Abstract
Biochar application to soil is currently widely advocated for a variety of reasons related to sustainability. However, the synergistic effects of biochar combined with mineral or organic fertilizer on soil N2O emissions, NH3 volatilization, and plant N uptake are poorly documented. Field plot experiments planted with peanut were conducted under the application of biochar (derived from rice husk and cottonseed husk, 50 t ha−1) with organic or mineral fertilizer. It was found that biochar increased soil nutrient availability and decreased surface soil bulk density, demonstrating that biochar could improve the soil quality especially in the 0–20-cm profile. The total N content of the plant changed little with treatments, but the kernel N concentration increased significantly when biochar was applied with organic fertilizer. Peanut yield increased with biochar amendment while no significant difference was observed in plant biomass, suggesting biochar had a positive effect on belowground biomass. Peanut N uptake was also increased following biochar amendment with either organic or mineral fertilizers. While biochar amendment had no significant effect on soil NH3 volatilization, it did decrease the cumulative N2O emission by 36.3% on average with organic fertilizer, and by 32.6% with mineral fertilizer, respectively (p < 0.05). The copy numbers of 16S rDNA, nifH, nirK, and nirS were not influenced by the application of biochar; however, the copy number of nosZ was significantly increased under biochar plus mineral fertilizer treatment. The results imply that biochar application can suppress N2O emissions, as a result of abiotic factors and enhanced peanut N uptake rather than changes of denitrification genes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Managing nitrogen (N) dynamics has received much attention in agricultural systems due to the increased application of N fertilizer. According to Liu et al. (2010), only 55% of the global applied N is taken up by crops while 14% is lost in gaseous emissions. Nitrous oxide (N2O) emissions and ammonia (NH3) volatilization are two of the main pathways for gaseous N losses from agricultural system, and result from excessive fertilizer. N2O is a potential greenhouse gas (GHG) that has a global warming potential 298 times that of carbon dioxide (CO2) over a 100-year time period (Davidson 2009). Agricultural land contributes approximately 60% to global anthropogenic N2O emissions (Reay et al. 2012). NH3 volatilization can contribute to the formation of atmospheric aerosol, and acidification and eutrophication of water systems (Howarth et al. 2002). It is also an indirect source of N2O and NO (Mosier et al. 1998). The loss of N through gaseous emissions (N2O, NH3 and N2) from soil decreases the amount of available N for crop growth and causes environmental degradation.
Biochar is a solid carbon-rich organic material generated by pyrolysing biomass. Biochar is typically alkaline, highly porous, with considerable amount of functional groups and large specific surface area (Tan et al. 2016). As a soil amendment, biochar can potentially increase soil N retention through several mechanisms: adsorption of NO3−, NH4+ and organic N, increase biological N fixation and change microbial community dynamics related to N transformation (Rondon et al. 2006; Kammann et al. 2017). Applying biochar with N fertilizers in agricultural ecosystem may be an effective method to increase soil C storage and enhance N use efficiency thereby reducing environmental impacts and increase crop productivity.
N2O emissions can be produced from three main processes in soil: nitrification, denitrification and dissimilatory nitrate reduction to ammonia (Baggs 2011). Both soil characteristics (texture, pH, aeration, C:N ratio and microbial diversity) and prevailing environmental conditions (temperature and rainfall) influence the contribution of each process to the total N2O emission flux (Cayuela et al. 2014). Reductions in soil N2O emissions after biochar application are widely reported (Case et al. 2012; Xu et al. 2014; Case et al. 2015), for which several mechanisms have been proposed: (i) biochar improves soil aeration thus reduces denitrification (Yanai et al. 2007); (ii) biochar liming effect could drive denitrification thorough to N2 (Castaldi et al. 2011); (iii) biochar increases the adsorption of NO3−, thus decreases substrate availability for denitrification (Mukherjee et al. 2014); (iv) biochar reduces the availability of organic C for soil microorganisms through sorption, thus decreasing the formation of N2O (Cayuela et al. 2014); (v) biochar could sorb N2O directly (Cornelissen et al. 2013); (vi) biochar participates in abiotic reduction of nitrate/nitrite to N2 (Thomazini et al. 2015); and (vii) biochar releases inhibitory or toxic compounds that inhibit nitrification (Freddo et al. 2012) thereby decreasing the formation of N2O. Most of these studies were conducted in the laboratory. More studies are needed to elucidate the effects of biochar when applied with fertilizers under field conditions. Furthermore, little information is available about the effects of biochar on microbial functional genes related to N2O emissions.
NH3 volatilization occurs when ammonium-N in fertilizer or solution is converted to dissolved ammonia gas at neutral to alkaline pH (Rochette et al. 2013). Soil NH3 volatilization is influenced by the combined effects of various factors including pH, aeration, N sources, moisture content and temperature (Mandal et al. 2016). Contradictory results have been reported about the effect of biochar on soil NH3 volatilization. Doydora et al. (2011) demonstrated that soil amended with acidified biochar could reduce NH3 volatilization from poultry litter. Taghizadeh-Toosi et al. (2012) showed that NH3 volatilization was reduced by 45% from ruminant urine when biochar had been incorporated in the soil. Similarly, Mandal et al. (2016) also reported that soil NH3 volatilization was reduced by approximately 70% under biochar amendment with fertilizer in an incubation experiment. However, Jones et al. (2012b) demonstrated that biochar had no significant effect on soil NH3 volatilization or soil NH4+ concentrations. Sun et al. (2014) reported that NH3 volatilization increased after applying biochar to an agricultural soil in an incubation experiment. Still, more studies are needed regarding to the impact of biochar amendment with fertilizer on NH3 volatilization in agricultural soil, especially under in situ conditions. In addition, the synergistic effects of biochar on soil N2O emissions, NH3 volatilization and plant N uptake are poorly documented.
In the present study, two biochars (derived from rice husk or cottonseed husk, 50 t ha−1) were applied in combination with organic or mineral fertilizer to a fluvo-aquic soil planted with peanut during field plot experiments. The objectives of the present study were as follows: (i) to study the effects of biochar on soil physiochemical properties and peanut yield; (ii) to investigate the effects of biochar on soil NH3 volatilization, N2O emissions and crop N uptake; and (iii) to determine the mechanisms for the effect of biochar amendment on N2O emissions in fluvo-aquic soil. In particular, quantitative real-time PCR was employed to quantify the copy numbers of soil N-fixing gene marker (nifH), nitrification functional markers (archaeal amoA and bacterial amoA) and denitrifying bacterial gene markers (nirS, nirK, and nosZ). We hypothesized that biochar amendment would improve peanut yield, mitigate greenhouse gas emissions and enhance peanut N uptake.
Materials and methods
Biochar characterization
Rice husk biochar (RH biochar) and cottonseed husk biochar (CH biochar) used in this experiment were supplied by the Sanli New Energy Company (Tianjin, China). The biochars were produced from rice husk (Oryza sativa L.) and cottonseed husk (Gossypium spp.) through pyrolyzation at 450 °C for 4.5 h in a vertical kiln made of refractory materials. The important characteristics of the biochars are provided in Table 1. Biochar pH was measured using a pH meter (Mettler Toledo Delta 320) with a biochar to deionized water ratio of 1:30 w/w, after being stirred for 1.5 min and equilibrated for 1 h. The cation exchange capacity (CEC) of the biochars were measured by a modified NH4+-acetate compulsory displacement method (Gai et al. 2014). The elemental composition (C, H, N, S) of the biochars were measured using an elemental analyzer (vario PYRO cube, Germany). Brunauer-Emmett-Teller (BET) specific surface area (SSA) of the biochars were determined using N2 gas on a Micrometrics ASAP 2010 system (Micrometrics, Norcross, GA, USA). Ash content was determined by combusting biochar at 750 °C for 6 h in open crucibles, on a dry weight basis. Fourier transform infrared (FTIR) spectra were recorded using a Thermo Nicolet 6700 FTIR spectrometer (Thermo Fisher, America) that was equipped with a TGS/PE detector and a silicon beam splitter with 4 cm−1 resolution. The infrared spectra were obtained in the range of 4000–400 cm−1 as shown in Fig. S1.
Site description and experimental design
The experiment was conducted from June to October, 2015 at the fluvo-aquic soil (Haplic Luvisol soil according to the Food and Agriculture Organization Soil Classification 2006) agricultural experimental station of Chinese Academy of Agricultural Sciences, Changping County, Beijing, China (40° 13′ N, 116° 14′ E, and 43.5 m above sea level). This area has a typical sub-humid temperate continental monsoon climate, with mean annual rainfall of 625 mm, mean annual temperature of 11.5 °C, featuring four distinct seasons. The main physicochemical characteristics of the soil are summarized in Table 1. Daily precipitation and the maximum and minimum air temperatures during the peanut growing season were recorded (Fig. S2).
Experimental units consisted of 10 m2 plots (each 2 m × 5 m) with seven different treatments (each in three replicates), resulting in a total of 21 plots. Plots were arranged in the field following a randomized complete block design. In each plot, the biochars were applied manually (50 t ha−1) and incorporated into the top 20 cm of soil. The application rate of organic fertilizer (chicken manure, Beijing Shiji Dade Environmental Protection Technology Co., Ltd., China) was 9 t ha−1. The application rate of mineral fertilizers was calculated to obtain the same level of nutrient content (NPK) as applied in the organic fertilizer. Mineral fertilizers applied in a dry form included CO(NH2)2 (urea, 0.89 t ha−1), P2O5 (0.34 t ha−1) and K2O (0.23 t ha−1). As a result, all the treatments were added with the same amount of nutrients except for the control (CK). Fertilizer was added in a single spring application before primary tillage, with surface broadcasting of fertilizers. Seven different treatments were included in the field plot experiments: CK, organic fertilizer only (OM), mineral fertilizer only (NPK), organic fertilizer plus RH biochar (ROM), organic fertilizer plus CH biochar (COM), mineral fertilizer plus RH biochar (RNPK), and mineral fertilizer plus CH biochar (CNPK). Plots were sown after application of the fertilizers and biochars on June 1. Peanut (Arachis hypogaea L.) was sown in each experimental plot; plots were overplanted and thinned to obtain a final density of approximately 75,000 plants ha−1. Plots were hand-weeded throughout the experiment. No pesticides or fungicides were applied and no irrigation water was supplied to the plants.
Soil and crop sampling and chemical analyses
Composite samples of bulk soil were collected with an Eijkelkamp soil core sampler from each plot after peanut harvest on October 7, 2015. Soil samples were immediately transported to the laboratory where they were sieved (2 mm) and processed for subsequent analyses. Soil samples were either: (1) dried at 60 °C until constant weight for determination of total nitrogen (TN), total phosphorus (TP), and total potassium (TK); (2) stored at 4 °C for the other physicochemical, biochemical, and microbial analyses; or (3) immediately frozen in liquid N2 and stored at − 80 °C until used for DNA extraction. All plants were harvested to calculate the aboveground biomass (plant biomass) and kernel weight (peanut yield). Aboveground biomass was oven-dried at 70 °C for 48 h before recording the plant biomass for the total plot. The kernels were air-dried for 1 week before recording the peanut yield for the total plot. One dried subsample of plant and kernel was selected at random and ground for determination of TN content using a Leco TruMac CNS Analyzer (LECO Corporation, USA). Plant N uptake was calculated using plant biomass multiplied by the TN of plant, and peanut N uptake was calculated using peanut yield multiplied by the TN of kernel. Except for those specified above, all the soil and biochar physicochemical properties were measured using the methods reported by Margesin and Schinner (2005), Xu et al. (2016) and Gai et al. (2016).
Measurements of NH3 volatilization
The NH3 volatilization flux was measured with a continuous airflow enclosure method using a chamber in each plot as described by Chen et al. (2015). The NH3 volatilization flux was measured in the morning daily, from 8:00 to 10:00 am after fertilization. A gas blanketing bottle filled with 60 ml of 10 g L−1 H3BO3 was used to absorb the ammonia gas. The air was pushed to flow through the 10 g L−1 H3BO3 for each treatment. Then the ammonia absorbents were brought to the laboratory immediately and titrated with diluted sulphuric acid (0.01 M). The daily NH3 volatilization flux was calculated as the average of the fluxes measured on each day.
Measurements of N2O emissions
The in situ N2O fluxes were measured using the closed chamber method (Pereira et al. 2015). The chambers consisted of a permanent base (bottom part) and a removable lid with a rubber septum for gas sampling, both made of polycarbonate engineering plastics. The lid was a cylindrical flux chamber of 30 cm in diameter and 60 cm in height. The permanent base was 15 cm in height with the same diameter as the lid. The base was inserted 10 cm into the soil immediately after fertilization to minimize the effects of disturbance. When the lid was closed, it was secured tightly to the base by a gas-tight rubber seal (8 cm wide). At sampling time, gas samples (50 ml) were drawn from the chambers with gas-tight syringes and stored in evacuated glass tubes at 0, 20, and 40 min after the chambers were closed. Samples were taken every day in the first 2 weeks after fertilization and then once every week over the 126 days growth period. The gas samples were analyzed for N2O concentration by electron capture gas chromatography (GC-2010 plus Shimadzu Gas Chromatograph, Kyoto, Japan). Standard N2O was used for calibration, and flux rates were calculated with the linear regression method.
Quantification of functional gene abundance
DNA was extracted from 0.5 g of soil using the FastDNA SPIN kit for Soil (MP Biomedicals, USA) according to the manufacturer’s instructions. The total amount of DNA was quantified using a NanoDrop ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies, USA) and stored at − 80 °C. Quantitative PCR (qPCR) was performed to assess the abundance of the following genes: 16S rRNA gene for total bacteria, amoA gene for archaeal ammonia oxidizers (AOA) and bacterial ammonia oxidizers (AOB), nifH (N fixation gene), nirK and nirS (nitrite reductase genes), and nosZ (nitrous oxide reductase gene). All qPCR reactions were conducted using a Bio-Rad CFX1000 Thermal Cycler (Bio-Rad, USA). Standard curves were obtained using 10-fold serial dilutions of plasmid DNA containing cloned genes of interest and spanning seven orders of magnitude. Single qPCR reaction was prepared in a total volume of 20 ml including 10 ml SYBR green PCR Master Mix (Takara SYBR Premix Ex Taq (perfect real time)), 0.4 ml of forward and reverse primers (10 mM), and approximately 5 ng of DNA. Melting curves and agarose gel running of PCR products were used at the end of each qPCR reaction to check amplification specificity and purity of negative controls. Specific primer combinations and thermal conditions were described in Table S1. Each PCR run included triplicate sample templates, calibration standard series and no template controls. The presence of PCR inhibitors in DNA extracted from soil was estimated by a 1:10 soil DNA dilution, and no inhibition was detected.
Statistical analysis
The soil physicochemical and N related results were expressed as means ± standard deviations. Statistical and correction analyses were performed using Statistical Product and Service Solutions 22.0 (SPSS Inc., Chicago, IL, USA). Significant differences were obtained by the one-way analysis of variance (ANOVA) with means compared using the Duncan’s multiple range test. The correlation was analyzed with the Pearson test (two-tailed) at p = 0.05. Any differences between the mean values at p < 0.05 were considered statistically significant.
Results
Soil physicochemical properties and peanut yield
Physiochemical properties of soil under different treatments were given in Table 2. Soil moisture content and pH both increased with biochar amendment (p < 0.05), while soil bulk density decreased (p < 0.05). Biochar amendment increased soil organic carbon (SOC) irrespective of organic or mineral fertilizer (p < 0.05). Neither soil CEC nor TP were influenced by treatments. Soil Olsen-P was significantly increased under CH biochar amendment compared to no biochar amendment with either organic or mineral fertilizer (p < 0.05), while the increase was not significant for RH biochar. Compared to the CK, soil TK was increased under the CNPK treatment (p < 0.05), while increasing only slightly under the other treatments. Soil available-K was increased with biochar amendment irrespective of organic or mineral fertilizer (p < 0.05). In addition, soil available-K increased with CH biochar amendment when compared with RH biochar (p < 0.05).
Plant biomass and peanut yield are shown in Fig. 1. Without biochar amendment, an increase in plant biomass, by 42.4 and 48.4% under OM (p > 0.05) and NPK treatments (p < 0.05), respectively, was observed when compared to CK (6.94 t ha−1). There were no significant differences in plant biomass due to biochar amendment. Without biochar amendment, increases in peanut yield, of 24.6 and 32.5% under OM and NPK treatments, respectively, were observed when compared to the CK (3.42 t ha−1) (p < 0.05). With biochar amendment, the peanut yield increased further, by 27.2 and 25.6% under ROM and COM treatments when compared to the OM (4.26 t ha−1), and by 16.8 and 14.4% under RNPK and CNPK treatments when compared to NPK (4.53 t ha−1), respectively (p < 0.05).
Plant biomass (a), peanut yield (b), plant N uptake (c), and peanut N uptake (d) under different treatments. (CK, the control; OM, organic fertilizer only; NPK, mineral fertilizer only; ROM, organic fertilizer plus rice husk biochar; COM, organic fertilizer plus cottonseed husk biochar; RNPK, mineral fertilizer plus rice husk biochar; CNPK, mineral fertilizer plus cottonseed husk biochar)
Soil N fractions, total N of peanut, and peanut N uptake
Soil N fractions and TN of peanut were shown in Table 3. In 0–20 cm profile, soil TN, NO3−, and NH4+ all increased with biochar amendment when compared to the mineral fertilizer treatment (p < 0.05); however, no obvious change was observed with biochar amendment when compared to the organic fertilizer treatment (p > 0.05). In the 20–40 cm profile, soil TN, NO3− and NH4+ were not affected by biochar or fertilizer treatments. There were no significant differences among CK, OM, and NPK treatments in the TN of plant or that of kernel. However, compared to CK (33.31 g kg1), the TN of kernel was significantly increased by 26.4 and 23.9% under COM and ROM, respectively (p < 0.05).
Plant N uptake and peanut N uptake are shown in Fig. 1. Compared to CK, plant N uptake was significantly increased under OM and NPK treatments. While there was no significant change in plant N uptake under biochar amendment when compared with either OM or NPK. However, peanut N uptake increased by 42.6 and 44.9% under ROM and COM treatments compared to OM (157.49 kg ha−1), and by 20.7 and 17.6% under RNPK and CNPK compared to NPK (167.38 kg ha−1), respectively (p < 0.05). In addition, when compared to CK, the peanut N uptake was significantly increased by 34.2 and 42.6% under OM and NPK, respectively (p < 0.05).
Soil NH3 volatilization and N2O emissions
Soil NH3 volatilization decreased rapidly after the mineral fertilizers were applied (Fig. S3). The cumulative NH3 volatilization over the first 4 days accounted for 67.4–68.5% of the total cumulative NH3 volatilization under mineral fertilizer treatments. Compared to the CK (1.38 N kg ha−1), the cumulative NH3 volatilization for NPK increased by 22.5 times (p < 0.05) according to Table 3. However, the NH3 volatilizations were minor for organic fertilizer treatments and not significantly different from that for CK. The application of biochar did not alter NH3 volatilization, either with mineral or organic fertilizers. There was no statistical difference in NH3 volatilization from OM, ROM, and COM treatments, or among those from NPK, RNPK, and CNPK treatments.
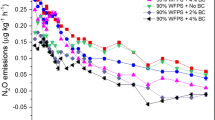
The N2O emissions (Fig. S4) throughout the whole peanut growing season were influenced by the prevailing environmental conditions such as temperature and rainfall (Fig. S2). In particular, N2O efflux declined for all treatments after September 1 as the temperature dropped. During the first half month, N2O efflux was higher under the mineral fertilizer treatments than others. N2O efflux ranged from 5.2 to 120.7 μg N m−2 h−1 and 2.2 to 65.1 μg N m−2 h−1 for mineral fertilizer treatments and organic fertilizer treatments, respectively. The N2O efflux from the control remained relatively stable and varied over the range of 2.3–16.2 μg N m−2 h−1. The cumulative N2O emissions decreased by 37.3 and 35.3% under ROM and COM treatments compared to OM (0.51 N kg ha−1), and by 33.7 and 31.5% under RNPK and CNPK treatments compared to NPK (0.89 N kg ha−1), respectively (p < 0.05). The NPK treatment recorded the highest cumulative N2O emission, 3.2 times higher than CK (0.21 N kg ha−1) and 75% higher than OM.
Abundance of functional genes involved in N cycle
Microbial gene copy numbers did not differ among treatments for nifH, nirK, and nirS (Table 4). Similarly, the copy numbers of AOB and AOA were not influenced by biochar amendment compared to no biochar treatment with either organic or mineral fertilizer (p > 0.05). However, biochar amendment with fertilizers increased the copy numbers of AOB and AOA compared to the control (p < 0.05). The copy numbers of nosZ increased under RNPK (6.51 × 104 copies g−1 dry soil) and CNPK (6.47 × 104 copies g−1 dry soil) compared to NPK (5.28 × 104 copies g−1 dry soil) (p < 0.05), but showed no obvious difference among ROM, COM, and OM.
Discussion
Biochar effect on peanut yield and total N of peanut
In accordance with previous reports (Agegnehu et al. 2015; Xu et al. 2015a), biochar significantly increased peanut yield. This result was mainly attributed to increased nutrient availability, such as SOC, Olsen-P and available-K, and to improved soil physical properties indicated by decreased soil bulk density and increased soil moisture content. As shown in Table 1 and Fig. S1, both RH biochar and CH biochar had large surface areas and quantity of functional groups which could potentially retain soil nutrients (Darby et al. 2016). The fact that peanut yield increased under biochar amendment, while plant biomass was not affected, suggests that biochar had a more positive effect on the belowground biomass.
The fact that both Olsen P and available-K increased under biochar treatments could contribute to improved TN of kernel as P is essential for the formation, development and function of nodules (Xu et al. 2015b) and K is essential for biological N2 fixation and enhanced competitive ability of legume species (Xu et al. 2015a). In addition, the application of organic fertilizer could input trace elements into the soil, such as molybdenum and boron which are essential for the growth of N-fixing microorganisms (Fageria et al. 2010). This could be one reason for the significant increase in the TN of kernel under ROM and COM treatments. Soil available-K was significantly improved under CH biochar amendments irrespective of organic fertilizer or mineral fertilizer compared to RH biochar amendments. This could be due to the relatively higher TK of CH biochar which was more than three times that of RH biochar. Similarly, soil Olsen-P was only significantly increased under CH biochar amendment irrespective of organic or mineral fertilizer. These results suggested that the changes of soil characteristics depended on the type of the biochar.
Biochar effect on soil N fraction and NH3 volatilization
Compared to CK, biochar significantly increased soil TN, NO3−, and NH4+ in 0–20 cm profile while no obvious changes were observed in 20–40 cm profile. As biochar was manually applied in the top 20 cm soil, this result indicated that biochar could immobilize soil N through adsorption directly.
The addition of mineral fertilizers significantly increased NH3 volatilization regardless of biochar application, which was predominantly due to the elevated NH4+ concentration in soil after the application of urea. The NH3 volatilization from organic fertilizer treated soils was very low, due to the slow mineralization of N from organic fertilizer (Mandal et al. 2016). No significant changes in NH3 volatilization were observed after biochar incorporation with either organic or mineral fertilizer, which were similar with (Jones et al. 2012a) who reported that biochar did not affect NH3 volatilization in situ. This outcome could be attributed to the balance of the contradictory effects of biochar on soil NH3 volatilization. The biochar alkalinity increased soil pH, and biochar porosity enhanced soil aeration, which were both beneficial to NH3 volatilization (Sun et al. 2014). On the contrary, biochar has the potential to adsorb both NH4+ and NH3 due to its large surface areas and the presence of various functional groups, which was unfavorable for NH3 volatilization (Mandal et al. 2016). As a result, soil NH3 volatilization was not influenced by biochar amendment.
Biochar effect on soil N2O emissions and peanut N uptake
Similar to soil NH3 volatilization, applying fertilizers only increased N2O emissions, which was due to higher N availability in those treatments compared to CK. However, biochar amendment significantly decreased N2O emissions under fertilizer treatments. The enhanced expression of nosZ under RNPK and CNPK compared to NPK suggested that biochar could mitigate N2O emissions by further reducing it to N2. Similarly, both Xu et al. (2014) and Harter et al. (2014) reported that biochar increased the copy number of NosZ while having no significant effect on nirK and nirS. Nonetheless, the copy number of nosZ was not significantly different among ROM, COM, and OM. On the other hand, soil pH increased under biochar amendment which could potentially favor the activity of N2O reductase from denitrifying microorganisms while inhibit the activity of reductases involved in the conversion of nitrite and nitrate to N2O (Yanai et al. 2007). Soil aeration improved as bulk density decreased which would also tend to limit the activity of denitrifiers in soil amended with biochar (Zhang et al. 2011), thereby decrease N2O emission. In addition, higher C:N ratio under biochar treatments could result in greater immobilization instead of mineralization (Ly et al. 2014) thus also inhibit N loss through N2O emission. These results indicated that denitrification gene abundances only play a partial role in the mitigation of N2O emissions in comparison with soil environmental factors. The copy number of nifH was not influenced by biochar amendment. However, it must be born in mind that this result was obtained from the bulk soil. Similar to Agegnehu et al. (2015), there was a significant increase in nodule number and size of peanut root under biochar amendment in the present study (data not shown). As the nodule number and size for leguminous plants were directly related to the ability of biological N2 fixation (Güereña et al. 2015), it was reasonable to believe that biochar enhanced the N2 fixation of peanut. Similarly, Rondon et al. (2006) demonstrated that biological N2 fixation by common beans increased under biochar addition. Compared to CK, the copy numbers of AOB and AOA significantly increased under biochar application implying that biochar could enhance microbial N transformation thereby improve plant N uptake and decrease N2O emissions.
Biochar amendment increased the peanut N uptake by 43.7% on average with organic fertilizer, and by 19.1% with mineral fertilizer, respectively (p < 0.05). Besides, plant N uptake was also slightly enhanced under biochar amendment. These results were similar with (Zhang et al. 2011) and (Pereira et al. 2015) which reported that N uptake was significantly enhanced under biochar amendment planted with maize and lettuce, respectively. In summary, as shown in Fig. 2, despite the insignificant effect on soil NH3 volatilization, biochar could adsorb soil NO3− and NH4+, decrease N2O emissions and increase the copy number of amoA and nosZ. Along with the improvement in soil nutrient pool, peanut yield, and N uptake were both enhanced under biochar amendment.
Conclusions
The present study investigated changes in soil properties, N2O emissions and the abundance of its related genes, NH3 volatilization, and peanut N uptake following biochar amendment with fertilizers in a field experiment planted with peanut. Soil physicochemical properties and nutrition availability were improved with the application of biochar. Compared to aboveground biomass (plant biomass), biochar had stronger positive effect on belowground biomass (peanut yield). Biochar had no influence on plant N content, but significantly increased kernel N content under the application with organic fertilizer. Although soil NH3 volatilization was not influenced by biochar, biochar application with fertilizers (mineral or organic) significantly mitigated N2O emissions in agricultural soil. The suppression of soil N2O emissions may be mainly due to abiotic factors (soil aeration, pH, and C:N) and peanut N uptake. Biochar addition could modulate soil N transformation and enhance peanut N uptake, resulting in increment in crop yield.
References
Agegnehu G, Bass AM, Nelson PN, Muirhead B, Wright G, Bird MI (2015) Biochar and biochar-compost as soil amendments: effects on peanut yield, soil properties and greenhouse gas emissions in tropical North Queensland, Australia. Agric Ecosyst Environ 213:72–85. https://doi.org/10.1016/j.agee.2015.07.027
Baggs EM (2011) Soil microbial sources of nitrous oxide: recent advances in knowledge, emerging challenges and future direction. Curr Opin Env Sust 3(5):321–327. https://doi.org/10.1016/j.cosust.2011.08.011
Case SDC, McNamara NP, Reay DS, Whitaker J (2012) The effect of biochar addition on N2O and CO2 emissions from a sandy loam soil – the role of soil aeration. Soil Biol Bioch 51:125–134. https://doi.org/10.1016/j.soilbio.2012.03.017
Case SDC, McNamara NP, Reay DS, Stott AW, Grant HK, Whitaker J (2015) Biochar suppresses N2O emissions while maintaining N availability in a sandy loam soil. Soil Biol Bioch 81:178–185. https://doi.org/10.1016/j.soilbio.2014.11.012
Castaldi S, Riondino M, Baronti S, Esposito FR, Marzaioli R, Rutigliano FA, Vaccari FP, Miglietta F (2011) Impact of biochar application to a Mediterranean wheat crop on soil microbial activity and greenhouse gas fluxes. Chemosphere 85(9):1464–1471. https://doi.org/10.1016/j.chemosphere.2011.08.031
Cayuela ML, van Zwieten L, Singh BP, Jeffery S, Roig A, Sánchez-Monedero MA (2014) Biochar's role in mitigating soil nitrous oxide emissions: a review and meta-analysis. Agric Ecosyst Environ 191:5–16. https://doi.org/10.1016/j.agee.2013.10.009
Chen A, Lei B, Hu W, Lu Y, Mao Y, Duan Z, Shi Z (2015) Characteristics of ammonia volatilization on rice grown under different nitrogen application rates and its quantitative predictions in Erhai Lake Watershed, China. Nutr Cycl Agroecosys 101(1):139–152. https://doi.org/10.1007/s10705-014-9660-7
Cornelissen G, Rutherford DW, Arp HP, Dörsch P, Kelly CN, Rostad CE (2013) Sorption of pure N2O to biochars and other organic and inorganic materials under anhydrous conditions. Environ Sci Tec 47(14):7704–7712. https://doi.org/10.1021/es400676q
Darby I, CY X, Wallace HM, Joseph S, Pace B, Bai SH (2016) Short-term dynamics of carbon and nitrogen using compost, compost-biochar mixture and organo-mineral biochar. Environ Sci Pollut Res 23(11):11267–11278. https://doi.org/10.1007/s11356-016-6336-7
Davidson EA (2009) The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat Geosci 2(9):659–662. https://doi.org/10.1038/ngeo608
Doydora SA, Cabrera ML, Das KC, Gaskin JW, Sonon LS, Miller WP (2011) Release of nitrogen and phosphorus from poultry litter amended with acidified biochar. Int J Environ Res Public Health 8:1491–1502
Fageria NK, Baligar VC, Jones CA (2010) Growth and mineral nutrition of field crops. CRC Press. https://doi.org/10.1201/b10160
Freddo A, Cai C, Reid BJ (2012) Environmental contextualisation of potential toxic elements and polycyclic aromatic hydrocarbons in biochar. Environ Pollut 171:18–24. https://doi.org/10.1016/j.envpol.2012.07.009
Gai X, Wang H, Liu J, Zhai L, Liu S, Ren T, Liu H (2014) Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS One 9(12):e113888. https://doi.org/10.1371/journal.pone.0113888
Gai X, Liu H, Zhai L, Tan G, Liu J, Ren T, Wang H (2016) Vegetable yields and soil biochemical properties as influenced by fertilization in Southern China. Appl Soil Ecol 107:170–181. https://doi.org/10.1016/j.apsoil.2016.06.001
Güereña DT, Lehmann J, Thies JE, Enders A, Karanja N, Neufeldt H (2015) Partitioning the contributions of biochar properties to enhanced biological nitrogen fixation in common bean (Phaseolus vulgaris). Biol Fert Soil 51:479–491
Harter J, Krause HM, Schuettler S, Ruser R, Fromme M, Scholten T, Kappler A, Behrens S (2014) Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. The ISME journal 8(3):660–674. https://doi.org/10.1038/ismej.2013.160
Howarth RW, Sharpley A, Dan W (2002) Sources of nutrient pollution to coastal waters in the United States: implications for achieving coastal water quality goals. Estuar Coast 25(4):656–676. https://doi.org/10.1007/BF02804898
Jones D, Rousk J, Edwards-Jones G, DeLuca T, Murphy D (2012a) Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol Bioch 45:113–124. https://doi.org/10.1016/j.soilbio.2011.10.012
Jones DL, Rousk J, Edwards-Jones G, DeLuca TH, Murphy DV (2012b) Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol Bioch 45:113–124. https://doi.org/10.1016/j.soilbio.2011.10.012
Kammann C, Ippolito J, Hagemann N, Borchard N, Cayuela ML, Estavillo JM, Fuertes-Mendizabal T, Jeffery S, Kern J, Novak J, Rasse D, Saarnio S, Schmidt H-P, Spokas K, Wrage-Mönnig N (2017) Biochar as a tool to reduce the agricultural greenhouse-gas burden – knowns, unknowns and future research needs. J Environ Eng Landsc 25(2):114–139. https://doi.org/10.3846/16486897.2017.1319375
Liu J, You L, Amini M, Obersteiner M, Herrero M, Zehnder AJ, Yang H (2010) A high-resolution assessment on global nitrogen flows in cropland. Proc Natl Acad Sci U S A 107(17):8035–8040. https://doi.org/10.1073/pnas.0913658107
Ly P, Duong VQ, Jensen LS, Pandey A, de Neergaard A (2014) Effects of rice straw, biochar and mineral fertiliser on methane (CH4) and nitrous oxide (N2O) emissions from rice (Oryza sativa L.) grown in a rain-fed lowland rice soil of Cambodia: a pot experiment. Paddy Water Environ 13:465–475
Mandal S, Thangarajan R, Bolan NS, Sarkar B, Khan N, Ok YS, Naidu R (2016) Biochar-induced concomitant decrease in ammonia volatilization and increase in nitrogen use efficiency by wheat. Chemosphere 142:120–127. https://doi.org/10.1016/j.chemosphere.2015.04.086
Margesin R, Schinner F (2005) Manual of soil analysis. Springer, Berlin
Mosier A, Kroeze C, Nevison C, Oenema O, Seitzinger S, Ovan C (1998) Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle: OECD/IPCC/IEA phase II development of IPCC guidelines for national greenhouse gas inventory methodology. Nutr Cycl Agroecosys 52(2/3):225–248. https://doi.org/10.1023/A:1009740530221
Mukherjee A, Lal R, Zimmerman AR (2014) Effects of biochar and other amendments on the physical properties and greenhouse gas emissions of an artificially degraded soil. Sci Total Environ 487:26–36. https://doi.org/10.1016/j.scitotenv.2014.03.141
Pereira EIP, Suddick EC, Mansour I, Mukome FND, Parikh SJ, Scow K, Six J (2015) Biochar alters nitrogen transformations but has minimal effects on nitrous oxide emissions in an organically managed lettuce mesocosm. Biol Fert Soil 51(5):573–582. https://doi.org/10.1007/s00374-015-1004-5
Reay DS, Davidson EA, Smith KA, Smith P, Melillo JM, Dentener F, Crutzen PJ (2012) Global agriculture and nitrous oxide emissions. Nat Clim Chang 2(6):410–416. https://doi.org/10.1038/nclimate1458
Rochette P, Angers DA, Chantigny MH, Gasser MO, Macdonald JD, Pelster DE, Bertrand N (2013) NH3 volatilization, soil NH4 + concentration and soil pH following subsurface banding of urea at increasing rates. Can J Soil Sci 93(2):261–268. https://doi.org/10.4141/cjss2012-095
Rondon MA, Lehmann J, Ramírez J, Hurtado M (2006) Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol Fert Soil 43:699–708
Sun L, Li L, Chen Z, Wang J, Xiong Z (2014) Combined effects of nitrogen deposition and biochar application on emissions of N2O, CO2 and NH3 from agricultural and forest soils. Soil Sci Plant Nutr 60(2):254–265. https://doi.org/10.1080/00380768.2014.885386
Taghizadeh-Toosi A, Clough TJ, Sherlock RR, Condron LM (2012) A wood based low-temperature biochar captures NH3-N generated from ruminant urine-N, retaining its bioavailability. Plant Soil 353(1-2):73–84. https://doi.org/10.1007/s11104-011-1010-9
Tan G, Sun W, Xu Y, Wang H, Xu N (2016) Sorption of mercury (II) and atrazine by biochar, modified biochars and biochar based activated carbon in aqueous solution. Bioresour Technol 211:727–735. https://doi.org/10.1016/j.biortech.2016.03.147
Thomazini A, Spokas K, Hall K, Ippolito J, Lentz R, Novak J (2015) GHG impacts of biochar: predictability for the same biochar. Agric Ecosyst Environ 207:183–191. https://doi.org/10.1016/j.agee.2015.04.012
Xu HJ, Wang XH, Li H, Yao HY, Su JQ, Zhu YG (2014) Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape. Environ Sci Tec 48(16):9391–9399. https://doi.org/10.1021/es5021058
Xu C-Y, Bai SH, Hao Y, Rachaputi RCN, Xu Z, Wallace HM (2015a) Peanut shell biochar improves soil properties and peanut kernel quality on a red Ferrosol. J Soil Sediment 15(11):2220–2231. https://doi.org/10.1007/s11368-015-1242-z
Xu C-Y, Hosseini-Bai S, Hao Y, Rachaputi RC, Wang H, Xu Z, Wallace H (2015b) Effect of biochar amendment on yield and photosynthesis of peanut on two types of soils. Environ Sci Pollut Res 22:6112–6125
Xu N, Tan G, Wang H, Gai X (2016) Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur J Soil Biol 74:1–8. https://doi.org/10.1016/j.ejsobi.2016.02.004
Yanai Y, Toyota K, Okazaki M (2007) Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil Sci Plant Nutr 53(2):181–188. https://doi.org/10.1111/j.1747-0765.2007.00123.x
Zhang A, Liu Y, Pan G, Hussain Q, Li L, Zheng J, Zhang X (2011) Effect of biochar amendment on maize yield and greenhouse gas emissions from a soil organic carbon poor calcareous loamy soil from Central China Plain. Plant Soil 351:263–275
Funding
This study was financially supported by the Special Fund for Agro-scientific Research in the Public Interest (201303095), the National Natural Science Foundation of China (41301311), the Fundamental Research Funds for Central Non-profit Scientific Institution (NO. 1610132016055), and the Newton Fund (Grant Ref: BB/N013484/1).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Hailong Wang
Electronic supplementary material
ESM 1
(DOCX 250 kb)
Rights and permissions
About this article
Cite this article
Tan, G., Wang, H., Xu, N. et al. Biochar amendment with fertilizers increases peanut N uptake, alleviates soil N2O emissions without affecting NH3 volatilization in field experiments. Environ Sci Pollut Res 25, 8817–8826 (2018). https://doi.org/10.1007/s11356-017-1116-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-1116-6