Abstract
Annaba Bay is a coastal area (southwestern Mediterranean Sea) that receives large diffuse inputs from the Seybouse and Bouhamra wadis, which are influenced by anthropogenic activities. Surface waters and sediments from these wadis were analyzed for polycyclic aromatic hydrocarbons (16 PAHs) and polychlorinated biphenyls (seven PCBs) by using gas chromatography-mass spectrometry (GC–MS). Total PAHs ranged from 0.183 to 0.503 µg l− 1, in water, and from 250.16 to 509.58 µg.kg− 1 dw in sediments. Total PCB levels ranged from 0 to 0.003 µg l− 1 in water, and from 2.15 to 6.37 µg.kg− 1 dw in sediments. In order to identify pollution emission sources of PAHs, different diagnostic ratios were used, including low molecular weight/high molecular weight indexes. The results show that the pollution origin was mainly due to pyrolytic inputs in waters and sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Located southwest of the Mediterranean Sea, Algeria is one of 131 “hotspots” listed by the Mediterranean Action Plan (MAP/MEDPOLL) and associated with large agglomerations, industrial sites, or river mouths (Khaled-Khodja 2016). Annaba is the fourth largest city in Algeria, with a population of over 1,000,000 inhabitants and covers about 1439 km2 of land. The city is located on the northeast coast extremity of Algeria, and it is an open bay oriented towards industrial, urban, and tourist activities (Ouali et al. 2008; Sifi et al. 2007). As a coastal city, Annaba suffers from a lack of water resources, and the wastewater is discharged into surface waters from urbanization, agriculture, and major industries (MATE 2002). Due to the lack of functional and effective water-treatment stations, all the telluric inputs are entering, without pretreatments, directly into littoral waters or conveyed by wadis to finish in the bay (Kherraz 2008; Khammar et al. 2009; Khaled-Khodja et al. 2014; Khaled-Khodja et al. 2016). Annaba Bay is a singular coastal area (southwestern Mediterranean Sea) that receives large diffuse inputs from the Seybouse and Bouhamra wadis and has also been subjected to severe pollution problems induced by direct domestic and industrial wastes (Khaled-Khodja et al. 2016; Ounissi et al. 2016). The coastal areas play an important role in the economic and social development at the local, national, and global scales. Coastal areas usually act as receptors for several types of discharges and dumping wastes containing high levels of persistent organic pollutants (POPs) coming from anthropogenic activities (Ziouch 2014; Merhaby et al. 2015). Organic compounds discharged into an aquatic environment can have negative impacts on aquatic ecosystems by direct and indirect toxic effects on organisms (Fleeger et al. 2003). Actually, organic contaminants are a major environmental concern due to their physicochemical properties, their ubiquity, their persistence, their long-range transportability, and fat solubility (Mzoughi et al. 2002; Bataille et al. 2010; Tiganus et al. 2013; Kanzari et al. 2014; Vane et al. 2014; Kong 2015; Merhaby et al. 2015; Net et al. 2015; Lukic et al. 2016). The compounds tend to bioaccumulate in fatty tissue and have potentially adverse effects on living organisms and human health via drinking water resources and the food chain (Fleeger et al. 2003; Fernandez et al. 2012; Net et al. 2015). Moreover, the contaminants are known or suspected as mutagenic and carcinogenic and their endocrine-disrupting activities in humans and wildlife have been recently reported for polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) (Brun et al. 2004; Merhaby et al. 2015). River water acts as receiving water for various kinds of organic contaminants originating from municipal and industrial wastewaters. For the ecosystem protection and to keep water resources clean, it is important to identify the nature of contaminants, their contamination levels, as well as their sources (Net et al. 2015).
PAHs are widely spread chemical pollutants released into the environment. The aromatic hydrocarbons can originate from the natural processes including biomass burning, volcanic eruptions, and diagenesis, or in anthropogenic inputs such as the incomplete combustion of wood, coal, industries, and vehicle emissions (Wang et al. 2007). They can also come from seepage of crude oil and coal or oil spills. Anthropogenic origins are generally the major sources of PAHs in the environment (Mirza et al. 2014). Hydrocarbons are highly lipophilic compounds, ubiquitous in the water column of coastal, estuarine, and river, as well as in sediments in which they tend to accumulate (Net et al. 2014). Due to their toxic, mutagenic, and carcinogenic effects (IARC 2010), 16 PAHs are listed as priority pollutants by the US Environmental Protection Agency (US EPA) (Cardellicchio et al. 2007; Tiganus et al. 2013; Kong 2015).
PCBs have commonly been used as dielectric fluids in capacitors and transformers, hydraulic fluids, lubricating oils, additives in pesticides, inks, and paints until the hazard posed to both the environment and human health by their use became evident (Fouial-Djebbar et al. 2010; Net et al. 2015). Anthropogenic pollutions particularly from shipping and industrial activities are responsible for the presence of PCBs in the environment (Hong et al. 2005, 2006). However, little is known about PCB pollution even if it is still a major concern due to the multiplicity of sources and transport mechanisms (Fernandez et al. 2012). However, recent studies on organic contamination are concentrated only on the northwestern part of the Mediterranean Sea and there is a great lack of information on the eastern and southern parts (Fouial-Djebbar et al. 2010; Merhaby et al. 2015). Algerian watersheds are poorly known and the few published data on nutrient levels and loads are very limited in time and space scales and concerned the distribution of nutrients in four coastal water sheds (Ounissi and Bouchareb 2013; Aounallah 2015). Moreover, data on organic contamination in coastal catchments and in freshwater ecosystems are nonexistent, except for the work of Fouial-Djebbar et al. (2010), which had only considered the level and distribution of PCBs in marine sediments collected from the seaside of Tamenfoust touristic resort located on the eastern side of Algiers Bay.
The objective of the present study was summarized as follows: (i) analysis of the degree of contamination by PAHs and PCBs in water and sediments from Bouhamra and Seybouse wadis, and (ii) determination of the possible sources of these organic contaminants.
Materials and methods
Description of Gulf of Annaba and the study sites
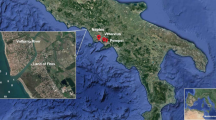
The Gulf of Annaba is situated at the extreme east of Algeria (36°50′-37°N; 7° 45′-8°15′E) (Fig. 1). In Annaba Bay, the modified Atlantic current moves eastward from the marine side and crosses the shelf of Annaba, which allows some renewing of the outer neritic waters. However, the inner part of the bay mostly influenced by continental inputs from the Seybouse and Bouhamra wadis and urban waste of about 1 million people in the city of Annaba and its surrounding villages, and industrial waste from Asmidal, a single large fertilizer factory (Ziouch 2014).
The selected study sites are the main sources of anthropic discharges at Annaba Bay. The first site is Bouhamra wadi (1) (Fig. 1), symbolized by Bouh. It represents the largest urban effluent of the city of Annaba. Bouhamra effluent, the major wastewater entering the bay, crosses the west plain of Annaba city and runs up to the sea. Before reaching the sea, the effluent receives multiple domestic sewage inputs from several sewer lift stations and many other connections to domestic sewages. It is estimated that the effluent brings the domestic waste of about 100,000 inhabitants. Outside the riverine water, the effluent had a flow varying generally within the day between 0.2 and 1 m3 s− 1. However, it is ultimately a real wastewater outfall (Khammar et al. 2009; Ziouch 2014). The second site is Seybouse wadi (3) (Fig. 1) situated at the northeast extremity of Algeria. This wadi is ranked in second place in the country, according to its vast drainage basin (6471 km2) and its length. However, it is considered as one of the most polluted wadis in Algeria (MATE (Ministère de l’Aménagement du Territoire et de l’Environnement) 2003). In the low plain of Seybouse, a multitude of anthropogenic activities takes place (heavy industry, national company of wood, regional company of cement, industrial group of paper and cellulose, the company of regional manufacturing of medical articles, complex of Asmidal, etc.). Agricultural activities are also present and very varied: cattle, mixed, truck farming, industrial crops (tomatoes and tobacco), forage crops, cereal and fruit mix cultivation according to the availability of water (Debieche 2002; Derradji et al. 2004; Abdelguerfi and Zeghida 2005). The wadi, 240 km long, crosses more than 86 municipalities and seven metropolises in which domestic wastewater are rejected, without pretreatments, due to the lack of treatment plants (Kherraz 2008). The various discards join at the level of Seybouse mouth before reaching the coast. Finally, both Bouhamra and Seybouse wadis are situated near a road that has intense traffic. Also, a gas station is situated next to Seybouse wadi.
Sampling
In order to estimate the concentrations of PAHs and PCBs in waters and sediments, a seasonal sampling was realized during the year 2009–2010: a campaign in October 2009 (autumn period), another one in February 2010 (winter period), a third one in May 2010 (spring period), and a last one in August 2010 (summer period). The samples of waters and sediments were taken in two stations. The first one is located downstream of the wadi Bouhamra (Bouh) where converges all urban waste from the western plain of the city to flow into the bay. The second station is situated approximately 100 m from the mouth of Seybouse wadi (Sey), where all the anthropic rejections (urban, agricultural, and industrial effluents) accumulate before reaching the sea.
Water samples were collected for PAHs and PCBs analyses in 1-l pre-cleaned glass amber bottles, with Teflon-lined screw caps from each site (Agence de l’eau Loire Bretagne 2006). Samples were manually collected from surface waters, no deeper than 1 m. All sampling vessels were pre-cleaned with acetone, purified water, and washed three times by sampled water.
Surface sediments (0–5 cm) were collected with a stainless-steel scoop (Schiavone and Coquery 2011) at the same stations as the water samples. Samples intended for determination of PAHs were stored in glass amber bottles. A calcined sheet of aluminum was placed between the plastic cork and the body of the glass flask. Samples intended for determination of PCBs were stored in glass jars washed with detergent (Decon, East Sussex, UK), rinsed with ultrapure water and acetone and finally dried at 120 °C prior to use.
After sampling the water and sediment, samples were transported in a cool box at 4 °C to the IDHESA LABOCEA (e.g., IDHESA Britain Oceane) laboratory, where they were stored at − 20 °C until analysis.
PAHs and PCBs extraction and analysis
Water samples
PAHs and PCBs were liquid–liquid extracted (Irace-Guigand and Aaron 2004) with hexane/dichloromethane mixture (50:50, v:v). After the separation, the organic extract was evaporated to the volume of 2–4 ml and then the micropollutants were separated with chromatography on activated silica gel. The fractions were concentrated under nitrogen vacuum and stored prior to analysis.
Sediment samples
Sediment samples were dried at 40 °C for 24 h, ground and sieved at 2 mm. In brief, 10–25 g of sieved samples, spiked with internal standards, was extracted by a Soxhlet system for 24 h with 300 ml of cyclohexane for PAHS analysis and 300 ml of n-hexane for PCBs analysis. The extracts were concentrated and purified by liquid chromatography on silica gel column. PAHS were recovered by elution with 40 ml of n-hexane/toluene mixture (7:3, v:v) and the PCBs were recovered by elution with 30 ml of diethyl ether/hexane mixture (1:10, v:v). Finally, the fractions were concentrated using a rotary evaporator followed by a slight stream of nitrogen and internal standards were added prior to quantification by instrumental analysis (Oehme 2001, 2003).
Separation, identification, and quantification of PAHS and PCBs in water and sediment were performed using gas chromatography coupled to mass spectrometry (GC–MS). This technique is widely applied in many branches of science and technology. For over half a century, gas chromatography (GC) has played a fundamental role in determining how many components and in what proportion they exist in a mixture. It is fast and sensitive and allows determination of thermally stable and volatile compounds. This makes it ideal for the analysis of the hundreds of relatively low molecular weight compounds found in environmental materials. However, the ability to establish the nature and chemical structure of these separated and quantified compounds is ambiguous and reduced, and requires a spectroscopic detection system. The most used is the mass spectrometric detector (MS), which allows obtaining the “fingerprint” of the molecule, i.e., its mass spectrum. Mass spectra provide information on the molecular weight, elemental composition, and if a high-resolution mass spectrometer is used, functional groups present, and, in some cases, the geometry and spatial isomerism of the molecule (Stashenko and Martinez 2014). The mass spectrum can be compared to reference libraries of known compounds in order to be identified.
Data analysis
The standards taken in term of evaluation of water and sediment quality were based on the French system of evaluation of streams water quality (SEQ-Eau) recommended by French water agencies (2003) (Table 1) and the circular of May 7, 2007 DCE/23 defining the “temporary environmental quality standards (NQEp)” for French superficial waters (Rodier et al. 2009).
Aromatic diagnostic criteria used in the interpretation of PAHs sources were: abundance ratio of 2–3 rings hydrocarbons to 4–6 rings hydrocarbons, low molecular weight/high molecular weight (LMW/HMW), Anthracene/(Anthracene + Phenanthrene) [Ant/(Ant + Phe)], Fluorine/(Flourine +Pyrene) [Flu/(Flu + Pyr)], Benzo(a)anthracene/(Benzo(a)anthracene + Chrysene) [BaA/(BaA + Chry)], Indeno(1,2,3-c,d)pyrene/[Indeno(1,2,3-c,d)pyrene + Benzo(ghi)perylene) (INP/(INP + BghiP)], Benzo(a)anthracene/Chrysene (BaA/Chry), Indeno(1,2,3-c,d)pyrene/Benzo(ghi)perylene (INP/BghiP) (Table 2).
Results and discussion
PAHs and PCBs concentration in the water
Sixteen PAHs were analyzed for station of water sample; their concentrations ranged from 0.003 to 0.084 µg.l− 1 (Table 3).
Among the 16 studied PAHs, ten hydrocarbons exceed the recommended values (Table 3). The water of wadi Bouhamra presents a poor quality for biological life of sensitive species (SEQ-Eau 2003). This degradation of the water quality is also confirmed by the NQEp, which attributes to the wadi a bad biological potential. This change in water is mainly due to the presence of the heavy PAHs (4–6 rings): Pyr > Chry > BghiP > Fluo > BaP > BaA = INP > BbF > DBA > BkF.
Half of the PAHs found in Seybouse exceed the standards (Table 3). The BaP recorded the highest content and so attributes to the wadi a passable biological ability for pollution-sensitive species (SEQ-Eau 2003). The most discriminating PAHs are: BaP > BbF > INP = BghiP > BaA > BkF > Chry > DBA.
We can say a priori, that Bouhamra and Seybouse waters are mainly contaminated by the heavy PAHs.
Unlike the PAHs, the PCBs were only detected in Seybouse water. Three PCBs were found (Table 3). Considering PCBs individually, it can be noted that the concentrations of the pollutants are below the permissible values. However, their sum exceeds widely the limits fixed by the SEQ-Eau and the NQEp, and hence attributes to the water a poor biological capacity.
Distribution and sources of PAHs in water
Possible sources of the PAHs emission into environment can be found by the use of indices, which are the ratio of concentrations of some PAHs in the sample (Tiganus et al. 2013; Botta et al. 2014; Kanzari et al. 2014).
The abundance ratio of two- and three-ring hydrocarbons (LMW) to four- to six-ring hydrocarbons (HMW) PAHs is a commonly used one to help determine the petrogenic and pyrolytic sources. Values below 1 are considered as combustion sources and values above 1 are considered for petrogenic contributions (Kanzari et al. 2014). As shown in Table 4, the value of LMW/HMW index at the two stations ranges from 0.62 to 0.86. These values indicate pyrogenic (pyrolytic) origins.
Table 4 shows that the ratio Ant/(Ant + Phen) in the two stations ranges from 0.31 to 0.57, which indicates a pyrolytic origin. For Flu/(Flu + Pyr), the value within the range from 0.4–0.5 is typical for pollution by combustion products of liquid fuel and oil. BaA/(BaA + Chry) ratio values higher than 0.35 indicate pollution by PAHs formed as a result of pyrolytic process. Values of INP/(INP + BghiP) ratio are between 0.42 and 0.5 confirm vehicle emissions related to fossil oil combustion. At all stations, the ratios Ant/(Ant + Phen), Flu/(Flu + Pyr), BaA/(BaA + Chry) and INP/(INP + BghiP) confirm the pyrogenic origin of PAHs.
PAHs and PCBs concentrations in sediments
Among the 16 PAHs recommended by the EPA, all were found in the studied sediments. Their concentrations fluctuate between 3.33 and 62.49 µg.kg− 1 dry weight (dw) (Table 5).
In spite of the significant number of PAHs found in sediments (Table 5), the concentrations found stay in the limits recommended by SEQ-Eau, except for phenanthrene, which exceeds the standard and so attributes to Bouhamra sediment a passable quality.
The sums of PCBs (PCB 52, PCB 101, PCB 138, PCB 153, PCB 180) determined in the sediment samples were below the permissible values (Table 5).
Distribution and sources of PAHs in sediments
Seven molecular diagnostic ratios are used to identify pollution emission sources contributing to PAHs released in the Gulf of Annaba (Table 6).
For Ant/(Ant + Phen) ratios, the two stations values range between 0.29 and 0.37, which confirms a pyrolytic origin. Stations exhibit values of the Flu/(Flu + Pyr) ratio between 0.17 and 0.18, indicating a petrogenic inputs. The high molecular weight PAHs such as BaA, Chry, INP, and BghiP are generally minor constituents in crude oil and refined petroleum products and are generally present in significant quantities only in the high molecular fractions as asphalt and possibly in the bitumen or coal (Kanzari et al. 2014). As shown in Table 6, BaA/(BaA + Chry) ratio values higher than 0.35 were used as indicators of pyrolytic sources. Values of the INP/(INP + BghiP) ratio are between 0.2 and 0.5, and indicate the vehicle emissions related to fossil oil combustion. The stations have a low LMW/HMW ratios (< 1), indicating the pyrolytic sources of PAHs (Tiganus et al. 2013; Kanzari et al. 2014; Net et al. 2015; Manneh 2016).
Conclusions
The present paper is the first study on the evaluation of organic contamination level in water and sediment of two freshwater systems, Bouhamra and Seybouse wadis (northeast Algeria).
The surface waters are heavily polluted with PAHs and to a lesser extent by PCBs. Waters of both stations are of poor quality and are mainly polluted by the heavy PAHs. The PCB138, the PCB153, and the PCB180 were detected only in the water of Seybouse, and their concentrations attribute a passable quality to the biological life.
The 16 priority PAHs are present in the sediments. Their concentrations are below standards, except for Bouhamra sediment, which is contaminated with phenanthrene. The sediment quality is bad for aquatic life. The PCB levels are below required limits. Therefore, the ecotoxicological evaluation based on international sediment quality guidelines (SQGs) should be performed in order to establish the ecological risk for aquatic fauna.
Multiple sources can be associated to PAHs and PCBs pollution into Bouhamra and Seybouse wadis. However, the diagnostic ratios indicate that pyrolytic process is the dominant source. It is characterized by abundance of high molecular weight PAHs resulting from incomplete combustion of organic matter including biomass and fossil fuels. The minor contribution of petroleum product produced by sewage outfall and fishery activities is due to the vulnerability of petroleum PAHs for degradation.
Finally, much remains to be done in the area and complementary studies are needed to take measures to control urban, agricultural, and industrial effluents to avoid dispersal of these persistent toxic contaminants into the environment. A continuous monitoring of PAHs and PCBs in water and sediment is recommended.
References
Abdelguerfi A, Zeghida A (2005) Utilisation des engrais par culture en Algérie. Organisation des Nations Unies pour l’Alimentation et l’Agriculture, Rome
Agence de l’eau Loire Bretagne (2006) Le prélèvement d’échantillons en rivière. Techniques d’échantillonnage en vue d’analyses physico-chimiques. Guide technique, Bretagne
Agences françaises de l’Eau (2003) Système d’évaluation de la qualité de l’eau des cours d’eau (SEQ-Eau). Grilles d’évaluation. version 2, France
Aounallah O (2015) Distribution and fluxes of biogeochemical variables in the Seybouse River Estuary, SW Mediterranean. Adv Environ Biol 9:101–108
Bataille T, Le Guyader C, Simon A (2010) Bilan national du réseau de surveillance de la qualité de l’eau et des sédiments des ports maritimes (RÉPOM) 1997-2006. XIème Journée Nationale de Génie Côtier, Brest, pp 851–858
Botta F, Léoz E, Albinet A, Ughetto E (2014) Origine des HAP dans les milieux aquatiques. Rapport final, Onema, p 46p
Brun GL et al (2004) Atmospheric deposition of polycyclic aromatic hydrocarbons to Atlantic, Canada: geographic and temporal distribution and trends 1980–2001. Environ Sci Technol 38(7):1941–1948
Cardellicchio N et al (2007) Organic pollutants (PAHs, PCBs) in sediments from the Mar Piccolo in Taranto (Ionian Sea, southern Italy). Mar Pollut Bull 55:451–458. https://doi.org/10.1016/j.marpolbul.2007.09.007
Debieche T H (2002) Évolution de la qualité des eaux (salinité, azote et métaux lourds) sous l’effet de la pollution saline, agricole et industrielle. Application à la basse plaine de la Seybouse—Nord-Est Algérien. Thèse de Doctorat, Université-Franche-Comté, France
Derradji F, Kherici N, Romeo M, Caruba R (2004) Aptitude des eaux de la vallée de la Seybouse à l’irrigation (Nord-est algérien). Sècheresse 15(4):353–360
Fernandez ACR et al (2012) 210Pb derived history of PAHs and PCB accumulation in sediments of a tropical inner lagoon (Las Matas, Gulf of Mexico) near a major oil refinery. Geochim Cosmochim Acta 82:136–153
Fleeger JW et al (2003) Indirect effects of contaminants in aquatic ecosystems. Sci Total Environ 317:207–233
Fouial-Djebbar D, Badjah Hadj Ahmed AY, Budzinski H (2010) Determination of organochlorine compounds in coastal marine sediments from southern west of Mediterranean Sea. Int J Environ Sci Tech 7:271–280
Hong SH et al (2005) Congener-specific survey for polychlorinated biphenyls in sediments of industrialized bays in Korea: regional characteristics and pollution sources. Environ Sci Technol 39(19):7380–7388
Hong SH et al (2006) Nationwide monitoring of polychlorinated biphenyls and organochlorine pesticides in sediments from coastal environment of Korea. Chemosphere 64(9):1479–1488
IARC (2010) Monographs on the evaluation of carcinogenic risks to humans: some non heterocyclic polycyclic aromatic hydrocarbons and some related exposures. International Agency for Research on Cancer, France
Irace-Guigand S, Aaron J-J (2004) L’analyse en chimie environnementale. L’Actua Chim 277:9–14
Kanzari F et al (2014) Distributions and sources of persistent organic pollutants (aliphatic hydrocarbons, PAHs, PCBs and pesticides) in surface sediments of an industrialized urban river (Huveaune), France. Sci Total Environ 478:141–151
Khaled-Khodja S (2016) Évaluation de la qualité physico-chimique des rejets anthropiques (urbains, agricoles et industriels) au Golfe d’Annaba. Thèse de Doctorat. Université Badji Mokhtar d’Annaba, Algérie, 186 p
Khaled-Khodja S, Samar M-EH, Ounissi M, Saker I-E, Kennouche M, Laabed S, Gouiez H (2014) Impacts des activités anthropiques sur la qualité des eaux de l’oued Seybouse (Annaba, Algérie). Ouvrage collectif aux éditions du Groupe Français des Pesticides, pp 51–55. ISBN 978-2-9545611-0-3
Khaled-Khodja S, Samar ME, Durand G (2016) Contamination métallique de l’eau et du sédiment d’oued Bouhamra. Rev. Sci. Technol. Synthèse 32:135–146
Khammar H et al (2009) Caractères chimiques des effluents urbains introduits au littoral d’Annaba (Nord Est Algérie). Actes du séminaire national sur l’eau et l’environement, Chlef, pp 127–135
Kherraz K (2008) Atelier sur la protection des eaux du bassin de la Seybouse contre la pollution. INECO, ABHCSM (Agence du bassin hydrographique constantinois-seybouse-mellegue). Annaba, pp 1–28
Kong L (2015) Monitoring of 1300 organic micro-pollutants in surface waters from Tianjin, North China. Chemosphere 122:125–130
Lukic B et al (2016) Evaluation of PAHs removal efficiency in an artificial soil amended with different types of organic wastes. Euro Mediterr J Environ Integr. https://doi.org/10.1007/s41207-016-0001x
Manneh R (2016) Analysis of polycyclic aromatic hydrocarbons (PAHs) in Lebanese surficial sediments: a focus on the regions of Tripoli, Jounieh, Dora, and Tyre. Mar Pollut Bull 110:578–583
MATE (Ministère de l’Aménagement du Territoire et de l’Environnement) (2002) Plan National d’Actions pour l’Environnement et le Développement Durable (PNAEDD), Edition du Ministère de l’Aménagement du Territoire et de l’Environnement. Algerie, pp 100–118
MATE (Ministère de l’Aménagement du Territoire et de l’Environnement) (2003) Rapport sur l’état et l’avenir de l’environnement, ONEDD (ed). Algérie, p 230–250
Merhaby D et al (2015) Organic pollution in surficial sediments of Tripoli harbour. Lebanon Mar Pollut Bull 93:284–293
Mirza R et al (2014) Distribution and sources of polycyclic aromatic hydrocarbons (PAHs) in surface sediments from the Northern Part of the Persian Gulf (Hormuzgan Province). Polycyclic Aromat Compd 34(4):343–355
Mzoughi N et al (2002) Méthodologie de l’extraction des hydrocarbures, application à des sédiments de la lagune de Bizerte (Tunisie). C R Géosci 334:893–901
Net et al (2014) Experimental design approach to the optimisation of hydrocarbons extraction from the sediment: method development and application. Appl Geochem 40:126–134
Net S et al (2015) Overview of persistent organic pollution (PAHs, Me-PAHs and PCBs) in freshwater sediments from Northern France. J Geochem Explor 148:181–188
Oehme M (2001) Analyse des hydrocarbures aromatiques polycycliques dans les sols par GC/MS. Méthode recommandée. In: Office Fédérale de l’Environnement, des Forêts et du Paysage (OFEFP), Berne, pp 1–29
Oehme M (2003) Analyse des biphényles polychlorés dans les sols par GC/MS. Méthode recommandée. In: Office Fédérale de l’Environnement, des Forêts et du Paysage (OFEFP), Berne, pp 1–28
Ouali N, Derradji F, Bouhedja Y, Kasdarli C (2008) Pollution du sédiment superficiel par neufs métaux traces: cas de la baie d’Annaba (Algérie—Méditerranée Sud-Occidental). Phys Chem News 42:139–143
Ounissi M, Bouchareb N (2013) Nutrient distribution and fluxes from three Mediterranean coastal rivers (NE Algeria) under large damming. C R Géosci 345:81–92
Ounissi M, Laskri H, Khélifi-Touhami M (2016) Net zooplankton abundance and biomass from Annaba Bay (SW Mediterranean Sea) under estuarine influences. Mediterr Mar Sci. https://doi.org/10.12681/mms.1474
Rodier J, Legube B, Merlet N (2009) L’analyse de l’eau. Dunod, Paris
Schiavone S, Coquery M (2011) Guide d’échantillonnage et de prétraitement des sédiments en milieu continental pour les analyses physico-chimiques de la DCE. Programme scientifique et technique. In: Action I-B-01: Appui aux donneurs d’ordre, surveillance milieux. Document final. Aquaref-Cemagref, France, pp 1–24
Sifi K, Chouahda S, Soltani N (2007) Biosurveillance de l’environnement par la mesure de biomarqueur chez Donax trunculus (L., 1758) dans le golfe d’Annaba, Algérie. Mésogée 63:11–18
Stashenko E, Martinez J R (2014) Gas chromatography-Mass Spectrometry. INTECH. https://doi.org/10.5772/57492
Tiganus D et al (2013) Identification of the sources of polycyclic aromatic hydrocarbons in sediments from the Romanian Black Sea sector. Cercet Mar 43:187–196
Vane CH et al (2014) Polycyclic aromatic hydrcarbons (PAH) and polychlorinated biphenyls (PCB) in urban soil of Greater London, UK. Appl Geochem 51:303–314
Wang Z et al (2007) Polycyclic aromatic hydrocarbons in Dalian soils: distribution and toxicity assessment. J Environ Monit 9:199–204
Ziouch OR (2014) Nutrient distribution in the Bay of Annaba under the influence of the Seybouse and the Mafragh estuaries inputs (southwestern Mediterranean). Doctoral thesis, Badji Mokhtar University of Annaba, Algeria, 117 p
Acknowledgements
The authors specially thank Mr. Ammar Rouibah for his priceless help and his constructive suggestions on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have no conflicts of interest to disclose.
Additional information
This paper was selected from the First International Symposium on Materials, Electrochemistry and Environment, September 2016, Tripoli (Lebanon). The evaluation process was monitored by Guest Editors Mishra Ajay (South Africa), Tarik Chafik (Morocco), Ahmad El Moll (Lebanon), Elke Fries (Germany), and Didier Hauchard (France).
Rights and permissions
About this article
Cite this article
Khaled-Khodja, S., Rouibah, K. Selected organic pollutants (PAHs, PCBs) in water and sediments of Annaba Bay, Algeria. Euro-Mediterr J Environ Integr 3, 23 (2018). https://doi.org/10.1007/s41207-018-0065-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41207-018-0065-x