Abstract
The Umgeni River is one of the main sources of water in KwaZulu-Natal, South Africa; however; there is currently a lack of information on the presence and distribution of polychlorinated biphenyls (PCBs) in its sediment, sediment pore water and surface water. This study aims to determine the occurrence and significance of selected PCBs in the surface water, sediment pore water and surface sediment samples from the Umgeni River. Liquid-liquid and soxhlet extractions were used for water or pore water, and sediments, respectively. Extracts were cleaned up using a florisil column and analysed by gas chromatography-mass spectrometry. The total concentrations of eight polychlorinated biphenyls were 6.91–21.69 ng/mL, 40.67–252.30 ng/mL and 102.60–427.80 ng/g (dry weight), in unfiltered surface water, unfiltered sediment pore water and surface sediments, respectively. The percentage contributions of various matrices were 4, 36 and 60 % for unfiltered surface water, unfiltered pore water and sediment, respectively. The highest concentrations of PCBs were found in water, pore water and sediment collected from sampling sites close to the Northern Wastewater Treatment Works. The highest chlorinated biphenyl, PCB 180, was the most abundant at almost all sampling sites. To our knowledge, this is the first report on occurrence of polychlorinated biphenyls in the Umgeni River water, pore water and sediment system and our results provide valuable information regarding the partitioning of the PCBs between the water and sediment systems as well as the organic chemical quality of the water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polychlorinated biphenyls (PCBs) constitute a group of organic pollutants characterised by their persistence, bioaccumulation and bioamplification in biota, toxicity and long-range transport (Pennington 2001; Sapozhnikova et al. 2004). PCBs are primarily industrial in origin and were intentionally produced and globally used in transformers and condensers; as flame-resistant dielectric insulating fluids; in mining as hydraulic oils; in printing ink, glues, resins, plasticisers, etc., (Manz et al. 2001; Samara et al. 2006). However, their emission also results from domestic waste incineration plants and rubbish dumps, plants processing industrial waste, waste-oil incineration plants and extensive application in agriculture (sewage, sludge) (Manz et al. 2001). They have carcinogenic, mutagenic as well as teratogenic effects (Lauby-Secretan et al. 2013). PCBs have been the subject and focus of extensive research and monitoring in the environment, due to their potential of deleterious effects in the development and reproduction in all biological species, fish behaviour and wildlife (Daouk et al. 2011; Cohn et al. 2011; Katarzyńska et al. 2015). PCBs have been detected in freshwater, wastewater and sediments in different parts of the world (Zhang et al. 2003, 2010; Sapozhnikova et al. 2004; Katsoyiannis and Samara 2004). Their presence in the environment is often related to surrounding activity in the area where they are found (Mwanamoki et al. 2014; Verhaert et al. 2013). Due to their various environmental hazards, they were banned by the United States Congress and the Stockholm Convention in 2001 and are included in the list of priority pollutants to be regularly analysed and monitored (USEPA 1996). Although they were restricted, their residues can still be found in different environmental matrices such as water, sediment, soil, air, biosolids, etc., due to their persistence.
In South Africa, less than a half of the rural population does not have access to clean and safe drinking water and about 12 million people who do not have access to clean water are left to access water from other sources such as rivers (Thwala 2010). Therefore, investigations into the quality of available water resources such as lakes, rivers, etc., are very important for protection of a scarce resource. Until now, many studies have focused on the investigation of heavy metals (Binning and Baird 2001; Fatoki and Awofolu 2003a; Pegram and Bath 1995) and only a few on organic pollution (Fatoki and Awofolu 2003b; Fatoki et al. 2010) in South African rivers.
The Umgeni River is one of the major rivers found in the province of KwaZulu-Natal in South Africa and is one of the main water sources, but the water is of poor quality due to dumping of domestic waste from informal settlements, seepage of industrial wastes, use of agricultural pesticides, etc., that make their way into this river. Studies showed that more than 85 % of contaminants in the Umgeni River basin were from non-point sources (Pegram and Bath 1995). However, some metals have been found in this river (Olaniran et al. 2014). In 2002, a survey of some persistent organic pollutants in major South African waters revealed the presence of some PCB congeners such as PCB 28, PCB 52, PCB 153, PCB 101 and PCB 138, in a sediment sample collected from the mouth of the Umgeni River (Vosloo and Bouwman 2005). No other information about the occurrence and significance of persistent organic pollutants such as PCBs in the Umgeni River has been found. Therefore, the aim of this work was to determine the occurrence, concentrations and significance of eight selected PCBs in water, pore water and sediment samples collected from 15 different sites along the Umgeni River. The water and pore water were analysed without being filtered in order to determine the total concentration of PCBs in water (freely dissolved + dissolved organic carbon + total suspended solids). The Umgeni River water is used by informal settlements along the river for various household activities such as washing, bathing, cleaning and irrigating as well as a source of drinking water for livestock. In order to determine the concentrations that the residents of the informal settlements and the livestock are exposed to, it was necessary to investigate the unfiltered water. The structures of the eight selected PCBs are shown in Fig. 1. These particular PCBs were chosen because some of them are among the most toxic congeners and recommended by the World Health Organization for monitoring (PCB 77 and PCB 105), other PCBs were chosen for the study because they are indicator PCBs (PCB 28, PCB 52, PCB 101, PCB 138, PCB 153 and PCB 180) and are recommended by the European Union for assessing PCB pollution (EC 1999), and this work aimed to determine their presence and quantity in the Umgeni River catchment.
Materials and methods
Physical parameters of the sampling sites
Ambient and water temperature, pH of water (model IQ150, handheld pH/mv/temperature meter), conductivity and total dissolved solids (TDSs) (portable conductivity meter, Schott Handylab LF12) were determined for each sample at the sampling site. Table 1 shows the physical parameters that were recorded at the different sampling sites during winter 2013.
Reagents and standards
High-performance liquid chromatography (HPLC) grade solvents, namely hexane, dichloromethane (DCM) and toluene, and florisil (MgO3Si residue analysis grade, mesh 60–100, pore size 60 Å) as well as the following PCB standards: PCB 28 (2,4,4′-trichlorobiphenyl), PCB 52 (2,2′,5,5′-tetrachlorobiphenyl), PCB 77 (3,3′,4,4′-tetrachlorobiphenyl), PCB 101 (2,2′,4,5,5′-pentachlorobiphenyl), PCB 105 (2,3,3′,4,4′-pentachlorobiphenyl), PCB 138 (2,2′,3,4,4′,5′-hexachlorobiphenyl), PCB 153 (2,2′,4,4′,5,5′-hexachlorobiphenyl) and PCB 180 (2,2′,3,4,4′,5,5′-heptachlorobiphenyl) were purchased from Sigma-Aldrich (South Africa). Anhydrous sodium sulfate (Na2SO4) gold line (CP) and silicon carbide (CSi) boiling stones were obtained from Associated Chemical Enterprises (ACE, South Africa), and sulfuric acid (98 %) was obtained from Promark Chemicals (USA).
Sample collection
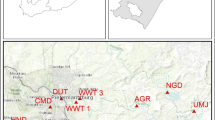
Sampling was carried out during the winter period from 15th to 17th of July 2013 and targeted 15 sampling sites including 12 sites selected along the Umgeni River and 3 sites at the Northern Wastewater Treatment Works and discharge point into the Umgeni River. Sampling sites were selected based on the close proximity of industrial, residential or agricultural activities along the river.
The sampling locations and coordinates are shown in Table 1 and Fig. 2. Water and sediment samples were collected at the same sites. The pore water samples were obtained from the sediment after centrifugation (DuPont® instruments, SS-automatic centrifuge).
Water samples were collected in 2.5-L amber Winchester bottles previously washed with hot water and detergent and rinsed three times with deionised water and sulfuric acid, respectively. At the site, the sampling bottles were rinsed three times with the river water to be collected. The bottles were then filled to overflowing, leaving no headspace.
After water collection, the bottles were closed with caps that were lined with aluminum foil to prevent contamination with phthalates and plasticisers from the lids. A 1-mL aliquot of H2SO4 (50 %) was transferred to each water sample to reduce microbial activity.
Sediment was collected using a grab sampler and transferred to glass bottles that had been previously washed and rinsed as mentioned above. All the samples were kept in a cooler box containing ice and transported to the laboratory where they were kept in a fridge at 4 °C until extraction which was carried out within 3 days.
Sample preparation and cleanup
The water samples were extracted using liquid-liquid extraction (EPA method 3510-C) (EPA 1996a). One litre of water was transferred into a separatory funnel and extracted with a 50-mL aliquot of HPLC grade DCM. This process was repeated six times for each sample with fresh aliquots of DCM each time to increase recovery. All fractions were then combined and transferred into a round-bottom flask and concentrated by rotary evaporation (Heidolph Instruments GmbH & Co. KG) to approximately 5 mL. The concentrated extract was then transferred to a florisil (activated at 130 °C for 12 h) column containing 5 g of anhydrous sodium sulfate as a top layer and eluted sequentially with 5 mL of hexane:DCM (94:6, 85:15 and 50:50) and DCM (100 %) (modified EPA method 3620-C) (EPA 2007). The increasing polarity of the hexane-DCM solvent system allowed elution of different PCBs having different polarity indexes and solubilities with respect to hexane and DCM. All fractions were combined and concentrated with a rotary evaporator and finally air-dried and stored in a fridge at 4 °C until analysis.
After sampling, the sediment samples were subjected to centrifugation using 10 × 1000 revolutions per min (rpm) for 15 min to separate the pore water (Zhang et al. 2003; Ankley and Schubauer-Berigan 1994). The pore water samples were treated as per water samples, extracting 100 mL of pore water with 10 mL of DCM. The centrifuged sediment was transferred onto aluminum foil and air-dried before being ground with a mortar and a pestle and sieved (laboratory test sieves: ss 200 mmϕ × 100 μm to ss 200 mmϕ × 600 μm purchased from DLD Scientific) for homogenisation and to increase the surface area. The sieved sediment (60 g) underwent soxhlet extraction for 24 h with 300 mL of toluene (EPA method 3540-C) (EPA 1996b). The obtained extracts were concentrated with a rotary evaporator to nearly 5 mL. The cleanup and concentration procedures were carried out as mentioned above with a florisil column containing 10 g of anhydrous Na2SO4 as a top layer and eluting with four aliquots of 20 mL of hexane-DCM solvent system. The extracts were air-dried and made up to 2 mL with DCM for analysis using gas chromatography-mass spectrometry (GC-MS).
Sample analysis
Sample analyses were carried out in triplicate using an Agilent 6890 series gas chromatography system attached to a mass spectrometer detector (MSD 5973). The GC system was equipped with a ZB-5MS Capillary column, 0.25 mm i.d., 0.25 μm film thickness and 30 m length (Hewlett Packard, Houston, TX, USA). The MS was operated using the selective ion monitoring (SIM) acquisition mode. The carrier gas was purified helium. A 2-μL injection volume was used on a splitless mode with injector and detector temperatures set at 250 and 280 °C, respectively. The oven temperature for analysis of PCBs was initially 120 °C and then increased to 290 °C at a ramping rate of 14 °C/min and held for 2 min. The total run time was 14.14 min. The MS source was operated at 250 °C and the quad at 200 °C. The electron ionisation energy was 70 eV.
Target analytes were quantified based on peak areas and using an external calibration technique with the following six calibration concentrations: 0.25, 0.5, 1, 2, 4 and 8 μg/mL. The actual samples were spiked with a known concentration of the analytes and analysed as total concentration (actual + spiked) which fell in the range of the calibration standards. The actual concentration of the analytes in the real samples was calculated by subtracting the known spiked concentration from the total concentration. The identification of analytes of interest was achieved by comparison of retention times of sample analytes with those of reference standards and using the NIST library mass spectral data by monitoring three ions for each analyte (see Figs. S1 and S2 in supplementary information (SI)).
Quality assurance
For water and pore water samples, the extraction recoveries were obtained by spiking tap water (three samples) (Meharg et al. 2003; Hellar-Kihampa et al. 2013) with known concentrations of standards and percentage recoveries were calculated (APHA et al. 1999; USEPA 2008) (Table 2). For sediment sample recoveries, real sediment samples (three samples from three different sites) were subdivided into two subsamples whereby one was spiked with known concentrations of standards before extraction and the other was left unspiked. The recoveries were obtained by subtracting non-spiked subsample concentrations from spiked subsample concentrations (Harry et al. 2008) (Table 2). The recovery samples were analysed at the same time with the actual samples. Procedural lab blanks (three to five) were used throughout all extractions and analyses for all matrices. There were no detectable levels of analytes of interest in the extracted blank samples. The solvent blanks and standards were regularly analysed on the GC-MS to monitor the presence of interferences and the condition of the column and instrument. To ensure minimal variation from the initial calibration standards, a calibration standard (0.5 μg/mL) was analysed after each batch of sample to ensure consistency of results. The identification of all analytes was possible using the base peak and two other confirming fragments including molecular ion. The limits of detection and quantification were calculated as three times and ten times, respectively, the signal-to-noise ratio using the standard deviation of the three calibration intercepts divided by the slope (Table 2). All data were processed using Microsoft Excel (version 2010).
Results and discussion
Polychlorinated biphenyls in surface water
The concentrations of different PCB congeners investigated are shown in Table 3 and Fig. 3. All investigated PCBs were detected in all sites. The concentration of PCB 180 (log K ow = 6.82) was highest in all sites, while that of PCB 28 (log K ow = 5.71) was lowest (see Fig. S3 in SI). The relatively high log K ow value for PCB 180 corresponds to low solubility in water, and it was therefore expected to be present in lower concentrations in water. However, the higher concentrations of PCB 180 in water may be explained by the strong affinity of this high molecular weight PCB with total suspended solids (TSSs) and dissolved organic carbon (DOC). The water samples were unfiltered in order to determine the concentrations that animals and humans are exposed to when they directly consume it. Hence, the water samples contained high TSS and DOC to which PCB 180 would partition to resulting in its high concentrations in water (Aparna et al. 2014; Matyas et al. 2015; Zhang et al. 2011). Furthermore, PCB 180 has a higher number of chlorine atoms (seven) compared to other investigated congeners and, consequently, was more difficult to degrade, lasting longer in the aquatic environment (de Voogt et al. 1990; Nhan et al. 2001). This suggests that its presence in the environment was due to accumulation over time rather than point source entry. This is in contrast to PCB 28 which has three chlorine atoms and was found to be present in the lowest concentration. The lowest concentration for PCB 28 was observed at Howick Falls (HOF) (0.42 ng/mL), and the highest concentration of PCB 180 was observed at Northern Wastewater Treatment Works influent (NWTI) (7.34 ng/mL).
The mean concentration of individual PCBs in water was 1.36 ± 0.07 ng/mL. The high concentration at NWTI was expected because this plant receives residential and industrial waste from the surrounding area which may contain high levels of PCBs. The conductivity was 970.00 μs/cm (Table 1) at this site, which is the second highest value observed, and indicates content high in dissolved ions, and the TDS value was also high at this site (568 mg/L) (Table 1). This suggests a highly contaminated site which again provides organic content in the water onto which PCB 180 can adsorb, resulting in its high concentration at this site (ter Laak Thomas et al. 2009). In addition, oxygen is required for the proliferation of water-dwelling animals and microorganisms. A high TDS content interferes with oxygen transfer (Pophali et al. 2003), leading to a reduced growth of microbes. As a result, the reduction in microbial growth results in little or no microbial degradation of PCBs which may also contribute to the high levels of PCBs observed at this sampling site (Rein et al. 2007; Chang et al. 2001). The total concentration of PCBs in water samples varied from 6.91 to 21.69 ng/mL with a mean concentration of 10.90 ± 0.56 ng/mL (Table S1 in SI, Fig. 4). This high level of PCBs in this river water may also be explained partly by the leaching of PCBs from urban activities at Durban as was demonstrated by Chevreuil and Granier who showed that the main cause of high levels of PCB in the basin of river Seine in France was leaching (Chevreuil and Granier 1985; Kim et al. 2007).
The wastewater treatment process showed some reduction in the concentrations of the PCBs in the water when it reached the Northern Wastewater Treatment Works after treatment (NWTT) compared to the NWTI (Table S2 in SI, Fig. 3). The treatment process was most able to reduce PCB 180, PCB 101 and PCB 77 by 76.29, 72.32 and 55.44 %, respectively, of the amount received by the plant at the influent (Table S2). This reduction may be attributed to either the actual chemical treatment used in the plant process or the hydrophobic PCBs partitioning itself into the phase containing the organic particles which then settle in the sedimentation step, during the wastewater treatment process. In a study on activated sludge treatment process, Katsoyiannis and Samara found that WWTP sludge contains between 39 and 98 % of persistent organic pollutants (POPs) and the fraction remaining in treated water was due to adsorption of these pollutants onto non-settleable solids (Katsoyiannis and Samara 2005). Concerning the Northern Wastewater Treatment Works (NWWTW), mentioned in this study, more investigation is needed to determine the annual loading of POPs, their accumulation in the sludge and their remaining fraction in treated water or if the addition of a chemical coagulation agent affects the partitioning of POPs between the water and biosolids. Studies on wastewater treatment plants also showed that in general, persistent organic pollutants were in higher concentrations in the influent than effluent (Mowery and Loganathan 2007).
Polychlorinated biphenyls in sediment pore water
The concentrations of PCBs in pore water ranged from not detectable level for PCB 28 at MDO to 52.30 ng/mL for PCB 77 at Umgeni Business Park (UBP) (Table 4) with an average concentration of 14.60 ± 0.81 ng/mL. The concentrations were generally lower towards the source of the river and increased towards the mouth (Table 4, Fig. 5). This may be due to the increase of industrial activities as the river flows down towards Howick and the city of Durban or also an accumulation effect of the PCBs as the river flows downstream towards the mouth carrying with it TSS and DOC with PCBs partitioned to it. PCB 180 was again the most abundant PCB in pore water in almost all sampling sites (Table 4, Fig. 5), confirming its strong affinity with TSS and DOC whose concentrations were greater in unfiltered pore water than unfiltered surface water (Aparna et al. 2014; Matyas et al. 2015; Zhang et al. 2011). Another possible reason is its highly chlorinated structure which makes it less volatile and lipophilic, allowing it to be preferentially retained in the sediment pore water (de Voogt et al. 1990).
The total concentrations of congeners at each site (Table 4) showed that the levels of PCBs were highest at NWTT and NWTE due to the accumulation of contaminants from different sources that make their way to the treatment plant. The higher levels of total PCBs at sites Inanda Dam outlet (IDO) and UBP were attributed to the low water flow rate at these sites, allowing time for the TSS onto which the pollutants were partitioned, to settle in sediment and therefore be extracted in its pore water. PCB 77 was in high concentrations at UBP (Table 4, Fig. 5, Fig. S4 in SI) which suggested a possible input of this congener at this site from sources such as transformer liquids, incineration of waste or from construction material (this site is currently under construction and is being used by heavy machinery).
The total PCB levels in pore water varied from 40.67 to 252.37 ng/mL with a mean of 116.77 ng/mL (Table S1, Fig. 4), which is higher than that in water. The higher concentrations in pore water are expected because PCBs are hydrophobic and tend to associate with organic materials found in sediments rather than dissolve in water (Julia et al. 2012). However, the hydrophobic PCBs in sediment tend to re-suspend from sedimentary phase to the pore water (Zhang et al. 2003) which may be the case for the present study. Studies on sediment pore water distribution models of POPs have also confirmed higher POP concentrations in pore water than in water (Perssona et al. 2005).
Polychlorinated biphenyls in surface sediments
The concentrations along the river ranged from 10.16 ng/g (PCB 105 at IDO) to 93.74 ng/g (PCB 28 at NWTT) with an average of 24.31 ± 1.10 ng/g (dry weight (dw)) (Table 5, Fig. 6). All the PCB congeners investigated were detected in all sediment samples. This may be attributed to the strong affinity that exists between the hydrophobic pollutants and sediment organic carbon (Kookana 2011). As in water and pore water, the level of PCB 180 was highest in all sediment samples (Table S1) due to its hydrophobicity in the aquatic environment which is related to its K ow value and, therefore, its sorption to the organic matter in sediment (Zhou et al. 2005). The distribution of PCB congeners in the aquatic systems may also be assigned to losses of less chlorinated congeners through volatilisation, sedimentation and degradation by microbial activity as well as thermal and UV light degradation (Brown et al. 1987; Quensen et al. 1988; MacDonald et al. 1992; De et al. 2006). PCBs 28 and 77 were in unusually high concentrations at NWTT (Fig. S5 in SI). This suggested that apart from the waste received by the plant, there may be another input of these two congeners at that site such as industrial effluent received by the plant (Gioia et al. 2014). The lowest total concentrations of PCBs were observed at IDO, while the highest was recorded at NWTT. The relatively low concentrations at the IDO sampling site could be because most of the PCBs had already been extracted from the sediment in the pore water (Table 4). Zhao and his co-workers showed that high PCB levels occur in the fraction of sediment with grain size of 31 to 63 μm (Zhao et al. 2010). In addition, studies on sorption of hydrophobic pollutants on natural sediment demonstrated that the sand fraction (>50 μm) is considerably less effective in adsorption of hydrophobic pollutants (Karickhoff et al. 1979; Ke-xin et al. 2003; Carro et al. 2002). Therefore, since more than 52 % of IDO sediment size was higher than 300 μm (particle sizes determined during grinding and sieving steps of sample preparation), it could not retain much pollutant and, hence, pore water was more concentrated than the sediment itself at this site. For the NWTT, the high concentration of PCBs in its biosolid was expected since this site continually receives treated water before being discharged. The treated water still has considerable amounts of PCBs which eventually partition itself in the biosolid sampled at the NWTT site resulting in its increased concentration (Table 5). The levels of PCBs in the biosolids of NWTI were generally lower than those in the biosolids of NWTT (Fig. 6). This was because the biosolid at NWTI was fresh and occasional, while the biosolid at NWTT accumulated over time at that sampling point. Note that in all matrices, the levels of PCB concentrations at sites close to and exiting the NWWTW were high. Other studies have also found that wastewater treatment plants are important point sources of POP contamination (Samara et al. 2006).
Comparison of sediment total PCBs with levels found elsewhere in the world
The total concentration of PCB congeners in sediment fluctuated between 102.62 and 427.83 ng/g with a mean concentration of 194.50 ± 8.79 ng/g (dry weight) (Table S1, Fig. 4). Concentrations of PCBs in sediment were higher than those in pore water and much higher than those in water (Fig. 4). This was expected because PCBs are hydrophobic and tend to be adsorbed onto solid organic particles and sediment (Bazzanti et al. 1997). Compared to PCBs detected elsewhere, this level was higher than that detected in the sediments obtained from the Yamuna River in Delhi in India (0.20–21.16 ng/g) with a mean of 6.63 ± 0.69 ng/g (Kumar et al. 2013). The levels in this study were also found to be higher than those found in Vietnam (Hanoi region) (0.47–28.10 ng/g, dw) (Nhan et al. 2001) or those in the Bahlui River, in Eastern Romania (24–158 ng/g with a mean of 59 ng/g) (Dragan et al. 2006). However, the present results were in the same range as those obtained in surface sediments of an industrialised urban river (Huveaune) in France (2.8–435 ng/g) having an average concentration of 148 ± 64 ng/g (Kanzan et al. 2014) and lower than PCB levels found in the Keelung River in Taiwan (mean 230 ng/g, dw) (Iwata et al. 1994), Minjiang Estuary (985.2 ng/g), Pearl Estuary (635.7 ng/g), Jiaozhou Bay (273.3 ng/g) (Xing et al. 2005) and upper Sheboygan River, Wisconsin in USA (1000–104,000 ng/g) (Li et al. 2005). No comparison could be made on the temporal PCB concentration trends, as no data were available on the past PCB levels for the investigated river in this study. Note that the current study revealed that the total average PCB level in the Umgeni River sediment was very much lower than the interim freshwater sediment quality guidelines (ISQG) of 21,500 ng/g (dw) and probable effect level (PEL) of 189,000 ng/g (dw) permitted by the Canadian quality sediment guidelines (CCME 2002). According to Ontario sediment quality guidelines, however, the total average of PCBs in the sediment of Umgeni River was found to be higher than the lowest effect level (LEL) (70 ng/g, dw) and far less than the severe effect level (SEL) (530,000 ng/g, dw) (Persaud et al. 1993).
Conclusion
The present study has provided data on the levels of PCBs in water, pore water and sediments from 15 sampling sites along the Umgeni River. All the eight selected PCB congeners were found in all 15 sites investigated and in all matrices. The concentrations of PCBs increased clearly at the sites of the Wastewater Treatment Works. The total concentrations of eight polychlorinated biphenyls were 6.91–21.69 ng/mL, 40.67–252.30 ng/mL and 102.60–427.80 ng/g (dry weight) in surface water, pore water and surface sediments, respectively. The PCB levels were higher in sediment than in water and pore water. In general, PCB 180 is the most abundant PCB in water, pore water and sediment samples which was expected because PCB 180 has the highest molecular weight in this study’s set of investigated analytes and has a K ow value that shows its preference to adsorb onto organic particles which was present in high amounts in the unfiltered samples. Our results suggest that the Umgeni River water quality is poor with regard to pollution by PCBs and may represent a risk to human health and aquatic environment. The findings of this study indicate that there is a need to establish a reliable system of monitoring polychlorinated biphenyls and other organochlorinated compounds in order to take appropriate action to maintain environmental water quality standards in the Umgeni River. Further research is needed on levels of organochlorines in aquatic biota such as fish and aquatic plants in the Umgeni River to allow a complete assessment of the risks these contaminants have on aquatic life and human health.
References
Ankley, G. T., & Schubauer-Berigan, M. K. (1994). Comparison of techniques for the isolation of sediment pore water for toxicity testing. Archives of Environmental Contamination and Toxicology, 27, 507–512.
Aparna, B., Nathan, H. L., & Hanadi, R. S. (2014). Polychlorinated biphenyls (PCBs) in industrial and municipal effluents: concentrations, congener profiles, and partitioning onto particulates and organic carbon. Science of the Total Environment, 473–474, 702–713.
APHA, AWWA & WEF. (1999). Standard methods for the examination of water and wastewater [online]. American Public Health Association; American Water Works Association and Water Environmental Association. Available: www.mwa.co.th/download/file_upload/SMWW_1000-3000.pdf. Accessed 4 Aug 2014.
Bazzanti, M., Chiavarini, S., Crimiseni, C. & Soldati, P. (1997). Distribution of PCB congeners in aquatic ecosystems: A case study. Environmental International, 23, 799--813.
Binning, K., & Baird, D. (2001). Survey of heavy metals in the sediments of the Swartkops River estuary, Port Elizabeth, South Africa. Water SA, 27, 461–466.
Brown, J. F., Bedar, D. L., Brennan, M. J., Granhan, J. C., Feng, H., & Wagner, R. E. (1987). Polychlorinated biphenyl dechlorination in aquatic sediments. Science, 236, 709–712.
Carro, N., Garcia, I., Ignacio, M., & Mouteira, A. (2002). Levels and distribution patterns of polychlorinated biphenyl congeners in surface sediments from Galicia coast (North-Western, Spain) according to granulometric characteristics. Journal of Environmental Technology, 23, 919–930.
CCME. (2002). Canadian sediment quality guidelines for the protection of aquatic life [online]. Canadian Council of Ministers of the Environment. Available: http://www.pla.co.uk/Environment/Canadian-Sediment-Quality-Guidelines-for-the-Protection-of-Aquatic-Life. Accessed 29 Aug 2014
Chang, B. V., Liu, W. G., & Yuan, S. Y. (2001). Microbial dechlorination of three PCB congeners in river sediment. Chemosphere, 45, 849–856.
Chevreuil, M., & Granier, L. (1985). Seasonal cycle of polychlorinated biphenyl in the waters of the catchment basin of the river Seine (France). Aquatic Toxicology, 7, 217–229.
Cohn, A. B., Cirillo, M. P., Sholtz, I. R., Ferrara, A., Parkc, J.-S., & Schwingl, J. P. (2011). Polychlorinated biphenyl (PCB) exposure in mothers and time to pregnancy in daughters. Reproductive Toxicology, 31, 290–296.
Daouk, T., Larcher, T., Roupsard, F., Lyphout, L., Rigaud, C., Ledevin, M., Loizeau, V., & Cousin, X. (2011). Long-term food-exposure of zebrafish to PCB mixtures mimicking some environmental situations induces ovary pathology and impairs reproduction ability. Aquatic Toxicology, 105, 270–278.
DE Voogt, P., Wells, D. E., Reutergardh, L., & Brinkman, U. A. T. (1990). Biological activity, determination and occurrence of planar, mono-ortho and di-ortho PCBs. International Journal of Environmental Analytical Chemistry, 40, 1–46.
De, S., Perkins, M., & Dutta, S. K. (2006). Nitrate reductase gene involvement in hexachlorobiphenyl dechlorination by Phanerochaete chrysosporium. Journal of Hazardous Materials, B135, 350–354.
Dragan, D., Cucu-Man, S., Dirtu, A. C., Mocanu, R., Vaeck, L. V., & Covaci, A. (2006). Occurrence of organochlorine pesticides and polychlorinated biphenyls in soils and sediments from Eastern Romania. International Journal of Environmental Analytical Chemistry, 86, 833–842.
EC. (1999). EU commission decision 1999/788/EC of 3 December 1999 on protective measures with regard to contamination by dioxins of certain products of porcine and poultry origin intended for human or animal consumption. Official Journal of the European Union, L310, 62–70.
EPA. (1996a). Method 3510 C, separatory funnel liquid-liquid extraction [online]. Available: http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/3510c.pdf. Accessed 9 Aug 2014.
EPA. (1996b). Method 3540C: soxhlet extraction [online]. Available: http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/3540c.pdf. Accessed 10 Mar 2014.
EPA. (2007). Method 3620: florisil cleanup [online]. Available: http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/3620c.pdf. Accessed 10 Mar 2014.
Fatoki, O. S., & Awofolu, O. R. (2003a). Levels of Cd, Hg and Zn in some surface waters from the Eastern Cape Province, South Africa. Water SA, 29, 375–380.
Fatoki, O. S., & Awofolu, O. R. (2003b). Persistent organochlorine pesticides residues in freshwater systems and sediments from the Eastern Cape, South Africa. Water SA, 29, 323–330.
Fatoki, O. S., Bornman, M., Ravandhalala, L., Chimuka, L., Genth, B., & Adeniyi, A. (2010). Phthalate ester plasticizers in freshwater systems of Venda, South Africa and potential health effects. Water SA, 36, 117–125.
Gioia, R., Akindele, A. J., Adebusoye, S. A., Asante, K. A., Tanabe, S., Buekens, A., & Sasco, A. J. (2014). Polychlorinated biphenyls (PCBs) in Africa: a review of environmental levels. Environmental Science Pollution Research, 21, 6278–6289.
Harry, B. M., Lynn, S. W. & Judith, A. S. (2008). Alternative approaches to collecting and interpreting matrix spike data. 27th annual EPA conference on managing environmental quality systems [online]. Available: http://www.epa.gov/QUALITY/qs-2008/alternative.pdf. Accessed 12 June 2014.
Hellar-Kihampa, De Wael, K., Lugwisha, E., Malarvannan, G., & Covacid, A. R. V. G. (2013). Spatial monitoring of organohalogen compounds in surface water and sediments of a rural–urban river basin in Tanzania. Science of the Total Environment, 447, 186–197.
Iwata, H., Tanabe, S., Sakai, N., Nishimura, A., & Tatsukawa, R. (1994). Distribution of persistent organochlorines in the oceanic air and surface sea water and the role of ocean on their global transport and fate. Environmental Pollution, 85, 15–33.
Julia, F., Ralph, M., Sebastian, H., Evelyn, C. & S, C. E. W. (2012). Organic carbon source in formulated sediments influences life traits and gene expression of Caenorhabditis elegans. Journal of Ecotoxicology, 21, 557-568
Kanzan, F., Syakti, A. D., Asia, L., Malleret, L., Piram, A., Mille, G., & Doumenq, P. (2014). Distributions and sources of persistent organic pollutants (aliphatic hydrocarbons, PAHs, PCBs and pesticides) in surface sediments of an industrialised urban river (Huveaune). France Science of the Total Environment, 478, 141–151.
Karickhoff, S. W., Brown, D. S., & Scott, T. A. (1979). Sorption of hydrophobic pollutants on natural sediments. Water Research, 13, 241–248.
Katarzyńska, D., Hrabia, A., Kowalik, K., & Sechman, A. (2015). Comparison of the in vitro effects of TCDD, PCB 126 and PCB 153 on thyroid-restricted gene expression and thyroid hormone secretion by the chicken thyroid gland. Environmental Toxicology and Pharmacology, 39, 496–503.
Katsoyiannis, A., & Samara, C. (2004). Persistent organic pollutants (POPs) in the sewage treatment plant of Thessaloniki, Northern Greece: occurrence and removal. Water Research, 38, 2685–2698.
Katsoyiannis, A., & Samara, C. (2005). Persistent organic pollutants (POPs) in the conventional activated sludge treatment process: fate and mass balance. Environmental Research, 97, 245–257.
Ke-Xin, J., Bi-Xian, M., Guo-Ying, S., Jia-Mo, F., & Ping-an, P. (2003). Grain-size distribution of polychlorobiphenyls in sediments of the Pearl River. Diqiu Huaxue, 32, 606–612.
Kim, J. G., Kim, K. S., Oh, J. G., Choi, K. H., & Yoon, J. H. (2007). Sources of CO-PCBs in sediments of Han River in the South Korea. Organohalogen Compounds, 69, 551–554.
Kookana, R. S. (2011). The role of black carbon in environmental fate of persistent organic pollutants (POPs) in soils and their effect on food safety [online]. Australia: CSIRO Land and Water, PMB 2, Glen Osmond, 5064. Available: http://www.fftc.agnet.org/htmlarea_file/library/20110804154803/eb621.pdf. Accessed 22 Feb 2015.
Kumar, B., Kumar, S., & Sharma, C. S. (2013). Ecotoxicological risk assessment of polychlorinated biphenyl (PCBs) in bank sediments from along the Yamuna River in Delhi, India. Human and Ecological Risk Assessment, 19, 1477–1487.
Lauby-Secretan, B., Loomis, D., Grosse, Y., EL Ghissassi, F., Bouvard, V., Benbrahim-Tallaa, L., Guha, N., Baan, R., Mattock, H., & Straif, K. (2013). Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncology, 14, 287–288.
Li, J., Mgonella, K. M., Bzdusek, A. P., & Christensen, E. R. (2005). PCB congeners and dechlorination in sediments of Upper Sheboygan River, Wisconsin. Journal of Great Lakes Research, 31, 174–186.
Macdonald, C. R., Metclafe, C. D., Metcalfe, T., & Baltch, G. C. (1992). Temporal trends and distribution of PCB congeners in a small contaminated lake in Ontario Canada. In F. A. P. C. Gobas & J. A. Mccorqodale (Eds.), Chemical dynamics in freshwater ecosystems. Boca Raton: FL Lewis.
Manz, M., Wenzel, K. D., Dietz, U., & Schuurmann, G. (2001). Persistent organic pollutants in agricultural soil of central Germany. Science of the Total Environmental, 277, 187–198.
Matyas, R., Joanna, P., Joao, F., Cathrin, V., & Pete, H. (2015). Dissolved organic carbon quality and sorption of organic pollutants in the Baltic Sea in light of future climate change. Environmental Science & Technology, 49, 1445–1452.
Meharg, A. A., Wright, J., Leeks, G. J. L., Wass, P. D., Owens, P. N., Walling, D. E., & Osborn, D. (2003). PCB congener dynamics in a heavily industrialized river catchment. Science of the Total Environment, 314–316, 439–450.
Mowery, H. R. & Loganathan, B. G. (2007). Persistent organic compounds in wastewater: azithromycin and urobilin concentrations in wastewater treatment plant samples from Murray, Kentucky, USA [online]. Murray, USA: Department of Chemistry and Center for Reservoir Research, Murray State University, Murray, KY 42071-3346, USA. Available: http://campus.murraystate.edu/services/URSA/FINAL_PAPER_Holly_Mowery.pdf. Accessed 19 Aug 2014
Mwanamoki, P. M., Devarajan, N., Thevenon, F., Birane, N., de Alencastro, L. F., Grandjean, D., Mpiana, P. T., Prabakar, K., Mubedi, J. I., Kabele, C. G., Wildi, W., & Poté, J. (2014). Trace metals and persistent organic pollutants in sediments from river-reservoir systems in Democratic Republic of Congon (DRC): spatial distribution and potential ecotoxicological effects. Chemosphere, 111, 485–492.
Nhan, D., Carvarho, F., & Am, N. (2001). Chlorinated pesticides and PCBs in sediments and molluscs from freshwater canals in the Hanoi region. Environmental Pollution, 112, 311–320.
Olaniran, A. O., Naiker, K., & Pillay, B. (2014). Assessment of physical-chemical qualities and heavy metal concentrations of Umgeni and Umdloti Rivers in Durban, South Africa. Journal of Environmental Monitoring and Assessment, 186, 2629–2639.
Pegram, G. C., & Bath, A. J. (1995). Role of non-point sources in the development of a water quality management plan for the Mgeni River catchment. Water Science & Technology, 32, 175–182.
Pennington, D. W. (2001). An evaluation of chemical persistence screening approaches. Chemosphere, 44, 1589–1601.
Persaud, D., Jaagumagi, R. & Hayton, A. (1993). Guidelines for the protection and management of aquatic sediment quality in Ontario [online]. Ontario Ministry of Environment & Energy. Standard development branch and environmental monitoring and reporting branch. Available: http://www.itrcweb.org/contseds-bioavailability/References/guide_aquatic_sed93.pdf. Accessed 29 Aug 2014.
Perssona, N. J., Buchelia, T. D., Gustafssona, O., Bromana, D., Naesb, K., Ishaqa, R. & Zebuhra, Y. (2005). Testing common sediment-porewater distribution models for their ability to predict dissolved concentrations of POPs in The Grenlandsfjords, Norway. Chemosphere, 59, 1475--1485.
Pophali, G. R., Kaul, S. N., & Mathur, S. (2003). Influence of hydraulic shock loads and TDS on the performance of large-scale CETPs treating textile effluents in India. Water Research, 37, 353–361.
Quensen, J. F., Tiedje, J. M., & Boyd, S. A. (1988). Reductive dechlorination of polychlorinated biphenyls by anaerobic microorganisms from sediments. Science, 242, 752–754.
Rein, A., Fernqvist, M. M., Mayer, P., Trapp, S., Bittens, M., & Karlson, G. U. (2007). Degradation of PCB congeners by bacterial strains—determination of kinetic parameters and modelling of rhizoremediation. Applied Microbiology and Biotechnology, 77, 469–481.
Samara, F., Tsai, C. W., & Aga, D. S. (2006). Determination of potential sources of PCBs and PBDEs in sediments of Niagara River. Environmental Pollution, 139, 489–497.
Sapozhnikova, Y., Dawardi, O., & Schlenk, D. (2004). Pesticides and PCBs in sediments and fish from Salton Sea, California, USA. Chemosphere, 55, 797–809.
Ter Laak Thomas, L., H, V. E. J. C., M, B. F. J., Van Leeuwen Herman, P. & M., H. J. L. (2009). Facilitated transport of polychlorinated biphenyls and polybrominated diphenyl ethers by dissolved organic matter. Environmental Science and Technology, 43, 1379-1385.
Thwala, D. W. (2010). Community participation is a necessity for project success: a case study of rural water supply project in Jeppes Reefs, South Africa. African Journal of Agricultural Research, 5, 970–979.
USEPA. (1996). Method 3630, revision C [online]. Washington, DC, USA: US Environmental Protection Agency. Available: http://www.epa.gov/solidwaste/hazard/testmethods/sw846/pdfs/3630c.pdf. Accessed 20 May 2015.
USEPA (2008). Method 1668B chlorinated biphenyl congeners in water, soil, sediment, biosolids, and tissue by HRGC/HRMS. Quantitative determination. Washington, DC 20460: Office of Science and Technology Engineering and Analysis Division (4303T), 1200 Pennsylvania Avenue, NW
Verhaert, V., Covaci, A., Bouillon, S., Abrantes, K., Musibono, D., Bervoets, L., Verheyen, E., & Blust, R. (2013). Baseline levels and trophic transfer of persistent organic pollutants in sediments and biota from the Congo River Basin (DR Congo). Environment International, 59, 290–302.
Vosloo, R. & Bouwman, H. (2005). Survey of certain persistent organic pollutants in major South African waters [online]. Available: http://www.wrc.org.za/Knowledge%20Hub%20Documents/Research%20Reports/1213-1-05.pdf. Accessed 27 Feb 2015.
Xing, Y., Lu, Y., Dawson, R. W., Shi, Y., Zhang, H., Wang, T., Liu, W., & Ren, H. (2005). A spatial temporal assessment of pollution from PCBs in China. Chemosphere, 60, 731–739.
Zhang, Z. L., Hong, H. S., Zhou, J. L., Huang, J., & Yu, J. (2003). Fate and assessment of persistent organic pollutants in water and sediment from Minjiang River Estuary, Southeast China. Chemosphere, 52, 1423–1430.
Zhang, H., Zhao, X., Ni, Y., Lu, X., Chen, J., Su, F., Zhao, L., Zhang, N., & Zhang, X. (2010). PCDD/Fs and PCBs in sediments of Liaohe River, China: levels, distribution and possible sources. Chemosphere, 79, 754–762.
Zhang, L., Shi, S., Dong, L., Zhang, T., Zhou, L., & Huang, Y. (2011). Concentrations and possible sources of polychlorinated biphenyls in the surface water of the Yangtze River Delta, China. Chemosphere, 85, 399–405.
Zhao, X., Zheng, B., Qin, Y., Jiao, L., & Zhang, L. (2010). Grain size effect on PBDE and PCB concentrations in sediments from the intertidal zone of Bohai Bay, China. Chemosphere, 81, 1022–1026.
Zhou, W., Zhai, Z., Wang, Z., & Wang, L. (2005). Estimation of n-octanol/water partition coefficients (Kow) of all PCB congeners by density functional theory. Journal of Molecular Structure, 755, 137–145.
Acknowledgments
We wish to thank the University of KwaZulu-Natal (UKZN) and the Water Research Commission (WRC) of South Africa for financially supporting this project. We are also grateful to the School of Chemistry and Physics at UKZN for laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 365 kb)
Rights and permissions
About this article
Cite this article
Gakuba, E., Moodley, B., Ndungu, P. et al. Occurrence and significance of polychlorinated biphenyls in water, sediment pore water and surface sediments of Umgeni River, KwaZulu-Natal, South Africa. Environ Monit Assess 187, 568 (2015). https://doi.org/10.1007/s10661-015-4790-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4790-1