on the plant’s oxidative defense system. Plant tolerance to heavy metal/metalloid mainly depends on effectiveness of uptake, translocation, and further restoration of these metals/metalloids in specified tissues or in trichomes in plant cells (Gupta et al. 2013a, c). When any metal is complexed and sequestered in cellular compartments, it becomes unavailable for translocation to other plant parts (Lasat et al. 1998). The cell wall is not only responsible for metal binding or metal immobilization to roots but is also a consequent reserve for ion translocation to shoots (Manara 2012). Vacuoles are commonly considered the main storage sites for metals in yeast and in plant cells. Furthermore, Yang et al. (2005) proved that phytochelatin-metal complexes are forced into the vacuoles in plants. Heavy metal ions can form complexes with ligands inside or outside of cells. Important ligands are chelators such as organic acids or low molecular binding peptides such as phytochelatins (PCs), methallothioneins (MTs), or glutathione (GSH) (Mello-Farias and Chaves 2008; Gupta et al. 2013a). In case of uranium reduction in the plant cell, GSH plays a central role in the defense mechanism by forming of complexes and later synthesizing phytochelatin (Viehweger et al. 2011).
Nitric oxide (NO) is a vital facilitator in an extensive choice of physiological and patho-physiological developments (Siddiqui et al. 2010; del Rio 2011). Numerous studies revealed that NO influences the extent of metal toxicity by controlling the plant reaction to heavy metals, and a robust association between reactive oxygen species (ROS) and NO has been established to control the cell response after exposure to different metals (Xiong et al. 2010; Sandalio et al. 2012).
In living cells, hydrogen peroxide (H2O2) is one of the key ROS molecules produced by numerous internal effects and reactions. H2O2 actively participates as a key signaling molecule in mediating hypersensitive reactions by triggering confined host cell death (Gupta K et al. 2016). In plants, photorespiration or C2 cycle is the major process that leads to the production of H2O2 comprising three different organelles—chloroplast, mitochondria, and peroxisome. Among the three organelles, mitochondrial and chloroplastidial electron transport chains and oxidation of fatty acids in the mitochondrial matrix play a vital role within the cells contributing to the H2O2 pool. To counteract oxidative stress, generally, plants produce metabolites and molecules like polyamines and H2O2 (Mittler et al. 2011).
When plants are exposed to ionizing radiation, molecular and cellular effects are induced either directly by energy transfers to macromolecules or indirectly via water radiolysis forming ROS (Gupta et al. 2016). Particularly, alpha particles and to some extent energetic electrons cause DNA strand breaks, lipid oxidation, or protein/enzyme denaturation by direct hits. However, water radiolysis and ROS production was also caused by macromolecules, indirectly inducing cellular damage in plant cells (Gupta and Walther 2014; Gupta et al. 2016). In plant cells, a wide range of enzymatic and non-enzymatic antioxidant schemes counteract ROS production right at the place of formation being a very effective mechanism to avoid the unwanted potentially negative effects of oxidative stress but conserving ROS’s signaling role (Corpas et al. 2015). In case of radioactive heavy metals, such as the actinides, chemical toxicity may cause similar or even stronger effects. Chemical production of ROS such as superoxide radicals (O2 •−), hydroxyl radicals (OH•-), and hydrogen peroxide molecule (H2O2) interferes strongly with metabolism and other biological activities of cells (Gupta et al. 2015).
To our knowledge, there is no information relating Pu-NO, and H2O2 localization in plants through microscopy yet, and neither are reports on histochemical staining of plants under Pu stress. This study mainly focuses on NO and H2O2 localization in potato (Solanum tuberosum) plants under different concentrations ([242Pu] = 100 and 500 nm) after 21 days of exposure. Besides, we also report the effect of Pu on macro/micronutrient uptake and accumulation (after 80 days of growth), histochemical parameters such as lipid peroxidation, and superoxide radical production as well as photosynthetic pigments alterations and antioxidative enzymes like catalase (CAT) and glutathione reductase (GR) activity in the tested plant after 21 d of Pu exposure. This study was a part of an integrated project focusing on disposal of high level radioactive waste (see, www.entria.de). 242Pu is very long lived and contributes more than 90% to the radiotoxicity of spent nuclear fuel after ca. 2000 years (up to almost 100,000 years). Depending on repository conditions and host rock, Pu might migrate out of the repository and enter the biosphere after these long years. Secondly, might be the release of Pu during handling or transport of Pu and direct release into the environment. In both cases, 239Pu and 240Pu will be of higher relevance than 242Pu. However, in the present study, we used the longer lived 242Pu to minimize the dose during handling in the laboratory.
Material and methods
Plant material, growth condition, and treatment
S. tuberosum L. germplasm were bought from Heilmann AG, Buchen, Germany. Initially, germplasm were kept in the dark and dry for 1 week for the initiation of sunken buds and later, these buds were transferred into the compost for 10 days for proper growth. After 10 days, healthy and equal size plantlets were transferred to hydroponics in plastic boxes containing 4 L of amended Hoagland medium at pH 6.2 (Gupta et al. 2013b). The plants were grown in a growth chamber at (22 ± 1) °C during 16/8 light/dark cycle with 120 μE m−2 s−1 of irradiance by cold fluorescent lamps for 2 weeks. For the treatment of plantlets, we dissolved appropriate amounts of 242Pu from our stock solution of 162 μmol L−1 (Eckert & Ziegler, Isotope Products, Valencia, CA, USA) in amended Hoagland solution (pH 5.5) to achieve concentrations of [Pu] = 100 and 500 nm respectively, (for 100 nm, we have given the treatment with 9.7E−05 g and for 500 nm, we used 4.8E−04 g Pu to fed the plants). After plant contact, the actual Pu concentration in solution decreases to a measured equilibrium value of 12 ± 2 nm for 100 nm and 20 ± 3 nm for 500 nm. And later when we calculated the total Pu concentration in plants, i.e., in 100-nm treatment (root = 10.2, shoot = 0.036, and in tubers = 0.0029 μg DW, respectively) and in 500-nm treatment (root = 95.2, shoot = 0.041, and in tubers = 0.025 μg DW, respectively). In our present study, we used 242Pu because of the long half-life period to minimize the dose. Oxidation state-pure Pu was prepared and stock solutions were measured by spectroscopy. Since the low concentrations can no longer be measured by UV-VIS, we did thermochemical modeling to make sure Pu was present in the oxidation state we wanted (either IV or in some cases V). Some samples were measured by capillary electrophoresis coupled to ICP-MS to consolidate the model data. We assume uptake of pure Pu(IV) does not play a role due to its very low solubility. Pu(V) which is a least partly present at the chosen oxidizing conditions should be much more plant available. In an amended medium, low phosphate concentrations were used to minimize the formation or precipitation of Pu phosphate phases. Plants without any treatment were used as control. The total life of potato plant is nearly 90–120 days. By other experimental analyses (previously on plant growth) on plants, we had an experience to know that after seedling growth, 21 days was best to do biochemical, histochemical, and microscopic studies that’s why we choose 21 days for these parameters. For each experiment, randomly first to fifth leafs of plantlets were selected for analysis. For metal analysis in plant tissue and in tubers, we choose 80 days because this was the last stage of plant having ripened potato and the metal which is accumulated in plant tissue was going to be remained there. All chemicals used were of analytical grade purchased from Sigma Chemical Company (USA).
Plant harvest
At harvest, the plants were divided into roots, shoots (including leaves), and tubers. The roots were rinsed twice for 15 min with MilliQ water to remove excess metal adhering to the root surface. Subsequently, biochemical parameters were determined. To obtain dry material for metal estimation, the roots and shoots and tubers were dried at 65 °C for constant weight.
Element determination
Plutonium concentration was measured in roots, tubers as well as in shoots (including leaves). Fifty to 100 mg of dry plant tissue was ground and digested with 5 ml of concentrated HNO3 using an open digestion system with heating block Velp Scientific (Milano, Italy). Heating was set at 130 °C for 2 h. Plastic caps were fit to the vessels to prevent loss by volatilization. After digestion, the samples were diluted five times with double distilled water resulting in a 50-ml volume. The Pu concentration in plants was measured by alpha spectrometry (Canberra 7200) by electrodeposition method. The Ca, Fe, K, Mg, P, S, Zn, and Cu concentration was determined by inductively coupled plasma optical emission spectrometry (ICP-OES), using an iCAP 6000 (Thermo Fisher, Germany) equipped with a cyclonic spray chamber and a concentric nebulizer.
NO in root and leaves detection through epifluorescence microscopy
NO was detected (Chaki et al. 2011) using the fluorescent reagent 10 μM DAF-2 DA (4,5-diaminofluorescein diacetate) prepared in 10 mM Tris-HCl (pH 7.4) and kept at 25 °C in the dark (Corpas et al. 2008). These probes are highly specific for NO. After 1 h, the samples were washed twice with the same buffer for 15 min each and mounted on a microscopic slide for examination with an epifluorescence microscope (Nikon GmbH) using standard filters and collection modalities for DAF-2 green fluorescence (excitation 495 nm; emission 515 nm).
H2O2 in root and leaves detection through epifluorescence microscopy
H2O2 was detected (Rodríguez-Serrano et al. 2006) using the fluorescent reagent 25 μM DCF- DA (2′,7′-dichlorofluorescin diacetate) prepared in 10 mM Tris-HCl (pH 7.4) and kept at 37 °C in the dark. These probes are highly specific for H2O2. After 1 h, samples were washed twice with the same buffer for 15 min each and mounted on a microscopic slide for examination with an epifluorescence microscope (Nikon GmbH) using standard filters and collection modalities for DCF-DA green fluorescence (excitation 485 nm; emission 530 nm).
Histochemical assays for superoxide radical and lipid peroxidation in leaves
Randomly selected five leaves (first to fifth expanded leaves) of the plant were employed for histochemical assays. The most representative images were shown.
Superoxide radical
In situ detection of superoxide radical (O2 •−) was done by Jabs et al. (1996). Selected detached leaves were vacuum infiltrated with 10 mM potassium phosphate buffer at pH 7.8, 10 mM NaN3, 0.1% (w/v) nitro blue tetrazolium (NBT), and 0.05% (v/v) Tween 20. The leaves were incubated overnight in the dark, and infiltrated and NBT-treated leaves were then maintained for 30 min under daylight condition prior to discoloration of leaves using boiling ethanol at 100 °C until the color disappeared.
Lipid peroxidation
Histochemical detection of lipid peroxidation was done with Schiff’s reagent, which detects aldehydes that can be generated from lipid peroxides (Leterrier et al. 2012). The leaves were incubated in Schiff’s reagent for 60 min and then were bleached by immersion in boiling ethanol at 100 °C until the color disappeared.
Determination of total chlorophyll and carotenoid concentration
Plant material (leaves, 100 mg) was grounded in 1 mL of chilled 80% acetone in the dark. After centrifugation at 10,000g for 10 min at 4 °C, absorbance of the supernatant was taken at 663, 645, 510, and 480 nm. The total chlorophyll concentration was determined by the method of Arnon (1949) and total carotenoid concentration by using the formula given by Duxbury and Yentsch (1956).
Enzymatic analysis
Frozen leaf tissues (300 mg) were ground in a mortar with liquid nitrogen in 600 μL of 50 mM Tris-HCl buffer (pH 7.5) containing 0.1 mM EDTA, 0.2% (v/v) Triton X-100, 2 mM DTT, and protease inhibitor cocktail (40 μg mL−1, Sigma) (Gupta et al. 2013b). The homogenate was centrifuged at 14,000g for 30 min and the supernatants were collected and used for enzyme assays. Catalase (CAT) activity was measured spectrophotometrically according to Aebi (1984) by monitoring the disappearance of H2O2 at 240 nm and 25 °C for 2 min (ε = 39.58 M−1 cm−1). The reaction mixture (1 mL) contained 50 mM potassium phosphate buffer (pH 7.0) and 10.6 mM H2O2. The reaction was started by adding 10 μL of the crude leaf extract. Glutathione reductase (GR) activity was measured following Edwards et al. (1990) by monitoring NADPH oxidation at 340 nm for 2 min at 25 °C (ε = 6.22 mM−1 cm−1). The reaction mixture (1 mL) consisted of 100 mM HEPES-NaOH (pH 7.8), 1 mM EDTA, 3 mM MgCl2, 0.5 mM GSSG, and 100 μL of enzyme extract. The reaction was started by adding 0.2 mM NADPH.
Protein determination and statistical analysis
Protein was determined by the method of Bradford (1976) using bovine serum albumin (BSA) as standard. All data were processed by statistical package SPSS (Version 11.0). All values are means of three independent replicates. Statistics was done separately for leaves and roots. Data were tested at significant levels of P < 0.05 and P < 0.01, respectively, using one-way ANOVA.
Results
Visual symptoms
After 21 days of low level Pu treatment in amended Hoagland solution, the plants did not show any clear symptoms of Pu toxicity, i.e., color change in root (white to pale yellow or brown) or necrosis of leaves in any of the Pu-treated plants (Supp. Fig. 1).
Plutonium, macro- and microelements
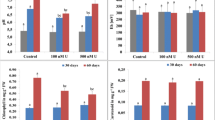
Due to precipitation, the effective Pu concentration in solution decreases during the weeks after plant contact reaching [Pu] < 20 nm. The Pu accumulation was analyzed after 80 days in roots, shoots (including leaves), and tubers (Fig. 1). The roots of plants in [Pu] = 500 nm medium showed maximum concentrations of CPu = (8460 ± 320) Bq g−1 DW (dry weight), which was 9.4 times higher than in the roots of plants grown in the [Pu] = 100 nm medium CPu = (900 ± 55) Bq g−1 DW. The translocation of Pu from root to shoot and tubers was about four magnitudes lower: In shoots of the 500 nm plants, the Pu concentration was CPu = (0.65 ± 0.05) Bq g−1 DW, 1.4 times higher than in the shoots of plants grown in the [Pu] = 100 nm medium. In tubers, the Pu concentration reached CPu = (0.96 ± 0.05) Bq g−1 DW in case of the higher concentration ([Pu] = 500 nm), exceeding the concentration in the shoots by a factor of 1.5. The lowest value CPu = (0.12 ± 0.01) Bq g−1 DW was measured in tubers of plants grown at [Pu] = 100 nm. The transfer factors (TFs) from solution to plant are as follows: 100 nm (root = 248, shoot = 0.12, and tuber = 0.034 Bq/kg_plant/Bq/kg solutions, respectively) and for 500 nm (root = 463, shoot = 0.04, and tuber = 0.053 Bq/kg_plant/Bq/kg solution, respectively).
Plutonium accumulation in shoots, roots, and in tubers of Solanum tuberosum plants treated for 80 days. Micro/macronutrient content in shoots and in roots of Solanum tuberosum plants treated for 80 days. Data for the nutrients are the mean of three independent replicates ± SD. The mean values followed by different letters (a, b, and ab) are significant difference at (P < 0.05 and P < 0.01)
The following effects were observed for the macro- and microelements: Ca, Cu, K, and Fe
The uncertainties for some of the elements were rather high (Fig. 1). For this reason, the comparison between the values should only represent trends. The shoots of plants treated by [Pu] = 500 nm contained CCa = (6200 ± 2200) μg kg−1 DW (×103), 2.1 times higher compared to control plants. The contents of the elements such as iron [Fe], Sulfur [S], and copper [Cu] were slightly higher (CFe(shoot) 2.2 times, CS(shoot) 2.3 times, CS(root) 1.6 times, CCu(shoot) 2.0 times, CCu(root) 1.4 times). In the roots of the plants treated by [Pu] = 100 nm, the potassium content was CK = (29,100 ± 2300) μg kg−1 DW (×103), 3.0 times higher compared to control plants. The Fe content in roots treated with [Pu] = 500 nm was CFe = (150 ± 30) μg kg−1 DW (×103), 1.5 times lower than the control.
The Zn, P, S, and Mg concentrations are not significantly altered or showed ambiguous behavior after the treatments. We observed only trends: A very small decrease of CZn in the roots of those plants treated with Pu (for both concentrations). The shoots showed a slight, not significant, increase of CZn only for the [Pu] = 500 nm treatment. The phosphorus concentration increased slightly in the roots in the case of the [Pu] = 500 nm treatment and decreased in shoots in comparison to that of the control. The magnesium concentration decreased in roots in case of [Pu] = 500 nm treatment. On the other hand, the magnesium concentration in shoots increased in both treatments in comparison to control. The highest magnesium concentration in the shoot was recorded for the [Pu] = 500 nm treatment (approx. 1.5 times). The treatment with [Pu] = 500 nm increased the sulfur content both in the roots by approx. 1.3 and in shoots by approx. 2.6 times. It is concluded from the present finding that, Pu in low concentration has no significant effects on the uptake of many trace and macroelements.
NO and H2O2 by epifluorescence microscopy
NO and H2O2 production was probed by epifluorescence microscopy in both the roots and leaves of Pu-treated S. tuberosum plants. As a central finding, we noted that in presence of different Pu concentrations (100 and 500 nm), NO production (in terms of green fluorescence) was reduced in both the leaves (Fig. 2a–c) and root (Fig. 2d–f), respectively, in comparison to that of control, after 21 days of treatment. The opposite trend was observed for H2O2 production; in both the leaves (Fig. 3a–c) and root (Fig. 3d–f), the concentration of H2O2 was higher (in terms of green fluorescence) in plants treated with [Pu] = 500 nm in comparison to that of control plants.
Representative images illustrating epifluorescence microscope detection of NO in the apex of Solanum tuberosum leaves/roots treated with or without plutonium for 21 days. The bright green fluorescence corresponds to the detection of NO in leaves/roots sections using fluorescence probe DAF-2 DA. Control leaves/root (a/d); (b/e) leaves/roots treated with [Pu] = 100 nm; (c/f) [Pu] = 500 nm-treated leaves/roots. The objectives size used 10× and the distance was 100 μm. The images are representative of five leaves/roots visualized
Representative images illustrating epifluorescence microscope detection of H2O2 in the apex of Solanum tuberosum leaves/roots treated with or without plutonium for 21 days. The bright green fluorescence corresponds to the detection of H2O2 in leaf sections using fluorescence probe DCF-DA. Control leaves/roots (a, d); (b, e) treated with [Pu] = 100 nm; (c,f) [Pu] = 500 nm-treated leaves/roots. The objectives size used 10× and the distance was 100 μm. The images are representative of five leaves/roots visualized
Photosynthetic pigments and enzymatic parameters under Pu treatment
We investigated the photosynthetic parameters chlorophyll and carotenoids in S. tuberosum plants treated with [Pu] = 100 and 500 nm in amended Hoagland solutions. There were no significant differences or changes noticed in any of the treated plants in comparison to the control for total chlorophyll (Fig. 4). On the other hand, the carotenoid content decreased significantly after both treatments in comparison to that of control (Fig. 4). The analysis of catalase (CAT) activity, one of the main antioxidative defense against H2O2, showed a statistically significant reduction in S. tuberosum plants treated with [Pu] = 100 and 500 nm in amended Hoagland solutions (Fig. 4). The activity of glutathione reductase (GR), a component of ASC-GSH cycle involved in H2O2 removal, catalyzing the reduction of GSSG to GSH, increased after 21 days in the plants treated with [Pu] = 100 nm and decreased in the plants treated with [Pu] = 500 nm in comparison to that of control plants (Fig. 4). However, the changes were not statistically significant.
Effect of plutonium on the total chlorophyll, carotenoids contents, and on catalase and glutathione reductase activity in Solanum tuberosum plants grown with or without [Pu] = 100 nm; [Pu] = 500 nm for 21 days. Data are the mean of three independent replicates ± SD. The mean values followed by different letters (a and b) are significant difference at (P < 0.05 and P < 0.01)
Superoxide radical and lipid peroxidation by histochemical methods
Histochemical techniques were used for the determination of superoxide radicals and lipid peroxidation in plant tissues (roots and leaves), which are subjected to Pu stress. Schiff’s reagent was used to detect aldehydes typically produced from lipid peroxides. In this method, a red/pink color that develops on tissue corresponds to the presence of aldehyde derived from oxidized lipid. Aldehyde involvement in cellular damage has been proven by the protective effects of the aldehyde-scavenging enzymes aldehyde dehydrogenase and aldehyde reductase to confer tolerance against various environmental stresses when they were overexpressed in plants. The Schiff’s reagent assay in leaves showed a higher upsurge in the pink color in [Pu] = 500 nm-treated plants in comparison to control leaves (Fig. 5a–c). The hydroxyl radical (OH•−) in the leaves had the highest reactivity and was able to react directly with biological membranes causing lipid peroxidation and to produce more color. A higher accumulation of superoxide radicals (with NBT) O2 •− was observed in the leaves (Fig. 5d–f) (dark blue patches) treated with [Pu] = 500 nm in S. tuberosum plant in comparison to control plants.
Histochemical localization of aldehydes derived from lipid peroxidation (LP) and O2 •− by NBT staining, in the fourth expanded young leaves of Solanum tuberosum plants grown in nutrient medium treated with or without plutonium for 21 days. a LP in control leaves. b Leaves treated with [Pu] = 100 nm. c [Pu] = 500 nm-treated leaves. d Accumulation of O2 •− in control leaves. e Leaves treated with [Pu] = 100 nm. f [Pu] = 500 nm-treated leaves. The images are representative of five leaves visualized
Discussion
Increasing the Pu concentration at this low level in nutrient solution did not inhibit S. tuberosum plant growth. Different types of results on plant responses to metal-induced stress were observed by Gupta et al. (2016) for U and Gupta et al. (2013a) for non-radioactive heavy metals like Hg, Pb, and As on Pisum sativum and Sedum alfredii plants for different durations under nutrient solution. In contrast to the present study, the metal concentrations of the previous study were much higher (micromolar range). At the nanomolar range, the translocation of Pu to the upper parts of plants is very low, indicating that the roots act as a barrier. Root exudates may also restrict uptake of metal through the roots into the plants (Degryse et al. 2008).
The effect of plant uptake on Pu transport into the aboveground tissues varies greatly and was influenced by the density of roots and other factors such as soil type, type of deposition, and of course, Pu species (Thompson et al. 2012). The Pu transport across root tissues strongly affects the overall transport and partitioning in the tested plants. Based on an experiment with As and Arabidopsis thaliana, Gupta et al. (2013b) hypothesized that metal detoxification mechanisms (e.g., production of phytochelatin) involve low molecular weight cytosolic proteins that bind toxic metal ions at the site of their uptake, restricting further mobility of metals to the upper part of plants (shoots). In the present work, plants were grown in hydroponic solution. Hence, Pu was in direct contact with the roots. Nevertheless, root to shoot transfer was very low, in accordance with previous work done be several authors on uranium uptake for different plants (Shahandeh and Hossner 2002; Aranjuelo et al. 2014; Gupta et al. 2016). It was also possible that the absorbed metals in the root tissue bind at substances of the cell wall and stay immobile in the apparent free space (AFS) (Lee et al. 2002).
Macro/micronutrients (elements) are generally required in different amounts by different plant species. Once these elements are taken up into the plants, long distance translocation of these metals plays a pivotal role in development and adaptation of plants in any condition. It is known that Ca2+ is able to sustain equilibrium and validity of cell membrane; on the other hand, it can act as a second envoy that transfers extracellular signals to the other parts of plants cells. In our experiment, the Ca concentration was increased slightly in both the root and shoot; in accordance with Siddiqui et al. (2012), they treated Vicia faba plants with Cd and noticed an increased level of Ca in the plant tissues. In another experiment, Gupta et al. (2013a) also noticed increase of Ca in roots of Pfaffia glomerata plants treated with [As] = 50 μM for 28 days. In the shoots, they noticed Ca increase in comparison to control after treatment by [Hg] = 1 μM for 28 days in the same plant. The Cu concentration in the tested plants increased in both root and shoots. It was in accordance with Gupta et al. (2013b); they also found an increase of Cu in A. thaliana plants treated with As in nutrient solution for various periods of time. It may be possible that Pu-dependent increase in [Fe] and [Cu] could provide oxidative damage by promoting OH•− formation through Fenton-type reactions. The Zn concentration decreased in the roots but increased in the shoots treated with Pu. Gupta et al. (2016) also found similar effects in roots of pea plants treated with different U concentrations. The reduction in essential nutrients will decrease the plant vitality and its ability to cope with heavy metal stress. Huang et al. explained this finding by convolution of zinc ions in the alleviating plasma membrane (Huang et al. 2008). Potassium [K] concentration increased in both root and shoot in comparison to control plants. This was in accordance with Kibria et al. (2009). They used two different genotypes of Amaranthus grown in the presence of lead and noticed increment of potassium in the plants.
Magnesium [Mg] activates numerous enzymes related to photosynthesis and respiration (Terry and Ulrich 1974) and stabilizes proteins and nucleic acids important for formation of chlorophyll molecules (Marschner 1986). In our experiment, the Mg content was not significantly influenced in roots or shoots. A reduction in root was observed by de Brito et al. (2016) under Pb treatment of Helianthus annuus L. plants and also Quzounidou et al. (1998) found reduction of [Mg] in roots in their experiment with spinach exposed to enhance copper concentrations. Gupta et al. (2016) noticed a reduction of [Mg] in both roots and shoots after 5 days in pea plants under different U treatments in nutrient solution.
Iron is an essential element for several enzymes and pigment generation in plants, and it assists in nitrate and sulfate reduction and also in energy production. In this experiment, the effects of Fe were small in the roots but higher in shoots. In the roots, it was also in accordance with the behavior of P. sativum plant under uranium treatment Gupta et al. (2016) and spinach treated with copper (Quzounidou et al. 1998). Both authors detected reduced Fe concentration in the roots. In the shoot, the Fe concentration increased upon addition of Pu in nutrient solution, in accordance with Gupta et al. (2013b), reporting increase of Fe in the leaves of A. thaliana treated with different concentrations of As in the nutrient solution. A slight tendency of [S] increase was observed in the present case, however, only statistically significant in roots. This was not unexpected, considering the contradictory findings in previous experiments; it was in accordance with Gupta et al. (2013b), who reported similar trends on A. thaliana treated with As in nutrient solution for 1 and 5 days. The opposite result (decrease) was reported by Gupta et al. (2016) in the case of pea plants treated with uranium for 5 days.
The main findings of the present work, however, concern the effect of the low amounts of Pu on NO concentration in the treated plants. It has been known that during different stress conditions, NO plays an important signaling role and regulates plant responses. It is also involved in various physiological processes of higher plants. Various authors report that NO protects against metal toxicity, see Gupta et al. (2013b) and Singh et al. (2009) for the effects of As on A. thaliana and Oryza sativa, as well as Wang and Yang (2005) for Cassia tora plants treated by enhanced aluminum concentrations. In our experiment, we found a reduction of NO for both high and low Pu concentrations after 21 days. It was in accordance with the observations of Gupta et al. (2016) for P. sativum plants after 5 days, however, at much higher concentration of the toxic metal uranium. Decrease of NO was also reported for treatment of Arabidopsis plants with 25 and 50-μM Cd solutions (Gupta et al. (2013b) and pea plants with 50-μM CdCl2 solutions (Rodriguez-Serrano et al. (2006). Jin et al. (2010) reported that reduction of NO by As or by a NO scavenger (PTIO) induced severe oxidative damage in tall fescue leaves, they further confirmed that NO plays an important role in the protection against As toxicity.
The observed increase in both H2O2 and malondialdehyde (MDA), seen oxidative damage to the leave membranes of S. tuberosum plants, suggested that even low levels of Pu may generate oxidative stress in tested plants. In our experiment, we also noticed induction of H2O2 in tested plants; one reason behind was that, the cellular damage and downstream signaling responses towards Pu-induced stress may cause higher production of H2O2, and it was in accordance with Gupta et al. (2016) with U and P. sativum plants treated for 5 days, and they also noticed induced level of H2O2 in the leaves. The increased level of ROS (H2O2) production and oxidative damage was also found after As treatment by Gupta et al. (2013b) in A. thaliana, Shaibur et al. (2006) in O. sativa, and Lin et al. (2008) in V. faba. It is also a well-established fact that H2O2 works as signal molecule during metal stress.
To assess further, how Pu in the nutrient medium affects the plant growth, we assessed the levels of photosynthetic pigments such as chlorophyll and carotenoids which are good indicators of photosynthetic capacity. The decreased total carotenoid (significant) and total chlorophyll (non-significant) contents in the leaves of Pu-treated plants were measured. A decrease in the level of photosynthetic pigments may be attributed to Pu-induced inhibition of chlorophyll and carotenoid biosynthesis that may be affected by the induced nutrient insufficiency. This was reported in case of iron (Vazquez et al. 1987) as well as it was also possible due to reduction in δ-ALA-D activity. Decrease of net photosynthesis might originate from the significantly reduced absorption of essential mineral nutrients, acting at the same time as an indirect reason for plant chlorosis and reduction in growth (Vazquez et al. 1987). We used very less concentration of Pu that is why we did not found any significant alteration in chlorophyll, but if we used higher concentration, we might see alteration. Beligni and Lamattina (2000) proposed after experiments with potato, lettuce, and Arabidopsis that the effect of NO on stomatal behavior could be another possible reason for the reduced photosynthesis rates. Tanyolac et al. (2007) reported similar results with Zea maize and copper contamination.
With the observed increased production of ROS but no significant changes in nutrient content of the plants, the question arises whether the effects are predominantly due to chemotoxicity or radiotoxicity of the Pu. Compared to U experiments with a 100 times higher concentration in solution (Gupta et al. 2016), the [Pu] = 500 nm solution contains a 360 times higher total alpha activity. In these experiments, U had in addition to the ROS production a significant influence on the nutrient content in different plant parts which is mainly referred to its chemotoxical property as a heavy metal. In case of Pu in low level concentration, the absence of significant chances in nutrient content refers to a ROS production which might mainly be induced by the Pu alpha decay. Further investigations are necessary.
The oxidative stress imposed by Pu was also mediated by disturbances of antioxidative defenses caused by the metal. In our experiment, CAT did not appear to be an efficient H2O2 scavenger under any Pu treatment because its activity was reduced in treated plants. The decline in CAT activity might be due to the inhibition of the enzyme by post-transcriptional alterations or downregulation of gene expression, as it was reported by Romero-Puertas et al. (2007) with cadmium-treated pea plants. Our result of decline in CAT activity was in accordance with As treatment to Phaseoulus aureus by Singh et al. (2007) and Taxithelium nepalense by Choudhury and Panda (2004); they also noticed the decline in CAT activity in their experiments. At oxidative stress, GR is a key enzyme whose function is to maintain the intracellular pool of GSH and contributes to H2O2 removal in the ASC-GSH cycle. Plutonium induces enhanced GR activity at low concentration ([Pu] = 100 nm) but shows the opposite effect at [Pu] = 500 nm. It suggests that GR participates in the general response to ROS induced by Pu but at higher concentration, its production is hindered. Smith et al. (1989) claimed that GR is highly sensitive to metal contamination due to the presence of thiol groups at the active site of the enzyme. Pu interaction might hence inhibit the functioning of the enzymes’ active site, explaining the reduced GR activity in this experiment.
The level of malondialdehyde (MDA) is a sign of lipid peroxidation in tissues and henceforth the oxidative stress. In our experiment, the increased levels of MDA at both Pu concentrations after 21 days of exposure were noticed. MDA is produced due to the peroxidation of membrane lipids and is an indicator of membrane damage. Uranium exposure caused increased levels of MDA and H2O2. Several groups reported heavy metal induced lipid peroxidation by formation of ROS. Examples are investigations on uranium-treated P. sativum plants Gupta et al. (2016), Cd-treated pea plants (Sandalio et al. 2001; Dixit et al. 2001), cadmium-treated rice (Hsu and Kao 2004), and arsenic-treated A. thaliana (Gupta et al. 2013b). Superoxide radical, accumulation of O2 •− was observed higher in both leaves treated with [Pu] = 100 or 500 nm in S. tuberosum plant samples. This was in accordance with findings of Gupta et al. (2016) on P. sativum treated with uranium [U] = 50 μM for 5 days and also with cadmium [Cd] = 50 μM for 14 days on pea by Rodriguez-Serrano et al. (2006), who also found higher accumulation of superoxide radicals in the leaves.
Conclusion
This result reports for the first time direct effects of low Pu concentrations on the regulation of plant nutrition. Pu affects the uptake of Ca, Cu, K, and Fe and to a lesser extent Zn, P, S, and Mg. The content of O2 •−, MDA, and H2O2 increased with increasing Pu concentration in the solution; while the opposite effect was seen on NO, CAT, and GR, showing decreased concentration or reduced activity. The nutrient content in plant parts was not influenced significantly. This suggests that at low concentration, the presence of Pu influences the production of ROS indirectly due to radiotoxicity and generates oxidative stress in the tested plants.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 52:121–126
Aranjuelo I, Doustaly F, Cela J, Porcel R, Müller M, Aroca R, Munné-Bosch S, Bourguignon J (2014) Glutathione and transpiration as key factors conditioning oxidative stress in Arabidopsis thaliana exposed to uranium. Planta 239:817–830
Arnon DI (1949) Copper enzyme in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Beligni MV, Lamattina L (2000) Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 210:215–221
Bisinger T, Hippler S, Michel R, Wacker L, Synal HA (2010) Determination of plutonium from different sources in environmental samples using alpha-spectrometry and AMS. Nucl Instrum Method Phys Res Sec B 268:1269–1272
Bondietti EA, Reynolds SA, Shanks MH (1976) Interaction of plutonium with complexing substances in soils and natural waters. IAEA-SM-199/51. pp 273–287.
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Brown KW, McFarlane JC (1978) Plutonium uptake by plants grown in soil containing plutonium-238-dioxide particles. Health Phys 35:481–485
Bunzl K, Kracke W, Schimmack W (1992) Vertical migration of plutonium-239+-240, americium-241 and cesium-137 fallout in a forest soil under spruce. Analyst 117:469–474
Chaki M, Valderrama R, Fernández-Ocaña AM, Carreras A, Gómez-Rodríguez MV, Pedrajas JR, Begara-Morales JC, Sánchez-Calvo B, Luque F, Leterrier M, Corpas FJ, Barroso JB (2011) Mechanical wounding induces a nitrosative stress by down-regulation of GSNO reductase and an increase in S-nitrosothiols in sunflower (Helianthus annuus) seedlings. J Exp Bot 62:1803–1813
Corpas FJ, Chaki M, Fernández-Ocaña A, Valderrama R, Palma JM, Carreras A, Begara-Morales JC, Airaki M, del Río LA, Barroso JB (2008) Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant Cell Physiol 49:1711–1722
Corpas FJ, Gupta DK, Palma JM (2015) Production sites of reactive oxygen species (ROS) in plants. In: Gupta DK, Palma JM, Corpas FJ (eds) Reactive oxygen species and oxidative damage in plants under stress. Springer Publication, Germany, pp 1–22
Choudhury S, Panda SK (2004) Induction of oxidative stress and ultrastructural changes in moss Taxithelium nepalense (Schwaegr.) Broth. under lead and arsenic phytotoxicity. Curr Sci 87:342–348
de Brito Abreu C, do Lima Sacramento B, Alves AT, Moura SC, Pinelli MS, ADA N (2016) Nutritional and biochemical changes induced by lead in sunflower (Helianthus annuus L.) Ciências Agrárias 37:1229–1242
Degryse F, Verma VK, Smolders E (2008) Mobilization of Cu and Zn by root exudates of dicotyledonous plants in resin-buffered solutions and in soil. Plant Soil 306:69–84
Dixit V, Pandey V, Shyam R (2001) Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J Exp Bot 52:1101–1109
Duxbury AC, Yentsch CS (1956) Plankton pigment monograph. J Mar Res 15:93–101
Edwards EA, Rawsthorne S, Mullineaux PM (1990) Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.) Planta 180:278–284
Entwistle JA, Flowers AG, Nageldiner G, Greenwood JC (2003) Identification and characterization of radioactive ‘hot’ particles in Chernobyl fallout-contaminated soils: the application of two novel approaches. Mineralog Magaz 67:183–204
Federov YA, Bakurov AS, Fedorova MN, Rasulev F (1986) Behavior of plutonium in the soil and its entry into plants. Agrokhimiya 12:83–88
Francis CW (1973) Plutonium mobility in soils and uptake by plants: a review. J Environ Qual 2:67–70
Francis AJ, Dodge CJ (2015) Microbial mobilization of plutonium and other actinides from contaminated soil. J Environ Radioact 150:277–285
Francis AJ, Dodge CJ, Gillow JB (2006) Biotransformation of plutonium complexed with citric acid. Radiochima Acta 94:731–737
Gupta DK, Walther C (2014) Radionuclide contamination and remediation through plants. Springer-Verlag, Germany
Gupta DK, Huang HG, Nicoloso FT, Schetinger MRC, Farias JG, Li TQ, Razafindrabe BHN, Aryal N, Inouhe M (2013a) Effect of Hg, As and Pb on biomass production, photosynthetic rate, nutrients uptake and phytochelatin induction in Pfaffia glomerata. Ecotoxicology 22:1403–1412
Gupta DK, Inouhe M, Rodríguez-Serrano M, Romero-Puerta MC, Sandalio LM (2013b) Oxidative stress and arsenic toxicity: role of NADPH oxidases. Chemosphere 90:1987–1996
Gupta DK, Corpas FJ, Palma JM (2013c) Heavy metal stress in plants. Springer-Verlag, Germany
Gupta DK, Palma JM, Corpas FJ (2015) Reactive oxygen species and oxidative damage in plants under stress. Springer-Verlag, Germany
Gupta DK, Tawussi F, Hamann L, Walther C (2016) Moderate uranium disturbs the nutritional status and induces oxidative stress in Pisum sativum L. J Plant Physiol Pathol 4:1
Gupta K, Sengupta A, Chakraborty M, Gupta B (2016) Hydrogen peroxide and polyamines act as double edged swards in plant abiotic stress responses. Front Plant Sci 7:1343
Hamilton TF, Jernströem J, Martinelli RE, Kehl SR, Eriksson M, Williams RW, Bielewski M, Rivers AN, Brown TA, Tumey SJ, Betti M (2009) Frequency distribution, isotopic composition and physical characterization of plutonium-bearing particles from the Fig-Quince zone on Runit Island, Enewetak Atoll. J Radioanalyt Nucl Chem 282:1019–1026
Haschke JM, Allen TH, Morales LA (2000) Surface and corrosion chemistry of plutonium. Los Alamos Sci 26:252–273
Hsu YT, Kao CH (2004) Cadmium toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regul 42:227–238
Huang H, Li T, Tian S, Gupta DK, Zhang X, Yang XE (2008) Role of EDTA in alleviating lead toxicity in accumulator species of Sedum alfredii H. Bioresour Technol 99:6088–6096
Jabs T, Dietrich RA, Dangl JL (1996) Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273:1853–1856
Jin JW, Xu YF, Huang YF (2010) Protective effect of nitric oxide against arsenic induced oxidative damage in tall fescue leaves. Afr J Biotechnol 9:1619–1627
Kashparov V, Ahamdach N, Levchuk S, Yoschenko V, Senko SF, Maloshtan I (2009) Dissolution of particles of irradiated nuclear fuel in the temporary storages of radioactive waste in Chernobyl zone: sources for radionuclides migration. In: Oughton DH, Kashparov V (eds) Radioactive particles in the environment. NATO Science for Peace and Security Series pp 139–156.
Kashparov V, Yoschenko V, Levchuk S, Bugai D, van Meir N, Simonucci C, Martin-Garin A (2012) Radionuclide migration in the experimental polygon of the red Forest waste site in the Chernobyl zone-part 1: characterization of the waste trench, fuel particle transformation processes in soils, biogenic fluxes and effects on biota. Appl Geochem 27:1348–1358
Kershaw PJ, Denoon DC, Woodhead DS (1999) Observations on the redistribution of plutonium and americium in the Irish Sea sediments, 1978 to 1996: concentrations and inventories. J Environ Radioact 44:191–221
Kersting AB, Efurd DW, Finnegan DL, Rokop DJ, Smith DK, Thompson JL (1999) Migration of plutonium in ground water at the Nevada test site. Nature 397:56–59
Kibria MG, Islam M, Osman KT (2009) Effects of lead on growth and mineral nutrition of Amaranthus gangeticus L. and Amaranthus oleracea L. Soil Environ 28:1–6
Kirsch R, Fellhauer D, Altmaier M, Neck V, Rossberg A, Fanghänel T, Charlet L, Scheinost AC (2011) Oxidation state and local structure of plutonium reacted with magnetite, Mackinawite, and Chukanovite. Environ Sci Technol 45:7267–7274
Lachner J, Christl M, Bisinger T, Michel R, Synal HA (2010) Isotopic signature of plutonium at Bikini atoll. App Rad Isotop 68:979–983
Lasat MM, Baker AJM, Kochian LV (1998) Altered Zn compartmentation in the root symplasm and stimulated Zn absorption into the leaf as mechanisms involved in Zn hyperaccumulation in Thlaspi caerulescens. Plant Physiol 118:875–883
Lee JH, Hossner LR, Attrep JRM, Kung KS (2002) Uptake and translocation of plutonium in two plant species using hydroponics. Environ Pollut 117:61–68
Leterrier M, Airaki M, Palma JM, Chaki M, Barroso JB, Corpas FJ (2012) Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environ Pollut 166:136–143
Lin A, Zhang X, Zhu YG, Zhao FJ (2008) Arsenate-induced toxicity: effects on antioxidative enzymes and DNA damage in Vicia faba. Environ Toxicol Chem 27:413–419
Lipton WV, Goldin AS (1976) Some factors influencing the uptake of plutonium-239 by pea plants. Health Phys 31:425–430
Little CA, Whicker FW, Winson TF (1980) Plutonium in a grassland ecosystem at rocky flats. J Environ Qual 9:350–354
Livens FR, Baxter MS, Allen SE (1987) Association of plutonium with soil organic matter. Soil Sci 144:24–28
Manara A (2012) Plant responses to heavy metal toxicity. In: Furini A (ed) Plants and heavy metals. SpringerBrief in Biometals pp 27-53.
Marschner H (1986) Mineral nutrition in higher plants. Academic press, London, U.K.
Mello-Farias PC, Chaves ALS (2008) Biochemical and molecular aspects of toxic metals phytoremediation using transgenic plants. In: Tiznado-Hernández ME, Troncoso-Rojas R, Rivera-Domínguez MA (eds) Transgenic approach in plant biochemistry and physiology. Research Signpost, India
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trend Plant Sci 16:300–309
Muller RN (1978) Chemical characterization of local and stratospheric plutonium in Ohio soils. Soil Sci 125:131–136
Nishita H, Hamilton M (1980) The influence of several soil components and their interaction on plutonium extractability from a calcareous soil. Soil Sci 13:56–59
Novikov AP, Kalmykov SN, Utsunomiya S, Ewing RC, Horreard F, Merkulov A, Clark SB, Tkachev VV, Myasoedov BF (2006) Colloid transport of plutonium in the far-field of the Mayak production association, Russia. Science 314:638–641
Ouzounidou G, Ilias I, Tranopoulou H, Karatalgis S (1998) Amelioration of copper toxicity by iron on spinach physiology. J Plant Nutr 21:2089–2101
Pinder JE 3rd, KW ML, Adriano DC, Corey JC, Boni AL (1990) Atmospheric deposition, resuspension and root uptake of Pu in corn and other grain producing agroecosystems near a nuclear fuel facility. Health Phys 59:853–867
Poinssot C, Geckeis H (2012) Radionuclide behaviour in the natural environment: science, implications and lessons for the nuclear industry. Woodhead Publishing Ltd., Cambridge
del Río LA (2011) Peroxisomes as a cellular source of reactive nitrogen species signal molecules. Arch Biochem Biophys 506:1–11
Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, del Río LA, Sandalio LM (2006) Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544
Romero-Puertas MC, Corpas FJ, Rodríguez-Serrano M, Gomez M, del Rio LA, Sandalio LM (2007) Differential expression and regulation of antioxidative enzymes by cadmium in pea plants. J Plant Physiol 164:1346–1357
Salbu B, Krekling T, Oughton DH, Østby G, Kashparov VA, Brand TL, Day JP (1994) Hot particles in accidental releases from Chernobyl and wind scale nuclear installations. Analyst 119:125–130
Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Río LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Sandalio LM, Rodríguez-Serrano M, Gupta DK, Archilla A, Romero-Puertas MC, del Río LA (2012) Reactive oxygen species and nitric oxide in plants under cadmium stress: from toxicity to signalling. In: Parvaiz A, Prasad MNV (eds) Environmental adaptations and stress tolerance of plants in the era of climate change. Springer, Heidelberg, pp 199–215
Shahandeh H, Hossner LR (2002) Role of soil properties in phytoaccumulation of uranium. Water Air Soil Pollut 141:165–180
Shaibur MR, Kitajima N, Sugawara R, Kondo T, Huq SMI, Kawai S (2006) Physiological and mineralogical properties of arsenic-induced chlorosis in rice seedlings grown hydroponically. Soil Sci Plant Nutr 52:691–700
Siddiqui MH, Al-Whaibi MH, Basalah MO (2010) Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 248:447–455
Siddiqui MH, Al-Whaibi MH, Ahmed MS, Basalah MO, Ali HM (2012) Effect of calcium and potassium on antioxidant system of Vicia faba L. under cadmium stress. Int J Mol Sci 13:6604–6619
Singh HP, Batish DR, Kohli RK, Arora K (2007) Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Reg 53:65–73
Singh HP, Kaur S, Batish DR, Sharma VP, Sharma N, Kohli RK (2009) Nitric oxide alleviates arsenic toxicity by reducing oxidative damage in the roots of Oryza sativa (rice). Nit Oxide 20:289–297
Smith IK, Vierheller TL, Thorne C (1989) Properties and functions of glutathione reductase in plants. Physiol Planta 77:449–456
Steinhauser G, Niisoe T, Harada KH, Shozugawa K, Schneider S, Synal HA, Walther C, Christl M, Nanba K, Ishikawa H, Koizumi A (2015) Post-accident sporadic release of airborne radionuclides from the Fukushima Daiichi nuclear power plant site. Environ Sci Technol 49:14028–14035
Tanyolac D, Ekmekci Y, Unalan S (2007) Changes in photochemical and antioxidant enzyme activities in maize (Zea maize L.) leaves exposed to excess copper. Chemosphere 67:89–98
Terry N, Ulrich A (1974) Effects of magnesium deficiency on the photosynthesis and respiration of leaves of sugar beet. Plant Physiol 54:379–381
Thompson SW, Molz FJ, Fjeld RA, Kaplan DI (2012) Uptake, distribution, and velocity of organically complexed plutonium in corn (Zea mays). J Environ Radioact 112:133–140
Vazquez MD, Poschenrieder C, Barcelo J (1987) Chromium (VI) induced structural and ultra-structural changes in bush bean plants (Phaseolus vulgaris). Ann Bot 59:427–438
Viehweger K, Geipel G, Bernhard G (2011) Impact of uranium (U) on the cellular glutathione pool and resultant consequences for the redox status of U. Biometals 24:1197–1204
Wang YS, Yang ZM (2005) Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant Cell Physiol 46:1915–1923
Xiong J, Fu G, Tao L, Zhu C (2010) Roles of nitric oxide in alleviating heavy metal toxicity in plants. Arch Biochem Biophys 497:13–20
Yang X, Feng Y, He Z, Stoffella P (2005) Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. J Trace Elem Med Biol 18:339–353
Zheng J, Yamada M (2006) Plutonium isotopes in settling particles: transport and scavenging of Pu in the western Northwest Pacific. Environ Sci Technol 40:4103–4108
Zheng J, Tagami K, Watanabe Y, Uchida S, Aono T, Ishii N, Yoshida S, Kubata Y, Fuma S, Ihara S (2012) Isotopic evidence of plutonium release in the environment from the Fukushima DNPP accident. Sci Report 2:304
Acknowledgements
We acknowledge financial support from the BMBF for FT (funding 02S9082A) and DG (funding 02S9276D). AH thanks the Sasse foundation for their continuous support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Georg Steinhauser
Highlights
1. First time reporting the information related to plutonium NO and H2O2 localization in plants through epifluorescence microscopy.
2. First time reporting histochemical staining of plants under Pu stress.
3. Plutonium in low concentration has no significant effects on the uptake of many trace and macroelements.
4. At low concentration, the presence of Pu influences the production of ROS indirectly due to radiotoxicity.
5. Our finding proves that even low concentration of Pu regulates ROS production and generates oxidative stress in S. tuberosum L.
Electronic supplementary material
Supplementary Figure 1
(DOCX 9475 kb).
Rights and permissions
About this article
Cite this article
Gupta, D.K., Tawussi, F., Hölzer, A. et al. Investigation of low-level 242Pu contamination on nutrition disturbance and oxidative stress in Solanum tuberosum L.. Environ Sci Pollut Res 24, 16050–16061 (2017). https://doi.org/10.1007/s11356-017-9071-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9071-9