Abstract
Semivolatile organic compounds (SVOCs) in surface microlayer (SML) and subsurface water (SSW) from Dianshan Lake were studied to investigate their occurrence, distributions, as well as enrichment and potential sources. A sample was concentrated by solid-phase micro-extraction (SPME). Identification and quantification were carried out by gas chromatography coupled to mass spectrometry (GC–MS). Total SVOCs concentrations ranged from 25.93 to 47.49 μg/L in SSW and 38.19 to 77.23 μg/L in SML. The phthalic acid esters (PAE) concentrations in both SSW and SML are the highest of the total SVOC. The enrichment factors (EFs) of total SVOCs ranged from 0.80 to 2.98, while the highest EF was found in benzyl phthalate and dibutyl phthalate, compounds of PAEs (4.06). The EFs values calculated in this study were consistent with the EFs reported for other water ecosystems. Compared with other place, the EF of PAHs were in the normal level (0.88–2.37). The results of correlation analysis, principal component analysis (PCA) suggested that at least three sources, i.e., agricultural residual pesticides, industrial sewage and miscellaneous sources, were responsible for the presence of SVOCs in Dianshan Lake examined, accounting for 94.16% of the total variance in the dataset. Environmental risk assessment revealed that a majority of SVOCs posed relatively low risks (the values of risk quotient were less than 0.1), while naphthalene, acenaphthene, 2,4-dinitrotoluene, and dibutyl phthalat exhibited moderate risks (values of risk quotient were more than 0.1 but less than 1fore) to aquatic organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The semivolatile organic compounds (SVOCs), including polycyclic aromatic hydrocarbons(PAHs), phthalic acid esters (PAEs), monoaromatic hydrocarbons (BTEXs), and some other compounds (Cai et al. 2007, Dai et al. 2007, Sanchez-Avila et al. 2009, Sanchez-Avila et al. 2011, Villar et al. 2006) are important groups of persistent organic pollutants (POPs). Since they are highly toxic and long distance transportable, and some of them such as organic chloride pesticides (OCPs) are recalcitrant to natural degradation, SVOCs have drawn much attention (Sun et al. 2014). Furthermore, majorities of SVOCs are lipophilic and bioaccumulative, these characters increase the risk to human exposure (Fang et al. 2012, Jones &de Voogt 1999, Sprovieri et al. 2007, Willett et al. 1998, Zhou et al. 2006).
Most of SVOCs are listed as priority pollutants both by China and US Environmental Protection Agency (EPA). For instance, 16 polycyclic aromatic hydrocarbons (PAHs) have been identified as priority environment pollutants by US EPA (Jiang et al. 2009), seven of them were considered as probable human carcinogens by US EPA (Portier &Schwartz 2005). Another class of SVOCs, phthalate esters (PAEs), is widely used in a wide variety of products ranging from industrial and commercial products, for example, pharmaceuticals pills, packaging, cosmetics, paints, and pesticides (Gao et al. 2014, Staples et al. 1997, Ye et al. 2015); however, diisobutyl phthalate (DiBP) has been reported to disrupt endocrine and cause severe adverse effects on the male rat reproductive development (Saillenfait et al. 2008). It is notable that the global production of PAEs is more than 8.0 million tons (Xue &Wang 2015). Due to extensive use of the substances containing SVOCs, this kind of chemical contaminant has been inevitably released into the environment, and eventually transferred into the urban water systems, resulting in the intermigration and recycling of these SVOC contaminations between atmosphere and watersheds in the city.
Surface microlayer (SML) of waters, whose thickness ranges from a few microns to hundreds of microns, is an interface layer between water and air. Compared with the subsurface water (SSW), the SML exhibits many unique properties. SML generally enriched in bacteria and microalgae could be considered as a unique ecosystem. Many plankton species which perch on the SML will interact with chemical substance in this layer. The SML is generally enriched in inorganic salts and different categories of organic pollutants (Guitart et al. 2007, Wang et al. 2014, Wurl &Obbard 2004), and also plays an important role in many biogeochemical processes, for example air-water exchanges, transport, accumulation, and degradation of organic pollutants (Cunliffe et al. 2013, del Vento &Dachs 2007, Guitart et al. 2007). As a consequence, understand the accumulation processes of SVOCs between SML and SSW is necessary. Some studies focused the different degrees of pollution in SML and SSW (Manodori et al. 2006, Wang et al. 2014). Few literature is available to reveal SVOCs pollution from the interfaces of freshwater (i.e. rivers, lakes and reservoirs), although freshwater is extremely significant to the transfer of SVOC contaminants in the city area.
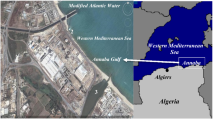
In the past several decades, Shanghai has experienced rapid industrial development and urbanization, and subsequently, the increased use of chemicals has caused many significant environmental problems. Dianshan Lake is the largest natural freshwater lake in Shanghai and the most important fresh water source for Shanghai City, which covers an area of 62 km2 and is located in the western of Shanghai, adjacent to Kunshan City of Jiangsu Province, a large industrial city of China. Therefore, identifying the occurrence and distributions of SVOCs in Dianshan Lake, the large natural reservoir in this area, could supply available information relating the pollution status and potential transfer of these contaminants.
Documented studies on SVOCs in SSW of Dianshan Lake are sparse. Moreover, there is no published report documenting the pollution level of SVOCs in the SML water in the Dianshan Lake. Thus, the main objectives of this study are focusing on (1) the occurrence and distributions of SVOCs in SML and SSW of Dianshan Lake, (2) understanding the SVOCs pollution situations in typical water system of Shanghai, and (3) evaluating the potential sources, fate, and ecological risks of SVOCs in this area. These findings could supply available information relating to the transferred characteristics of SVOCs in large cities.
Materials and methods
Chemicals and reagents
A composite stock standard solution (1000 g/mL in dichloromethane: benzene 4/1 v/v, 99.8% filtered purity) containing 62 SVOCs was used. Dichloromethane, hexane, acetone purchased from CNW Technologies GmbH (CNW, Germany) were of chromatographic grade. The HPLC-grade water used during the analysis was obtained from filtering through a Milli-Q-Plus ultra-pure water system (resistance >18.2 MΩ cm) from Milipore (Sartorius 611, Germany).
Sample collection
Ten water sampling sites were set up around the Dianshan Lake (Fig. 1). Some of the sampling sites are located in Shanghai and others are in Jiangsu Province. With the aid of global positioning system (GPS), ten sample spots were designated and their coordinates recorded in September 2015, and we have collected samples in SML and SSW in thoes 10 sample spots, respectively.
Screen sampler consists of the screen (a stainless steel mesh of 16 stainless steel per Screen sampler consists of the screen (a stainless steel mesh of 16 stainless steel per inch) and two handles attached to a stainless steel framework (40 × 40 cm) supporting the screen. SML water samples were kept horizontal first and collected by screen surface microlayer. Make sure the screen contact with the water at the surface and keep it horizontal. Then, pull up the screen slowly while it was still in the horizontal position. The large screen area and thin water film led to a significant gas exchange between atmosphere and the water. Time of the sampling process usually lasted for 10 s. The physical thickness of SML water samples was estimated to be typically 200 ± 10 μm. On the other hand, SSW samples were simultaneously taken from a depth of 20 to 25 cm by submerging a 500-mL glass bottle and opening and closing it under water (Zhang et al. 2008). A total of 20 samples were taken from the designated sampling sites.
Pretreatment of samples
Water samples were passed through Neutral filter paper and 0.7-μm glass fiber filters (Whatman, Maidstone, England) were pre-baked in muffle furnace during 6 h. All the water samples were preserved in the refrigerator at 4 °C and samples must be extracted in 6 days. Direct SPME mode was used to extraction. Each sample (10 mL) was injected into a 15-mL vial containing a magnetic stir rotor. Then, a Teflon septum was used to seal the vial tightly. The optimum conditions were determined and listed as follows.
A magnetic heated stirrer was put in the vial and 85 μm polyacrylate (PA) fiber was inserted in SPME handle. The extraction temperature of SPME was set as 50 °C, extraction time was 20 min and extraction speed was 200 rpm/min. As the extracting process was completed, remove the fiber from the vial and then insert it into the injection port of GC/MS system in manual injection mode for analysis. The injection time lasts for 5 min in general, and then the fiber is removed. In addition, before and after the sample desorption, the fiber was inserted into the injection port of GC/MS system for blank sample analysis. When testing the sample, blank sample was analyzed first and sample analysis program followed it. Each sample has 3 parallel samples. The peak area of the blank sample should be less than 0.05% of the test sample.
Quantification of SVOCs
The determining instrument was (5975C/7890A, Agilent). Analysis of individual compounds was performed by gas chromatography equipped with a mass spectrometer (GC-MS, 5975C/7890A, Agilent Technology).
The chromatographic conditions were described as follow. A DB-5MS 30 × 0.25 mm I.D., 0.25-μm film (Agilent Technology) fused silica capillary column was used (Qi et al. 2013). The GC oven temperature program was as follows: 40 °C hold for 1 min, 40–180 at 8 °C/min for 10 min, 180–310 at 15 °C/min for 10 min. Helium was the carrier gas at 0.615 mL/min. The injection was set on a splitless mode at 280 °C. The solvent delay was 5 min and a final runtime of 37 min.
High purity helium (>99.99%) at a flow rate of 0.615 mL/min was used as the carrier gas. The oven ramp was set to initial temperature of 40 °C (hold 1 min), increased to 180 °C at 8 °C/min for 10 min, to 310 °C at 15 °C/min for 10 min (hold for 10 min) (Clara et al. 2010). The mass spectrometry condition was electron ionization at selected ion monitoring (SIM) Mode. Using this method, 62 SVOCs had been detected with effective linear relation >0.99. The limits of detection range from 0.005 to 0.02 μg/L. The repeatability and the reproducibility of the method, expressed as a percentage of RSD were between 0.6 and 6.2% for reproducibility. Details of the quality assurance parameters were listed in Table S1.
Results and discussion
Occurrence of the SVOCs in SML and SSW
PAHs, BTEXs, PAEs, and some other SVOCs were detectable in all the SML and SSW samples (Fig. 1). Thirty-two SVOCs among 62 target compounds were detected, implying SVOCs were ubiquitous in the research area. The concentrations of SVOCs in SSW were lower than those in SML, which is agreeing with other researches aiming to PAHs (Table S2). The PAE concentrations are the highest of the total SVOCs both in SSW and SML. The PAHs and other SVOCs concentrations are lower than other components both in SSW and SML.
PAHs
2-Chloronaphthalene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, indeno(1,2,3-cd)pyrene, dibenz(a,h)anthracene, and benzo(g,h,i)perylene were not detected in SML and SSW samples analyzed. Phenanthrene was only detected at S5 and S6. The levels of phenanthrene detected were 0.036 and 0.054 μg/L in the subsurface water samples, and 0.048 and 0.064 μg/L in the surface microlayer water samples from S5 and S6 locations, respectively. The total concentrations of PAHs ranged from 2.187 to 4.055 μg/L in SSW samples, and 3.454 to 5.194 μg/L in SML samples. Anthracene and carbazole are predominant analogs among the target PAHs. The total concentration of these two contaminants accounts for 50% of the 9 PAHs both in SML and SSW. Anthracene is thought to be an especially toxic compound, which can occur naturally but is also formed when products like coal, oil, gas, and refuse undergo incomplete combustion (Sellami et al. 2015). Carbazole mainly exists in the industrial waste water of coke industry, petrochemical industry and dye industry (vanVlaardingen et al. 1996). Except S7, the total concentrations of PAHs in SML sample were higher than those in SSW from all the sampling sites. Remarkably, benzo(a)anthrancene was not detected in both SML and SSW in the present study, which is the only regulated compound among these target PAHs in the national environmental quality standard for surface water of China.
BTEXs
2-Chlorophenol, nitrobenzene, 2-nitrophenol, 2,4-dimethylphenol, 2,4-dichlorophenol, hexachlorobenzene, and pentachlorophenol were not detected in any sample. 1,2,4-Trichlocrobenzene was not detected in both surface microlayer and subsurface water samples from S2, while 4-chloro-3-methylphenol was not detected in surface microlayer and subsurface water samples from S5. The total concentrations of BTEXs (∑BTEXs) in the SML ranged from 7.210 to 10.517 μg/L in S4 and S10, respectively. And the levels of ∑BTEXs were 5.929 and 10.007 μg/L in the SSW from S5 and S10 locations, respectively. Phenol was the main pollutants in SSW, while 2,4-dinitrotoluene was the main pollutants in SML, which may be attributed to the different physicochemical properties between these two compounds. The higher lipotropy of 2,4-dinitrotoluene (log K ow = 1.98) than phenol (log K ow = 1.46) makes this pollutants easily transport and accumulate in SML, where lipids is abundant (Abdelwahab et al. 2009). The concentration of phenol accounts for about 25% in the 13 BTEXs in SSW, and the proportion of 2,4-dinitrotoluene in SML was as the same as phenol in BTEXs from SSW. Except S4 and S9, the total concentrations of BTEXs in the surface microlayer water sampler were always higher than those in the subsurface water samples.
PAEs
Dimethylphthalat, bis(2-ethlhexyl)phthalate and di-n-octylphthalate were not detected in any samples. And diethyl phthalate, dibutyl phthalate, benzyl phthalate were detected in SML and SSW samples analyzed. The total concentrations of PAEs ranged from 11.208 to 29.384 μg/L in SSW samples, and total concentrations of PAEs ranged from 20.103 to 58.935 μg/L in the SML samples. Dibutyl phthalate was the main pollutants both in SSW and SML, which accounts for 50% of the 3 PAE concentrations both in SML and SSW. Except S7, the total concentrations of PAEs in SML samples were higher than those in the SSW. Among the target PAEs, only di-n-butyl phthalate and di-2-ethylhexyl phthalate are the regulated contaminants listed in the national environmental quality standard for surface water of China (GB3838–2002). According to this restrict regulation, these two compounds were not detected in both SML and SSW.
Other SVOCs
The total concentrations of other SVOCs ranged from 2.655 to 4.921 μg/L in SSW, while those ranged from 3.101 to 5.167 μg/L in SML. The levels of bis(2-chloroisoprophyl)ether and azobenzene are higher than others. The total concentrations of these two pollutants account for 50% of the target compounds in both SML and SSW. The concentrations of other SVOCs in SSW from S1, S2, and S10 were higher than those in SML, while the opposite situation was found in other sampling sites.
Total SVOC
The total SVOC concentrations at the different sampling sites are illustrated in Fig. 1. The total SVOC concentrations in SSW ranged from 25.735 μg/L (S1) to 47.487 μg/L (S7), with the mean SVOC concentration in SSW at Dianshan Lake of 32.832 μg/L. The concentrations of PAEs in SSW are the highest among all the SVOC categories. The proportions of the PAEs in the SVOCs at the ten sampling sites were between 39.55% (S10) and 64.45% (S4), with the dibutyl phthalate concentrations the highest, accounting for between 22.56% at S10 and 41.42% at S2 of the ΣSVOCs in SSW at the sampling sites. The mean concentrations of ΣPAHs, ΣPAEs, ΣBTEXs, and Σother SVOCs in SSW were 2.734, 18.543, 7.934, and 3.621 μg/L, respectively.
The proportions of the four SVOC classes in SML at the different sampling sites are depicted in Fig. 1. The total SVOC concentration in SML ranged from 38.194 μg/L at S7 to 77.224 μg/L at S5. The mean SVOC concentration in SML at Dianshan Lake was 52.451 μg/L. The predominant components in SML are also PAEs, whose proportions were between 51.39% (S10) and 79.20% (S3). It was similar to the situation in SSW. The mean concentrations of ΣPAHs, ΣPAEs, ΣBTEXs and Σother SVOCs in SML were 4.094, 35.600, 8.601, and 4.156 μg/L, respectively. The mean concentrations of four SVOC classes in SML were higher than in SSW, implying SVOCs easily accumulate in SML of natural waters.
The enrichment of surface microlayer
SML enrichment may be explained by considering the hydrophobic character of POPs and their great affinity with surfactants collected at the air-water interface and it may be well represented by the enrichment factors (Manodori et al. 2006). Enrichment factors (EFs) were calculated as the ratio of the concentration of the respective parameter in the SML to that in the SSW. An EF >1.0 means enrichment in the SML relative to SSW; an EF < 1.0 means depletion in the SML relative to SSW (Wang et al. 2014).
The SML is generally enriched with PAHs, BTEXs, PAEs and other SVOCs (as shown in Fig. 2). For PAHs, except S7, the value of EF was greater than 1. The maximum enrichment of PAHs appeared at S5, with EF of 2.37. Except naphthalene and fluorine, the EFs of PAHs at S5 were higher than the EFs at other site. At S7 the EFs of PAHs were less than 1, except anthracene. For BTEXs, except S4 and S7, the value of EF was higher than 1. The maximum enrichment of BTEXs appeared at S5, with EF was 1.48. The EFs of 9 different categories of BTEXs were almost nearly 1, except 2, 4-dinitrotoluene. The EF of 2, 4-dinitrotoluene were range from 2.76 at S5 to 1.32 at S7. For PAEs, except S7, the value of EF was higher than 1. The maximum enrichment of PAEs appeared at S5, with EF of 4.02. The EF values of dibutyl phthalate and benzyl phthalate were higher than 1 at all samplings except S7. At S5 the EF values of dibutyl phthalate and benzyl phthalate were both 4.06, were the highest in SVOCs. For other SVOCs, except S1, S8 and S10, the value of EF was higher than 1. The maximum enrichment of other SVOCs appeared at S6, with EF was 1.70. The EFs of 7 different categories of other SVOCs were almost nearly 1, except hexachloroethane. The EF values of hexachloroethane were range from 2.70 at S7 to 0.74 at S10. Table S2 show the EFs and concentration of ΣSVOCs at all the locations respectively. The ΣSVOCs concentration in SML was higher than those in SSW, except S7. The concentration of ΣSVOCs in SSW ranged from 25.735 μg/L at S1 to 47.487 μg/L at S7, and the concentration of ΣSVOCs in SML ranged from 38.194 μg/L at S7 to 77.224 μg/L at S5. At S5 has a scallop cleaning workshop, and here were some suspended particulate matters in the water absorbing some SVOCs on the surface, and many small ship stop and transport, cracking process of petroleum fuel produce many SVOCs made the concentration of ΣSVOCs was higher than other place. The EFs of ΣSVOCs were higher than 1, except S7. The maximum enrichment of ΣSVOCs appeared at S5, with EF of 2.98.
The EFs of PAEs were almost higher than other SVOC compounds, while those of BTEXs were almost lower than others. This may be caused by the differences of the octyl alcohol–water partition coefficient (K ow) and the saturated vapor pressure of diffident categories of SVOCs.
It could be seen that the PAH pollution in the SML water along the coastal Dianshan Lake showed relatively low level. And the EFs of PAHs were in the normal level (Table 1). As a drinking water source area in Shanghai, the pollution level of PAHs in Dianshan Lake was lower than other areas (Table 1).
Interrelationships and source appointment
To understand the interrelationships between every single individual, Pearson correlation analysis was employed. The result matrix was shown in Table S3. A high correlation among indexes for organic pesticides might be associated with agricultural source. For example, the high positive correlations between acenaphthene and hexachloroethane (r = 0.94), and the moderate correlations between acenaphthene and phenol (r = 0.72), fluorene and carbazole (r = 0.68). Dibenzofuran, fluorine, and anthracene both had a significant correlation with 4-chloroaniline. These SVOCs were probably associated with discharges from the dyeing and printing enterprise. The correlation between 3-nitroaniline and hexachloroethane (r = 0.80) was related to the pharmaceutical industry.
In order to further explore the potential cluster source of SVOCs, principal component analysis (PCA) was performed. There were seven principal nutrients components extracted (Table S4). The PC1 showed very high correlations for 17 SVOCs, explaining 27.878% of the total variance. Dibenzofuran, anthracene, 4-methylphenol, 4-chloroaniline, 4-chloro-3-methylphenol and 4-chlorophenyl phenyl ether showed higher loadings (≥0.7) than 2-methylnaphthalene, acenaphthene, fluorine, carbazole, phenol, 2-methylphenol, 2-nitroaniline, 2,4-dinitrotoluene, 3-nitroaniline, diethyl phthalate and benzyl phthalate. Fluorine, 2-nitroaniline and 2-methylphenol were used in agriculture pesticide. Anthracene, 4-chloroaniline, 2-methylnaphthalene, acenaphthene, carbazole, phenol and 3-nitroaniline were important intermediates in the textile printing and dyeing. These organics were found in the sewage of these industries. Thus, PC1 can be selected to represent compound pollution factors including agricultural residual pesticides and industrial sewage.
The PC2 had important contributions (loadings >0.5) of acenaphthene, 1,2-dichlorobenzene, 3-nitroaniline, dibutyl phthalate, benzyl phthalate, hexachloroethane, hexachlorobutadiene, hexachlorocyclopentadiene, 2-methylnaphthalene, 1,3-dichlorobenzene and bis(2-chloroisoprophyl)ether, accounting for 26.342% of the total variance. Most SVOCs in PC2 had strong positive loadings in this factor associated with residual pesticides from agricultural source. Therefore, the PC2 could be considered as agricultural sources.
The PC3 was dominated by naphthalene, fluorine, phenol, 2-methylphenol, and 2,4-dinitrotoluene, accounting for 12.897% of the total variance. Naphthalene, phenol, and 2,4-dinitrotoluene, well known as chemicals in the textile printing and dyeing, and existed largely in the sewage of these industries. Thus, the PC5 could be considered as the industrial sewage.
The PC4 showed strong loadings for phenanthrene, fluoranthene and diethyl phthalate accounting for 8.111% of the total variance. The PC5 was correlated very strongly with 1,2,4-trichlorobenzene, which had a high loading value (more than 0.5), and explained 7.560% of the total variance. The PC6 explained 6.574% of the total variance and consisted of a good loading of 4-nitroaniline and bis(2-chloroisoprophyl)ether. The PC7 explained 4.797% of the total variance. These SVOCs were probably discharged from various industries and agriculture fields. Therefore, the PC4, PC5, PC6 and PC7 could be considered as miscellaneous sources.
Ecological risk assessment
As is well known, SVOCs are not only wide varieties, but also toxic, harmful and persistent in the environment. To assess the ecological risk can make us understand the pollution posing on the aquatic ecosystems.
Herein, the risk quotient (RQ) approach was performed, which was expressed as the ratio between the maximum measured environmental concentration (MEC) and the predicted no-effect concentration (PNEC) of an individual compound, as suggested by the EPI (US Environmental Protection Agency, 2012).
The PNEC value is often extrapolated from chronic toxicity data, or if no chronic data is available, from acute toxicity data. In practice, PNEC values used in the risk analysis are 1000 times lower than the lowest ecotoxicity concentration values found for three representative trophic levels of the ecosystem, fish, daphnia and algae (Dai et al. 2016). The potential environmental adverse effect on aquatic organisms based on the criteria adopted by several authors fell into three levels: RQ < 0.1, low risk; 0.1 ≤ RQ ≤ 1, medium risk; RQ ≥ 1, high risk (Hernando et al. 2006, Marcus et al. 2010, Sanchez-Avila et al. 2012).
The RQ values for individual contaminants were calculated as shown in Table 2. In this study, most RQ values were below 0.1 in the Dianshan Lake, which meant the current risk of SVOCs is not really high. However, the RQ value of naphthalene, acenaphthene, 2,4-dinitrotoluene and dibutyl phthalate were higher than 0.1, indicating relatively moderate risk. No contaminant is in high risk. However, due to the chronic toxicities and bioaccumulative properties exhibited by most of SVOCs detected in the research area, the potential ecological risks posed from some compounds, like acenaphthene, 2,4-dinitrotoluene, should also be taken attentions.
Conclusions
This study has provided the first data on the levels of SVOCs in the SML and SSW of Dianshan Lake. The total SVOC concentrations were in moderate level in both the surface microlayer and subsurface water of the Dianshan Lake. PAEs were ubiquitous and dominant in both SSW (ΣPAEs: 11.21 to 29.38 μg/L) and SML (ΣPAEs: 20.10 to 58.93 μg/L). The mean concentrations of SVOC in both SSW and SML observed from the highest to the lowest were in the order of: PAHs < Other SVOCs < BTEXs < PAHs. The results indicate that the concentration of SVOCs in SML were almost higher than in SSW. That means SML has good accumulation on SVOCs. The maximum enrichment factor was 2.98 for SVOCs at S5, 2.37 for PAHs, 1.48 for BTEXs, 4.02 for PAEs, 1.70 for other SVOCs, and the highest enrichment factor was 4.06 for benzyl phthalate and dibutyl phthalate at S5, suggesting concentration levels of majority SVOCs in SML of the Dianshan Lake were higher than in SSW. In order to further explore the potential cluster source of SVOCs, PCA was performed. There were seven principal nutrient components extracted. The PC1 showed very high correlations for 17 SVOCs, explaining 27.878% of the total variance. The PC2 showed correlations for 11 SVOCs, explaining 26.342% of the total variance. The PC3 was dominated by 5 SVOCs, accounting for 12.897% of the total variance. Whlie the PC4 accounted for 8.111% of the total variance, the PC5 accounted for 7.560% of the total variance, the PC6 accounted for 6.574% of the total variance, and the C7 explained 4.797% of the total variance. For the pollutants source identification, agricultural residual pesticides, industrial sewage could be considered. The environmental risk assessment shows most SVOCs were in low risk. Further research is needed to conduct regular monitoring of SVOCs pollution in the Dianshan Lake to prevent health risks to the millions of people relying on such important drinking water source.
References
Abdelwahab O, Amin NK, El-Ashtoukhy ESZ (2009) Electrochemical removal of phenol from oil refinery wastewater. J Hazard Mater 163:711–716
Benson NU, Essien JP, Asuquo FE, Eritobor AL (2014) Occurrence and distribution of polycyclic aromatic hydrocarbons in surface microlayer and subsurface seawater of Lagos lagoon, Nigeria. Environ Monit Assess 186:5519–5529
Burns KA, Codi S (1999) Non-volatile hydrocarbon chemistry studies around a production platform on Australia’s northwest shelf. Estuar Coast Shelf S 49:853–876
Cai QY, Mo CH, Wu QT, Zeng QY, Katsoyiannis A (2007) Occurrence of organic contaminants in sewage sludges from eleven wastewater treatment plants, China. Chemosphere 68:1751–1762
Clara M, Windhofer G, Hartl W, Braun K, Simon M, Gans O, Scheffknecht C, Chovanec A (2010) Occurrence of phthalates in surface runoff, untreated and treated wastewater and fate during wastewater treatment. Chemosphere 78:1078–1084
Cunliffe M, Engel A, Frka S, Gasparovic B, Guitart C, Murrell JC, Salter M, Stolle C, Upstill-Goddard R, Wurl O (2013) Sea surface microlayers: a unified physicochemical and biological perspective of the air-ocean interface. Prog Oceanogr 109:104–116
Dai G, Wang B, Fu C, Dong R, Huang J, Deng S, Wang Y, Yu G (2016): Pharmaceuticals and personal care products (PPCPs) in urban and suburban rivers of Beijing, China: occurrence, source apportionment and potential ecological risk. Environmental science. Processes & impacts
Dai JY, Xu MQ, Chen JP, Yang XP, Ke ZS (2007) PCDD/F, PAH and heavy metals in the sewage sludge from six wastewater treatment plants in Beijing, China. Chemosphere 66:353–361
del Vento S, Dachs J (2007) Influence of the surface microlayer on atmospheric deposition of aerosols and polycyclic aromatic hydrocarbons. Atmos Environ 41:4920–4930
Fang MD, Lee CL, Jiang JJ, Ko FC, Baker JE (2012) Diffusive exchange of PAHs across the air-water interface of the Kaohsiung Harbor lagoon, Taiwan. J Environ Manag 110:179–187
Gao DW, Li Z, Wen ZD, Ren NQ (2014) Occurrence and fate of phthalate esters in full-scale domestic wastewater treatment plants and their impact on receiving waters along the Songhua River in China. Chemosphere 95:24–32
Guitart C, Garcia-Flor N, Bayona JM, Albaiges J (2007) Occurrence and fate of polycyclic aromatic hydrocarbons in the coastal surface microlayer. Mar Pollut Bull 54:186–194
Hernando MD, Mezcua M, Fernandez-Alba AR, Barcelo D (2006) Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 69:334–342
Jiang YF, Wang XT, Wang F, Jia Y, Wu MH, Sheng GY, Fu JM (2009) Levels, composition profiles and sources of polycyclic aromatic hydrocarbons in urban soil of Shanghai, China. Chemosphere 75:1112–1118
Jones KC, de Voogt P (1999) Persistent organic pollutants (POPs): state of the science. Environ Pollut 100:209–221
Manodori L, Gambaro A, Piazza R, Ferrari S, Stortini AM, Moret I, Capodaglio G (2006) PCBs and PAHs in sea-surface microlayer and sub-surface water samples of the Venice lagoon (Italy). Mar Pollut Bull 52:184–192
Marcus MD, Covington S, Liu BL, Smith NR (2010) Use of existing water, sediment, and tissue data to screen ecological risks to the endangered Rio Grande silvery minnow. Sci Total Environ 409:83–94
Portier CJ, Schwartz DA (2005) The NIEHS and the National Toxicology Program: an integrated scientific vision. Environ Health Persp 113:A440–A440
Qi WX, Liu HJ, Pernet-Coudrier B, Qu JH (2013) Polycyclic aromatic hydrocarbons in wastewater, WWTPs effluents and in the recipient waters of Beijing, China. Environ Sci Pollut R 20:4254–4260
Saillenfait AM, Sabate JP, Gallissot F (2008) Diisobutyl phthalate impairs the androgen-dependent reproductive development of the male rat. Reprod Toxicol 26:107–115
Sanchez-Avila J, Bonet J, Velasco G, Lacorte S (2009) Determination and occurrence of phthalates, alkylphenols, bisphenol A, PBDEs, PCBs and PAHs in an industrial sewage grid discharging to a municipal wastewater treatment plant. Sci Total Environ 407:4157–4167
Sanchez-Avila J, Fernandez-Sanjuan M, Vicente J, Lacorte S (2011) Development of a multi-residue method for the determination of organic micropollutants in water, sediment and mussels using gas chromatography-tandem mass spectrometry. J Chromatogr A 1218:6799–6811
Sanchez-Avila J, Tauler R, Lacorte S (2012) Organic micropollutants in coastal waters from NW Mediterranean Sea: sources distribution and potential risk. Environ Int 46:50–62
Sellami B, Khazri A, Louati H, Dellali M, Driss MR, Aissa P, Mahmoudi E, Hamouda B, Coelho AV, Sheehan D (2015) Effects of anthracene on filtration rates, antioxidant defense system, and redox proteomics in the Mediterranean clam Ruditapes decussatus (Mollusca: Bivalvia). Environ Sci Pollut R 22:10956–10968
Sprovieri M, Feo ML, Prevedello L, Manta DS, Sammartino S, Tamburrino S, Marsella E (2007) Heavy metals, polycyclic aromatic hydrocarbons and polychlorinated biphenyls in surface sediments of the Naples harbour (southern Italy). Chemosphere 67:998–1009
Staples CA, Peterson DR, Parkerton TF, Adams WJ (1997) The environmental fate of phthalate esters: a literature review. Chemosphere 35:667–749
Stortini AM, Martellini T, Del Bubba M, Lepri L, Capodaglio G, Cincinelli A (2009) n-Alkanes, PAHs and surfactants in the sea surface microlayer and sea water samples of the Gerlache Inlet sea (Antarctica). Microchem J 92:37–43
Sun H, An T, Li G, Qiao M, Wei D (2014) Distribution, possible sources, and health risk assessment of SVOC pollution in small streams in Pearl River Delta, China. Environ Sci Pollut Res Int 21:10083–10095
US Environmental Protection Agency (2012): Tools EA Models, Estimation Program Interface (EPI) Suite, version 4.11. Office of Pollution Prevention and Toxics: Washington, DC
vanVlaardingen PLA, Steinhoff WJ, deVoogt P, Admiraal WA (1996) Property-toxicity relationships of azaarenes to the green alga Scenedesmus acuminatus. Environ Toxicol Chem 15:2035–2042
Villar P, Callejon M, Alonso E, Jimenez JC, Guiraum A (2006) Temporal evolution of polycyclic aromatic hydrocarbons (PAHs) in sludge from wastewater treatment plants: comparison between PAHs and heavy metals. Chemosphere 64:535–541
Wang Y, Zhao J, Yuan Y, Liu S, Feng Z, Zhao Y (2014) Synthesis of maleimido-substituted aromatic s-triazine and its application in flame-retarded epoxy resins. Polym Degrad Stab 99:27–34
Willett KL, Ulrich EM, Hites RA (1998) Differential toxicity and environmental fates of hexachlorocyclohexane isomers. Environ Sci Technol 32:2197–2207
Wurl O, Obbard JP (2004) A review of pollutants in the sea-surface microlayer (SML): a unique habitat for marine organisms. Mar Pollut Bull 48:1016–1030
Xue Y, Wang X (2015) The effects of Ag doping on crystalline structure and photocatalytic properties of BiVO 4. Int J Hydrog Energy 40:5878–5888
Ya ML, Wang XH, Wu YL, Ye CX, Li YY (2014) Enrichment and partitioning of polycyclic aromatic hydrocarbons in the sea surface microlayer and subsurface water along the coast of Xiamen Island, China. Mar Pollut Bull 78:110–117
Ye K-H, Yu X, Qiu Z, Zhu Y, Lu X, Zhang Y (2015) Facile synthesis of bismuth oxide/bismuth vanadate heterostructures for efficient photoelectrochemical cells. RSC Adv 5:34152–34156
Zhang HH, Yang GP, Zhu T (2008) Distribution and cycling of dimethylsulfide (DMS) and dimethylsulfoniopropionate (DMSP) in the sea-surface microlayer of the Yellow Sea, China, in spring. Cont Shelf Res 28:2417–2427
Zhou RB, Zhu LZ, Yang K, Chen YY (2006) Distribution of organochlorine pesticides in surface water and sediments from Qiantang River, East China. J Hazard Mater 137:68–75
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 41573096, 41273126, 41273141, and 41430644) and Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13078).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 74 kb)
Rights and permissions
About this article
Cite this article
Wu, Mh., Yang, Xx., Xu, G. et al. Semivolatile organic compounds in surface microlayer and subsurface water of Dianshan Lake, Shanghai, China: implications for accumulation and interrelationship. Environ Sci Pollut Res 24, 6572–6580 (2017). https://doi.org/10.1007/s11356-016-8308-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8308-3