Abstract
Due to excessive mining and use of radionuclide especially uranium (U) and its fission products, numerous health hazards as well as environmental contamination worldwide have been created. The present study focused on demonstrating whether low concentration of U treatment in liquid nutient medium may translocate traces of U in plants and in fruits of Pisum sativum after 30 and 60 days of exposure for the safe use as a food supplement for human/animals. Hydroponically grown plants (in amended Hoagland medium) were treated with two different concentrations of uranium ([U] = 100 and 500 nM, respectively). Plants showed a decrease in total chlorophyll after 60 days of treatment. On the other hand, Eh of the nutrient medium was not affected from the initial days till 60 days of treatment, but pH of nutrient medium was increased upon durations, highest at 60 days of treatment. In seeds, micro/macro elements were under limit as well as U concentration was also under detection limit. We did not observe any U in the above ground parts (shoots/seeds) of the plant, i.e., under detection limit. Our observation suggests that P. sativum plants may be useful to grow at low radionuclide [U]-contaminated areas for safe human/animal use, but for other fission products, we have to investigate further for the safe human/animal use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium (U) is a natural radioactive material, extensively spread in the earth’s crust, with an average of less than 4 mg kg−1 concentration. The main health risk of U intake approaches is lungs and kidney damage, and also due to alpha radiation, one should also get risk of cancer (Gupta et al. 2016a). There are several factors from which a normal man is getting affected by the contamination of U, i.e., due to unsafe handling and transportation.
Nowadays, a number of remediation techniques are in use for remediation of contaminated land and water. Among them, soil excavation is the most collective method for radioactively contaminated soils as well as encapsulation (Evans 2000). Cleaning of the environment by using green plants generates assorted numerous environmental pollution complications through direct uptake of toxic chemical(s), followed by consequent alteration, conveyance, and their accumulation in less toxic forms (Schnoor et al. 1995). Macek et al. (2000) described that remediation practices are being upgraded by plant root discharge exudates and enzymes that may encourage microbial assortment at rhizosphere and biochemical movement in bulk soil. Marschner (1995) also hypothesized that plant roots discharge exudates in the neighboring soil matrix that may help in chelation of unwanted metals to escape transportation inside the plant cell, such as root exudates like histidine (His) inhibit Ni uptake from the soil (Salt et al. 2000); however, pectic sites and diverse extracellular carbohydrate particles present on the cell wall also show their role in accumulation of toxic heavy metal ions (Manara 2012). In plants, when growth is hindered by any toxic compounds, it is known as phytotoxicity, sometimes the damage may be caused by an extensive multiplicity of compounds, including heavy metals, salinity, pesticides/insecticides, phytotoxins, and radionuclides. Phytoremediation methods are attractive substitute towards traditional energy and instrument intensive, chemical-based expensive renewal techniques of massive polluted areas of land and water (Padmavathiamma and Li 2007; Lone et al. 2008). Immersion and circulation of contamination in plants may take place either through straight or secondary means, which may vary significantly in different plant species principally in case of long-lived radionuclides (Din et al. 2010). Transfer of vitality from soil to plant is normally calculated for trace materials, whose behavior in the soil-plant-rhizosphere scheme principally depends on the concentrations of macro-nutrients present there. It has been already observed that plants are taking up many cations existing in their root zone; it is also a manifestation that whenever plants are exposed to ionizing radiation, some molecular and cellular effects are immediately induced straight towards impairment of macromolecules or parenthetically through water radiolytic reactions (Gupta and Walther 2014; Gupta et al. 2016a). Generally, plants employ numerous tactics to manage toxic effects of radionuclides which comprises synchronization of complex functional and biochemical procedures. Plants are generally resistant to radionuclide anxieties either by monitoring uptake or by enduring in existence of high internal absorption. However, biosorption to cell walls, extracellular precipitation, reduced uptake, or amplified efflux are collective mechanism from which plants check abiotic stress and also decrease the absorption of metal inflow in cells (Gupta and Walther 2014; Walther and Gupta 2015). Though in plants, tolerance to stress comprises complicated physiological reactions such as production of amino acids, organic acid generation, glutathione/or metal-binding ligand production, and up-regulation of antioxidant resistance systems (Gupta and Sandalio 2012), it was reported by Ebbs et al. (1998) that adding of phosphate to a U containing solution condensed the noxious effects of U in Pisum sativum; the reason behind was that U makes complex with phosphate, and it may likely be due to the fact that complexation decreases the bioavailability of U in the solution. It is well known that plants take up P to variable amounts from substrates in which they are rooted. Colle et al. (2009) proved that plants get direct contamination if their above-ground parts seize radionuclides or toxic complexes through contaminated rain/sprinkling irrigation. It is acclaimed that if only one part of the plants is edible, it is obligatory to take into explanation that the translocation procedure throughout the plant might allocate toxins from contaminated leaves to the comestible parts; it might be conceivable that the circulation from foliar deposition and absorption by the leaves. It was hypothesized by Chamel et al. (1991) that in plants, permeation through cuticle consists of four phases, i.e., sorption, dispersal, desorption in the apoplast, and absorption by the subjacent cells. Subsequently after infusion phase, active transport within cells, conveyance in the vascular arrangement, and conveyance between phloem and xylem interfere (Lauchli 1972; Carini and Bengtsson 2001).
When any contaminated land with radioactively contaminated substances is being remediated with the aim of returning for farming use, measures for reducing of radionuclide transfer from soil to vegetation are obligatory. Radionuclides commonly migrate from soil to vegetation through ground solutions. Nowadays, best method for reducing of radionuclide migration from soil to vegetation is tilling of radioactively contaminated deposits (Voronina et al. 2015). This present study focused on demonstrating whether low concentration of U may translocate in the fruits of pea (P. sativum) under different concentrations of U after 30 and 60 days of exposure for the safe use as a food supplement for human and for animals. Furthermore, we address the effect of U on macro/micro nutrient uptake and accumulation, photosynthetic parameters and also checked toxic symptoms of radionuclide (U) in tested plants as well as change in Eh and pH upon different durations.
Material and methods
Plant material, growth condition, and treatment
Pisum sativum L. var. Boretta seeds were bought from N. L. Chrestensen, Erfurt, Germany. Initially, seeds were soaked in distilled water for 12 h and again kept in wet blotting paper for 48 h for seed germination in the dark; healthy and equal size seedlings were transferred to hydroponics in plastic boxes containing 5 L of amended Hoagland medium pH 6.2 (Gupta et al. 2013b). Plants were grown in a growth chamber at 22 ± 1 °C during 16/8 light/dark cycle with 120 μE m−2 s−1 of irradiance by cold fluorescent lamps for 3 weeks. For the treatment of plantlets, we dissolved appropriate amounts of UO2(NO3)2 in the amended Hoagland solution (pH 5.5) to achieve concentrations of [U] = 100 and 500 nM, respectively. In modified nutrient solution, low phosphate concentrations were used to avoid precipitation of U as U-phosphate complexes. Plants without any U treatment were used as control. U-treated plants were harvested totally at random after 30 and 60 days of treatment. In each experiment, randomly leaves, root, and seeds were selected. All chemicals used were of analytical grade purchased from Sigma Chemical Company (USA).
Eh and pH analysis
At every harvest (60 and 90 days), Eh and pH concentration was measured in the nutrient medium with the help of Eh and pH meter. Eh and pH values were also measured before adding U concentration in the liquid medium and served as an initial control.
Determination of total chlorophyll and carotenoid concentration
Plant material (0.1 mg) was grounded in chilled 80% acetone in dark. After centrifugation at 10,000g for 10 min at 4 °C, absorbance of the supernatant was taken at 663, 645, 510, and 480 nm. The concentration of total chlorophyll was calculated by the method of Arnon (1949) and carotenoid concentration by Duxbury and Yentsch (1956).
Element determination
Uranium and other micro/macro nutrient concentration were measured in all root, shoot (including leafs), and in seeds. Between 0.01 and 0.25 g of dry plant tissue and seeds were grounded and digested in 5 ml of concentrated HNO3, using an open digestion system with heating block Velp Scientific (Milano, Italy). Heating was set at 130 °C for 2 h. Plastic caps were fitted to the vessels to prevent loss by volatilization. After digestion, the rest of the materials was diluted five times with MilleQ water and make up to 50 ml. The U, Ca, K, Mg, P, S, Zn, and Cu concentration was determined by inductively coupled plasma optical emission spectrometry (ICP-OES), using a PerkinElmer Optima 4300 DV (Shelton, USA) equipped with a cyclonic spray chamber and a concentric nebulizer (Gupta et al. 2013a).
Statistical analysis
All data were processed by statistical package SPSS (Version 11.0). All values are means of three independent replicates. Data were tested at significant levels of P < 0.05 (b) and P < 0.01 (bc), respectively, using one-way ANOVA.
Results
Visually, there were no phytotoxic symptoms shown in plants after 30 days (Supplementary Fig. 1). The plants did not show any symptom of U toxicity at initial days (30 days), but at 60 days, plants were getting yellow leaves that may be due to aging. We also noticed that there were no effects of U concentration on the production of seed quality or on the size of seeds.
Eh and pH of nutrient solution
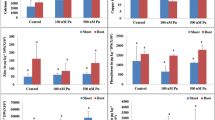
The initial Hoagland solution has a pH of 5.5. The interdependency with P. sativum showed an increase of pH in control medium, i.e., 6.9 ± 0.1 after 30 days up to 7.6 ± 0.1 after 60 days. At low U concentration (100 and 500 nM), it does not influence pH at initial solution. After 30 days, the pH of the U-treated solution increased only up to 6.4 ± 0.1 at 500 nM concentrations, and after 60 days, the pH of 100 nM U-treated solution was 6.6 ± 0.1 and 500 nM was 7.3 ± 0.4 (Fig. 1). The redox potential (Eh) showed no significant change since beginning of the experiment till last (Fig. 1).
Effect of uranium on Eh and pH concentration, total chlorophyll, and carotenoid contents in Pisum sativum L. plants after 0, 30, and 60 days of treatment in nutrient solution. Data are the mean of three independent replicates ± SD. Mean values followed by different letters (bc) are significant difference at P < 0.01 by one-way ANOVA
Chlorophyll and carotenoid
After 30 days of U treatment in modified Hoagland nutrient solution ([U] = 500 nM), the chlorophyll content was noticed 1.2 times higher compared to control plant. In case of 60-day harvest, U-treated plants ([U] = 500 nM) showed 1.6 times less chlorophyll content than control plants. One reason behind decrease of chlorophyll is that the plants came to their maturity of life cycle (generally, pea plants have the total life cycle of 60–90 days) (Fig. 1). The carotenoid showed no significant influence by any of the U treatment at any days (Fig. 1).
Uranium, macro and micro elements in solution
The U concentration in modified Hoagland solution after 30 days was about 4.5 times less than the initial 500 nM U solution. After 60 days, it was 7.1 times less than the initial concentration. In case of macro elements such as Ca, K, Mg, and S, the concentration in solution decreases strongly after 30 days of treatment that was due to the fact that plants initially require these elements for growth. In comparison to control solution, after 30 days of treatment, Ca concentration in 500 nM U solution was 3.4 times less, K concentration was 2.0 times less, Mg concentration was 2.3 times less, and S concentration was 1.5 times less. After 60 days, Ca concentration in 500 nM U solutions was 2.4 times less compared to control solution, K concentration was 1.5 times less, Mg concentration was 3.1 times less, and S concentration was 1.2 times less (Fig. 2).
In case of micro elements, Cu concentration was 1.3 times higher after 30 days in comparison to control solution, and Zn concentration was about 2.0 times less. After 60 days, Cu and Zn concentration in nutrient solution was below detection limit in all samples. The phosphorus concentration in the initial solution was increased upon addition of U concentration in comparison to control. One reason behind is that the low concentration of U in the liquid medium provokes the P at initial stage, but after 30 and 60 days, it was lowered due to plant uptake (Fig. 2).
Uranium, macro and micro elements in root, shoots, and in seeds
Uranium accumulation was analyzed in root, shoots (30 and 60 days), as well as in the seeds after their maturity (60 days). The highest recorded U amount was in the roots of 500 nM U treatment after 60 days, i.e., 0.38 ± 0.07 mg U g−1 of root (DW) (Fig. 3). This U content was approximately 10 times less, if we compared with Pisum sativum in 100 times higher U concentration (50 μM) for 5-day treatment (Gupta et al. 2016b). For shoots and seeds, the U content was below detection limit (the detection limit for root, shoots, and seeds was 0.01 mg U g−1 DW). This shows that the U translocation from root to the upper parts of the plant was very less.
Effect of uranium on accumulation of macronutrient/micro nutrient content in shoots and roots of Pisum sativum L. plants treated for 30 and 60 days. Data are the mean of three independent replicates ± SD. Mean values followed by different letters (b and bc) are significant difference at P < 0.05 and P < 0.01 by one-way ANOVA
Among macro elements, Ca was approximately 1.4 times higher in the roots of [U] = 500 nM treated plants after 60 days in comparison to control (same trend as it was for 50 μM U treatment, Gupta et al. 2016b). In case of shoots, Ca content in [U] = 100 nM treated plants was 2.5 times higher than the control after 60 days, the shoots of [U] = 500 nM treated plants also shows some increment of Ca but was lesser than [U] = 100 nM treated plants, but it was significantly different to control. The same trend was also observed for K, Mg, and S in shoots after 60 days. The P content in both root and shoot was strongly induced due to the presence of U. In root, it was approximately 21.7 times higher, in shoots 6.0 times higher, for [U] = 500 nM treated plants after 30 days in comparison to control. After 60-day treatment, the P content was less, but still 4.8 times higher in root and 4.5 times higher in shoots compared to control. In case of micro element, Zn and Cu concentration decreased in both root (Zn: 1.3, Cu: 1.3 times lower) and shoots (Zn: 1.8, Cu: 1.2 times lower) upon addition of U (500 nM, 60 days) in nutrient medium in comparison to control (Fig. 3). In seeds, only P and S content was higher at 500 nM U treatment plants than control, i.e., 1.9 and 0.5 times higher, respectively, and significantly different. Other macro and micro elements were not significantly higher in comparison to control. U concentration in the seeds was below detection limit. From this result, the seeds have less metal accumulation, so that it is useful for safe human/animal consumption. Only P was high, because of Pisum plants enriched in phosphorus, so that in many countries, it is used as P supplement.
Discussion
In general, like heavy metals, radionuclides may not be obviously or synthetically degraded (Gupta and Walther 2014). For living creatures, all heavy metals are noxious and affect rigorously to all vital biological processes such as photosynthesis, respiration, metabolism, and reproduction and also effect total injury to the individual or even to cell death (Anderson 2003). The concentration of radionuclides and its movement and bioavailability principally inclined by initial excellence, quantity, and the rate of discharge at the point source; it also depends upon diverse hydrological features, like circulation, advection, reduction, and also to geochemical procedures such as complexation at diverse pH, solid/liquid distribution constant, aqueous phase, reduction/oxidation (redox), precipitation/dissolution, diffusion, colloid-facilitated transport, and of course organic matter substances (Gupta and Walther 2014).
The pH value and redox potential (Eh) of the soil are predictable as major leading factors in radionuclide chemical speciation. All redox developments in soil occur in the existence of water. The redox potential and the pH values are negatively connected, i.e., the pH will increase with the decrease of Eh. In our present experiment at 30 days, the Eh value was increased (not significant) in U treatment, but at 60 days, it again came to its initial value what we used for the experiment, but in case of pH, it was always increased from 30 to 60 days and the maximum was at 60 days in control plants (Fig. 1). In U-treated plants, it was also increased but not like control plants; it shows that there is some limiting factor that makes some redox reactions in the liquid medium. It was in accordance with Koch-Steindl and Pröhl (2001); they hypothesized that oxygen supply controls the redox responses in the soil and the radionuclide chemical speciation, thus creating the Eh-pH conditions. For U availability, data shows that the movement and bioavailability of U were inclined by the oxygen content, organic material content, pH, parent material and weathering, and also comprises texture and clay content. By an experiment, Sheppard and Evenden (1987) showed that U retaining in soils increased with higher soil cation-exchange ability, while the U movement was increased by the presence of carbonate, concluded formation of anionic U and CO3 complexes. At low pH values (pH < 3), U has a low sorption in soils, while for pH ranging from 5 to 7, the sorption was maximum and decreases again at pH above 7, due to the increasing number of negatively charged binding sites (EPA 1999).
Increase of photosynthetic pigments (total chlorophyll) in our experiment at 30 days attributed to U-induced biosynthesis (Fig. 1), but at 60 days, it was declined that may cause by the induced nutrient deficiency, like as iron as well as due to the decline in δ-ALA-D activity. Similar type of result was reported by Vazquez et al. (1987) with Phaseolus vulgaris plants treated with chromium and Gupta et al. (2017) also got reduction of chlorophyll in Solanum tuberosum plants treated with low level of plutonium in nutrient medium. They also proposed that decrease in net photosynthesis as a significance of reduced absorption of vital mineral nutrients as an accidental reason for plant chlorosis; Tanyolac et al. (2007) has the same type of experience with Zea maize plants treated with Cu. Rebechini and Hanzely (1974) proposed that decrease of chlorophyll was due to injury in chloroplast in relation to grana and stroma in Ceratophyllum demersum. Sharma and Dubey (2005) assumed that diminished uptake of vital elements such as Mn and Fe injures the photosynthetic device, and due to that, chlorophyll deficit happened by amplified chlorophyllase movement. One more reason what we proposed at 60 days, that the plant came to its life maturity that also ascribed to the reduction in photosynthesis. In case of carotenoids, there was no change observed at both treatment and duration in comparison to control; it attributes to U that it does not induce any inhibition (Fig. 1). It was in accordance with Pallett and Young (1993) that carotenoid contents were not going to change or small raise will be noticed; it might be that carotenoid acts as a defense device for the plant to cope up with metal stress.
In general, a decreasing trend was noticed in all other nutrient components (after exposure to 30 and 60 days) except S and P (Fig. 2), which could be elucidated through the fact that the added nutrients in the solution are adequately used by the plants for their growth and expansion and to cope up from the metal persuaded toxicity. S plays a pivotal role because sulfate transporters can mediate the entry of sulfate-analogs into the cells and S-containing complexes like glutathione and phytochelatins, which may amplify the tolerance of plants to metals through complexation.
The U concentration was increased upon concentration and duration dependent in the nutrient solution from root to shoot after 30 and 60 days, but the transfer of U from root to shoot is less (Fig. 3). With an experiment, Singh et al. (2005) hypothesized that uptake and dispersal of U generally vary from plant age and species. U accumulation mainly in the roots and less transfer to shoot was reported by various authors (Ramaswami et al. 2001; Vandenhove et al. 2006; Gupta et al. 2016b). For the usual plant growth and development, mineral nutrients (macro/micro) are very necessary; for the general plant metabolism, a limited amount of mineral nutrients is vital day to day. For the long-term experiments, a decrease of mineral nutrient was noticed due to extensive use in plant expansion and to cope up from the toxic element influence on growth.
Macro nutrients, such as Ca content in the root, were increased till 60 days of treatment, but in shoots, it was increased till 30 days of treatment in [U] = 100 nM concentration (Fig. 3). Oxidative stress and antioxidant system in plants have been shown to have some relations with Ca2+ and calmodulin (Huang et al. 2008). Ca and Zn ions are involved in stabilizing plasma membrane; Ca and Zn deficiency has been described in previous results of augmentation and may lead to membrane leakage of cell solutes (Marschner et al. 1997). Our results are in accordance with Smeets et al. (2008) following exposure to cadmium. Cations, like Ca and Mg, are commonly thought to alleviate toxicities of heavy metals through site-specific competition. Kopittke et al. (2011) hypothesized that Ca alleviated Pb toxicity through a specific effect in plants. Straczek et al. (2009) and Vanhoudt et al. (2011) also observed similar Ca concentration increments in Carrot and in Arabidopsis plant exposure to different U treatment.
K concentration decreases at the final harvest at 60 days [U] = 500 nM treatment in both root and shoots (Fig. 3). This was due to the U, which caused membrane damage that result in K leakage for the integrity of the cells. Hernandez et al. (1996) with Cd and pea plant, Gupta et al. (2016b) in pea plant, and Vanhoudht et al. (2011) in Arabidopsis also observe K decreases under U exposure. Mg concentration was also decreased in our plant samples after final harvest in roots (Fig. 3). It is also in accordance with Vanhoudht et al. (2011) and Gupta et al. (2016b) following exposure to U decreases Mg concentration in root at [U] = 100 and 50 μM treatment for 7 and 5 days, respectively. In another experiment, Huang et al. (2008) also notice that Mg concentration was reduced upon addition of [Pb] = 200 and 400 μM to Sedum alfredii plants for 21 days. Sulfur content was increased upon addition of U in tested plants after exposure of 30 and 60 days in both root and shoots (Fig. 3). It is in agreement with Gupta et al. (2013b) subsequent exposure to arsenic increases S in Arabidopsis wild-type plants upon addition of 25 and 50 μM As for 5 days. But Vanhoudt et al. (2011) got opposite trends upon addition of either U low or high (0.1–100 μM) concentration in Arabidopsis plants, i.e., decrease of S after 7 days of treatment. Gupta et al. (2016b) also got the opposite trend when pea plants were treated with [U] = 25 and 50 μM for 5 days. It is well known that S is an essential component of amino acids and proteins, and the shortage of S may cause injury to biomolecules.
Phosphorus concentration was increased till 30 days, and after that decreased in both the treatments at 60 days of exposure in both root and shoots, but higher than control (Fig. 3). One reason behind is that U binds phosphate to form uranyl phosphate complexes which are going to be the steadier and may form insoluble precipitates. It was in agreement with Vanhoudt et al. (2011) following the exposure of U to Arabidopsis plants for 7 days under various treatments. Smeets et al. (2008) also observed in 3-week-old Arabidopsis plants a lessening of P after Cd exposure for 1 day.
The absorption of Cu was reduced upon addition of higher U concentration in contrast to control in both root and shoots (Fig. 3). It is in agreement with Vanhoudt et al. (2011) following the contact of U decreases Cu content in both root and shoots after 1, 3, and 7 days in Arabidopsis plants; Gupta et al. (2016b) have the same understanding with pea plants treated with [U] = 25 and 50 μM for 5 days. Smith et al. (2009) described that in the leaf tissues, As was specially positioned in the leaf veins and co-located with Cu. Nevertheless, he stressed that the motive for the co-localization of Cu with As (or vice versa) is unknown at present. Fascinatingly, in the contemporary study, in both root and shoots, Cu concentration was reduced compared to control. The lessening in essential nutrient will decrease the plant strength and their aptitude to cope with (metal) stress; one of the conceivable explanations may be that Zn ions are intricate in alleviating plasma membrane (Huang et al. 2008). The pragmatic decline in the present experiment for Zn can also cause electron outflow due to plasma membrane deterioration (Fig. 3). Williams et al. (2009) institute that Zn content in rice grain considerably declined with cumulative As content, and the association between As in shoots and Zn in grains was sturdier than that between As and Zn in grains. Vanhoudt et al. (2011) also acquired the analogous type of result after 7 days in Arabidopsis plant exposure to [U] = 100 μM. To make accessible vital micronutrients like Fe, Mn, Zn, and Cu in metallic soils, roots also release metal chelators that liberate the metals from the soil particles and boost the movement and solubility of the same (Bertin et al. 2003; Lakshmanan et al. 2012).
The mature seeds of P. sativum plants have accumulated appreciable amount of metal which followed the order K > P > S > Mg > Ca > Zn > Cu > U (Fig. 4). Since the accumulation of U was considerably low (under detection limit 0.01 mg g−1) in the seed samples of [U] = 500 nM treatment, showing safe for human/animal consumption besides considerable accumulation of other essential metals like K, Mg, and P may be beneficial for consumption. Several metals such as Cu, Zn, and Mg are considered to be an important component of cells and are co-factor in several metalloenzymes in plants for plant growth and development. Comparable type of studies on accretion of heavy metal in seeds of Cicer arietinum has been conceded out by Gupta et al. (2006) and found that 10% fly-ash alterations with press mud for associate excellent growth of plants by keeping the level of toxic metals within a safe limit for human intake. Yang et al. (2011) assumed that surplus P reduced the circulation of Zn in grain, while Zn boosted the uptake of Zn and P in grain. The collective solicitation of Zn fertilizer with the wide use of P fertilizer can successfully increase the P and Zn concentration and Zn bioavailability of wheat grain, and hence Zn nutritional eminence. In a recent report by Singh et al. (2016), higher concentration of fly-ash amendments (50%) with soil may depreciate the soil quality, and the production of rice is affected by heavy metal which leads to contamination of toxic metals in the grain and are not safe for human intake.
Effect of uranium on accumulation of macro/micro elements in the seeds of Pisum sativum L. plants after ripening of pods (60 days). Data are the mean of three independent replicates ± SD. Mean values followed by different letters (b and bc) are significant difference at P < 0.05 and P < 0.01 by one-way ANOVA
Conclusion
The present study concludes that the P. sativum plants may be useful to grow at low radionuclide [U]-contaminated areas for safe human/animal use, but for other fission products, we have to investigate further for the safe consumption. In future studies, an experiment with contaminated soil should be carried out, in order to get information on soil-plant relationships and uranium uptake under actual growing conditions in the field.
References
Anderson D (2003) Introduction to heavy metal monitoring. European Environment Agency (EEA), environmental assessment report no. 10, “Europe’s environment: the 3rd assessment”, published on the web by EEA
Arnon DI (1949) Copper enzyme in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Bertin C, Yang XH, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256:67–83
Carini F, Bengtsson G (2001) Post-deposition transport of radionuclides in fruit. J Environ Radio 52:215–236
Chamel A, Gaillardon P, Gauvrit C (1991) La penetration foliaire des herbicides. Les herbicides: mode d’action et principes d’utilisation sous la direction de R. Scalla. Institut National DE LA RecherCHE Agronomique. 147, rue de l’Universite, 75007 PARIS, 8–49
Colle C, Madoz-Escande C, Leclerc E (2009) Foliar transfer into the biosphere: review of translocation factors to cereal grains. J Environ Radioact 100:683–689
Din KS, Harb S, Abbady A, Saad N (2010) The distribution of the radionuclides Ra-226, Th-232 and K-40 in various parts of the alfalfa plant. Tenth radiation physics and protection conference, 27-30 November, Nasr City-Cairo, Egypt. (web: http://www.rphysp.com/s7/distribution.pdf)
Duxbury AC, Yentsch CS (1956) Plantkton pigment monograph. J Mar Res 15:93–101
Ebbs SD, Brady DJ, Kochian LV (1998) Role of uranium speciation in the uptake and translocation of uranium by plants. J Exp Bot 49:1183–1190
EPA (1999) Understanding variation in partition coefficient Kd values volume II: review of geochemistry and available Kd values for cadmium, cesium, chromium, lead, plutonium, radon, strontium, thorium, tritium (3H) and uranium, US Environmental Protection Agency, report US EPA 402-R-99-004B, Washington DC
Evans KG (2000) Methods for assessing mine site rehabilitation design for erosion impact. Aust J Soil Res 38:231–248
Gupta DK, Tripathi RD, Rai UN, Dwivedi S, Srivastava S, Mishra S, Inouhe M (2006) Changes in amino acid profile and metal content in seeds of Cicer arietinum L. grown under various fly-ash amendments. Chemosphere 65:939–945
Gupta DK, Sandalio LM (2012) Metal toxicity in plants: perception, signaling and remediation. Springer, Germany
Gupta DK, Huang HG, Nicoloso FT, Schetinger MRC, Farias JG, Li TQ, Razafindrabe BHN, Aryal N, Inouhe M (2013a) Effect of hg, as and Pb on biomass production, photosynthetic rate, nutrients uptake and phytochelatin induction in Pfaffia glomerata. Ecotoxicology 22:1403–1412
Gupta DK, Inouhe M, Rodríguez-Serrano M, Romero-Puerta MC, Sandalio LM (2013b) Oxidative stress and arsenic toxicity: role of NADPH oxidases. Chemosphere 90:1987–1996
Gupta DK, Walther C (2014) Radionuclide contamination and remediation through plants. Springer, Germany
Gupta DK, Chatterjee S, Dutta S, Voronina AV, Walther C (2016a) Radionuclides: accumulation and transport in plants. Rev Environ Cont Toxicology 398:1–22
Gupta DK, Tawussi F, Hamann L, Walther C (2016b) Moderate uranium disturbs the nutritional status and induces oxidative stress in Pisum sativum L. J Plant Physiol Pathol 4:1
Gupta DK, Tawussi F, Holzer A, Hamann L, Walther C (2017) Investigation of low-level 242Pu contamination on nutrient disturbance and oxidative stress in Solanum Tuberosum L. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-9071-9
Hernandez LE, Carpena-Ruiz R, Garate A (1996) Alterations in the mineral nutrition of pea seedlings exposed to cadmium. J Plant Nutri 19:1581–1598
Huang H, Li TX, Tian S, Gupta DK, Zhang X, Yang XE (2008) Role of EDTA in alleviating lead toxicity in accumulator species of Sedum alfredii H. Bioresour Technol 99:6088–6096
Koch-Steindl H, Pröhl G (2001) Considerations on the behaviour of long-lived radionuclides in the soil. Radiat Environ Biophys 40:93–104
Kopittke PM, Kinraide TB, Wang P, Blarney FPC, Reichman SM, Menzies NW (2011) Alleviation of Cu and Pb rhizotoxicities in Cowpea (Vigna unguiculata) as related to ion activities at rootcell plasma membrane surface. Environ Sci Technol 45:4966–4973
Lakshmanan V, Kitto SL, Caplan JL, Hsueh YH, Kearns DB, YS W, Bais HP (2012) Microbe-associated molecular patterns-triggered root responses mediate beneficial rhizobacterial recruitment in Arabidopsis. Plant Physiol 160:1642–1661
Lauchli A (1972) Translocation of inorganic solutes. Annu Rev Plant Physiol 23:197–218
Lone MI, He ZH, Stoffella J, Yang X (2008) Phytoremediation of heavy metal polluted soils and water: progresses and perspectives. J Zhejiang Univ Sci B 9:210–220
Macek T, Mackova M, Kas J (2000) Exploitation of plants for the removal of organics in environmental remediation. Biotechnol Adv 18:23–34
Manara A (2012) Plant responses to heavy metal toxicity. In: Furini A (ed), Plants and heavy metals. Springer briefs in Biometals 27–53
Marschner H (1995) Mineral nutrition for higher plants. 2ndedn. Academic press and Harcourt brace and co. publishers London-San Diego-New York-Boston-Sydney-Tokyo-Toronto
Marschner H, Kirkby EA, Engels C (1997) Importance of cycling and recycling of mineral nutrients within plants for growth and development. Bot Acta 110:265–273
Padmavathiamma PK, Li LY (2007) Phytoremediation technology: hyper-accumulation metals in plants. Water Air Soil Pollut 184:105–126
Pallett KE, Young AJ (1993) Carotenoids. In: Alscher RG, Hess JL (eds) Antioxidants in higher plants. CRC Press, 60–81
Ramaswami A, Carr P, Burkhardt M (2001) Plant-uptake of uranium: hydroponic and soil system studies. Int J Phytorem 3:189–201
Rebechini HM, Hanzely L (1974) Lead-induced ultrastructural changes in chloroplasts of the hydrophyte Ceratophyllum demersum. Z Pflanzenphysiol 73:377–386
Salt DE, Kato N, Kräme U, Smith RD, Raskin I (2000) The role of root exudates in nickel hyperaccumulation and tolerance in accumulator and nonaccumulator species of Thlaspi. In: Terry N, Banuelos G (eds) Phytoremediation of contaminated soil and water. CRC Press LLC, Boca Raton
Schnoor JL, Licht LA, McCutcheon SC, Wolfe NL, Carreira LH (1995) Phytoremediation of organic and nutrient contaminants. Environ Sci Technol 29:318–323
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17:35–52
Sheppard S, Evenden W (1987) Review of effects of soil on radionuclide uptake by plants. Research report prepared for the atomic energy control board, Ottawa, Canada, march 31, INFO-0230
Singh PK, Tripathi P, Dwivedi S, Awasthi S, Shri M, Chakrabarty D, Tripathi RD (2016) Fly-ash augmented soil enhances heavy metal accumulation and phytotoxicity in rice (Oryza sativa L.); a concern for fly-ash amendments in agriculture sector. Plant Growth Regul 78:21–30
Singh S, Malhotra R, Bajwa BS (2005) Uranium uptake studies in some plants. Radiat Meas 40:666–669
Smeets K, Ruytinx J, Semane B, Van Belleghem F, Remans T, Van Sanden S, Vangronsveld J, Cuypers A (2008) Cadmium-induced transcriptional and enzymatic alterations related to oxidative stress. Environ Exp Bot 63:1–8
Smith E, Kempson I, Juhasz AL, Weber J, Skinner WM, Grafe M (2009) Localization and speciation of arsenic and trace elements in rice tissues. Chemosphere 76:529–535
Straczek A, Wannijn J, Van Hees M, Thijs H, Thiry Y (2009) Tolerance of hairy roots of carrots to U chronic exposure in a standardized in vitro device. Environ Exp Bot 65:82–89
Tanyolac D, Ekmekci Y, Unalan S (2007) Changes in photochemical and antioxidant enzyme activities in maize (Zea maize L.) leaves exposed to excess copper. Chemosphere 67:89–98
Vandenhove H, Cuypers A, Van Hees M, Koppen G, Wannijn J (2006) Oxidative stress reactions induced in beans (Phaseolus vulgaris) following exposure to uranium. Plant Physiol Biochem 44:795–805
Vanhoudt N, Vandenhove H, Horemans N, Bello DM, Van Hees M, Wannijn J, Career R, Vangronsveld J, Cuypers A (2011) Uranium induced effects on development and nutrition of Arabidopsis thaliana. J Plant Nutr 34:1940–1956
Vazquez MD, Poschenrieder C, Barcelo J (1987) Chromium (VI) induced structural and ultra-structural changes in bush bean plants (Phaseolus vulgaris). Ann Bot 59:427–438
Voronina AV, Blinova MO, Semenischev VS, Gupta DK (2015) Returning lands, contaminated as a result of radiation accidents, to farming use. J Environ Radioact 144:103–112
Walther C, Gupta DK (2015) Chemistry of radionuclides in the environment: influence of chemical speciation and plant uptake on radionuclide migration. Springer, Germany
Williams PN, Islam S, Islam R, Jahiruddin M, Adomako E, Soliaman AR, Rahman GK, Lu Y, Deacon C, Zhu YG, Meharg AA (2009) Arsenic limits trace mineral nutrition (selenium, zinc, nickel) in Bangladesh rice grain. Environ Sci Technol 43:8430–8436
Yang XW, Tian XH, XC L, Cao YX, Chen ZH (2011) Impacts of phosphorus and zinc levels on phosphorus and zinc nutrition and phytic acid concentration in wheat (Triticum aestivum L.) J Sci Food Agric 91:2322–2328
Acknowledgements
FT is thankful to BMBF (funding 15S9082A) for providing fellowship for the Ph. D. studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Georg Steinhauser
Highlights
1. Pisum sativum L. plants are useful to grow at low uranium-contaminated areas for safe human/animal use.
2. No observation of uranium content in the above ground parts (shoots/seeds) of the plants.
3. In seeds, micro/macro elements were under limit.
4. Uranium concentration was also under detection limit.
Electronic supplementary material
Supplementary Figure 1
(DOCX 637 kb)
Rights and permissions
About this article
Cite this article
Tawussi, F., Walther, C. & Gupta, D.K. Does low uranium concentration generates phytotoxic symptoms in Pisum sativum L. in nutrient medium?. Environ Sci Pollut Res 24, 22741–22751 (2017). https://doi.org/10.1007/s11356-017-0056-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0056-5