Abstract
Suitable reservoirs and monitoring methods are needed to manage scarce water supplies in dry countries. We assessed here the impact on aquatic macroinvertebrates of the only dam on the Eerste River, which runs through the heart of a biodiversity hotspot, the Cape Floristic Region, South Africa. The dam and associated activities, were the only forms of disturbance in this otherwise pristine area. We sampled over 20,000 macroinvertebrate individuals and illustrated some categorical effects of the impoundment and its effects on macroinvertebrate assemblages. Macroinvertebrate species diversity below the dam was only half of that in the pristine catchment area above the dam. Furthermore, Ephemeroptera, Plecoptera and Trichoptera diversity and abundance dropped to almost zero as a result of the impoundment. In contrast, the abundance of the Diptera family Chironomidae increased substantially below the dam. These changes in macroinvertebrate diversity mirrored those recorded in biologically less diverse areas, but are of major concern in this biodiversity hotspot with its rich endemic fauna. We conclude that such an impoundment, while important for human welfare, results in a high price being paid in terms of loss of local biodiversity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rivers are highly vulnerable to anthropogenic change, and their flow is often manipulated to provide water for human use. To deal with the greater demands on the world’s freshwater (March et al. 2003), barriers are constructed for power generation, flood control and irrigation, resulting in widespread degradation of river ecosystems (Zwick 1992; Ward 1998; King and Schael 2001). River systems are affected severely by the disruption of the natural course and flow, altered water temperatures, redirection of river channels, transformation of floodplains, and disruption of river continuity (Petts 1984; Yeager 1994; Ligon et al. 1995; Ward and Stanford 1995; Stanford et al. 1996; Poff et al. 1997; Born et al. 1998). These major changes often transform and reduce the biological composition of rivers, isolating populations of wildlife and their habitats within a river (McIntosh et al. 2002; Barow et al. 2005). Studies on the effects of impoundments include Quinn and Hickey (1990), Winston et al. (1991), Doeg and Koehn (1994), Drinkwater and Frank (1994), Ligon et al. (1995), Ward and Stanford (1995), Malmqvist and Englund (1996), Kondolf (1997), while Bednarek (2001) studied the effect of removing impoundments.
The search for improved methods of monitoring water quality has led to intense development of techniques for rapid bioassessment of rivers and evaluation of water quality using benthic macroinvertebrates (Rosenberg and Resh 1993; Metcalf-Smith 1994; Resh 1995; Dickens and Graham 2002). These techniques are used for assessment of general river condition, and are implemented by many regulatory authorities for management of aquatic resources (Dickens and Graham 2002).
South Africa has scarce water supplies and unpredictable seasonal rainfall, with water demands expected to exceed supply within the next few years (Basson et al. 1997). Furthermore, the country is significant for global biodiversity, as it is home to three of the world’s 34 biodiversity hotspots (Myers et al. 2000; Mittermeier et al. 2004). This amplifies the importance of appropriate evaluation and management of riverine ecosystems in this area and which are under so much anthropogenic pressure, yet rich in irreplaceable biodiversity.
Monitoring methods determine the ecological status of rivers and provide a basis on which to develop practicable conservation strategies. These methods can be physical, chemical or biological. Abiotic and biotic factors along a stream vary greatly (Vannote et al. 1980). The purpose of biological assessment is to characterize the status of the water and to monitor variations in water condition associated with anthropogenic perturbation (Resh 1995). Any assessed river site can then be compared with similar, less-impacted ones, so as to provide a reference condition of how far removed from “natural” the site might be (King and Schael 2001).
Biological monitoring (biomonitoring) makes use of the living components of the studied environment, and indicates, as well as assesses, ecological degradation, transformation, improvement or other effects, resulting from a localized event or variable. Benthic macroinvertebrates, being widespread and sensitive to environmental changes, are widely used for assessment of freshwater resources (Resh 1995), and have been shown to be the most reliable of all the bioindicators used (Williams and Feltmate 1992; Rosenberg and Resh 1993; Rosenberg 1998; Roque et al. 2002; Iliopoulou-Georgudaki et al. 2003). Indeed, many species of macroinvertebrates are diagnostic of certain kinds of habitats and specific water quality (Mackie 1998). Their presence, abundance and activities reveal something about the state of the ecosystem in which they are found, and whether processes are operating according to expectations within normal bounds (Kevan 1999).
In South Africa, the South African Scoring System (SASS5), which is a modified version of methods used by the British Monitoring Working Party (BMWP) (Dickens and Graham 2002), is widely used for measuring water quality. This method has rarely been employed to study the impacts of impoundments on biodiversity, particularly in a biodiversity hotspot.
This study assesses the impact of the Kleinplaas Dam (storage capacity of 337,000 m3) (Brown and Dallas 1995) situated in the Eerste river, arising in the Hottentots Holland mountains in the heart of the Cape Floristic Region (CFR) biodiversity hotspot. The water system upstream of the impoundment is relatively pristine, whereas further downstream from the study area, the river is degraded due to multiple direct and indirect disturbances (Brown and Dallas 1995). Against this background, this study aimed to quantify the influence of the dam on benthic macroinvertebrate biodiversity.
Sites and methods
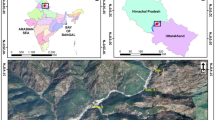
The study was upstream and downstream of the Kleinplaas dam on the Eerste River, Jonkershoek, Western Cape, South Africa (38 58′11″ S; 18 55′31″ E) (Fig. 1). Thirty lotic river sites (20 m) were chosen on 3rd–4th order streams for accessibility and to represent as many microhabitats as possible. Fifteen sites were downstream of the dam, at the Yellow foot trail station (referred to here as “downstream”), spread over 300 m, starting 800 m from the dam wall. Fifteen sites were upstream of the dam (referred to here as “upstream”). Nine of these upstream sites were farther up the river, at the White Bridge station (covering 350 m, starting 1,220 m from the dam inflow), and six were just above the dam at the Concrete Bridge station (covering 350 m, starting 600 m above the dam inflow). An experimental cage-trout farm was situated in the dam, covering 20% of its surface.
Based on the River Continuum Concept (Vannote et al. 1980), any macroinvertebrate community discontinuities at the point of the dam were considered here to be indicative of the immediate and direct effect of the dam. This was more appropriate than having another river acting as a control and where the biotic and abiotic variables may differ considerably from one river to another.
All sampling and data processing was done according to the stipulations of the South African Scoring System version 5 (SASS5) (Chutter 1994). At each site, macroinvertebrate samples were obtained using kick-sampling, a technique in which rocks and other benthic material are disturbed to encourage organisms to flow downstream into a soft, 1 mm-gauge mesh net, 30 cm in diameter. This was done in all possible microhabitats within a particular site over 15 min (Dickens and Graham 2002). The contents of each sample were then washed to the bottom of the net and inverted. All specimens were identified to family level or higher taxon in the case of non-insects according to SASS5 protocol. Percentages of macroinvertebrate taxa were calculated for each site for each sampling period, as well as for the total sampling period. Macroinvertebrate scores based on the species tolerance levels to toxic levels, derived from SASS5, were allocated for each taxon per sample (Dickens and Graham 2002), and added up to calculate the macroinvertebrate score. The Average Score Per Taxon (ASPT) was determined by dividing the macroinvertebrate scores by the number of taxa at each site. Samples were stored in 96% ethanol.
There were seven sampling periods: 6–8 October 2003, 16–18 February 2004, 1–3 March 2004, 15–17 March 2004, 29–31 March 2004, 10–12 May 2004 and 21–23 June 2004, resulting in 210 (30 sites × 7 occasions) sampling units (SUs).
During each sampling period, the physical state of the water was measured using a multi-probe system for pH, temperature, conductivity, and dissolved oxygen (in percentage as well as mg l−1). Considerable attention was given to shade as this is known to have a major impact on South African aquatic macroinvertebrates (Samways and Taylor 2004; Smith et al. 2007). Shade was categorized based on visual observation to one of three categories according to the extent to which tree canopy covered the surface of the water at midday (<33%; 33–66%; >66%).
ANOVA single factor analysis was undertaken on the data, from upstream and downstream areas for temperature, conductivity, dissolved oxygen, percentage dissolved oxygen, pH, macroinvertebrate scores, ASPT and number of individuals for each sampling period, as well as for the combined data. Sites were scored by hand for presence (1) or absence (0) of each family to create binary matrices from which a Bray-Curtis similarity dendrogram was created with the program, PRIMER 5 for Windows Version 5.2.9. Taxon diversity was calculated for each of the sites using the Simpson’s diversity index (Ds).
Regression analyses were done on the number of taxa and each of the variables, temperature, conductivity, dissolved oxygen, percentage dissolved oxygen as well as pH. Further regression analysis was done on the number of individuals for each of these same variables.
Results
Macroinvertebrate relative abundance and scores
A total of 20,760 invertebrate individuals were sampled, mainly in five insect orders: Ephemeroptera (48%), Diptera (24%), Coleoptera (10%), Trichoptera (7%) and Plecoptera (5%), with all other taxa comprising a total of 6%. Differences upstream versus downstream in relative abundance of Ephemeroptera, Plecoptera, Coleoptera, Trichoptera and Annelida were highly significant (Table 1). Differences in relative abundance of Odonata, Hemiptera, Diptera and Crustacea were not significant (Table 1).
There were significant differences upstream versus downstream for overall macroinvertebrate scores (Table 2) and ASPT (Table 3) (Fig. 2) for each sampling period as well as for the combined data set. The upstream variances were also greater than downstream, illustrating consistently reduced macroinvertebrate diversity as a result of the dam (Fig. 2). The number of individuals per sampling period was significantly different for all the sampling periods as well as the combined data set, except for the three sampling periods during March 2004 (Table 2).
Macroinvertebrate assemblage clustering
There were four main macroinvertebrate dendrogram clusters, two for upstream and two for downstream (Fig. 3). The White bridge upstream sites clustered together with Sites 21, 22 and 23 (73.4%), where it joins the cluster (74.1%) of the remaining upstream sites, as well as one of the downstream clusters (75.1%). The other downstream cluster (63.1%) joins the rest at a similarity of 48.8%.
The macroinvertebrate clustering appears to be largely driven by physico-chemical conditions (Fig. 4), with a large proportion of the 63.1% cluster in Fig. 3 in the 86 % cluster in Fig. 4. All abiotic variables upstream versus downstream differed significantly (Table 4). Physico-chemical conditions for downstream sites, with the exception of Site 1, clustered with a similarity of 84.7%. The upstream sites, with the exception of Site 24 clustered together with a similarity of 76.4%. This cluster joined the combination of Sites 1 and 24 at a similarity of 75.2% before joining the downstream cluster, to give an overall similarity for the data set of 71.5% (Fig. 4).
The downstream station had the lowest macroinvertebrate diversity with an average mean of 4.05 (Fig. 5). The two upstream stations, Concrete bridge and White bridge had average mean values of 6.01 and 5.7 respectively. The means of upstream sites (5.81) differed significantly from the downstream means (3.97) (P < 0.0001). The variances around the mean diversity measures was less downstream than upstream, reflecting a more homogeneous fauna downstream than upstream (Fig. 5).
Taxonomic interpretation of clustering
Further interpretation of Figs. 3 and 5 is as follows. The Ephemeroptera were mainly Baetidae, which preferred upstream sites, but were present also in lesser numbers downstream. Leptophlebiidae were mostly upstream, while Caenidae were mainly downstream at sites with much silt and sediment. High Diptera abundance was due to Chironomidae downstream, and Simuliidae and Blepharoceridae upstream. Trichoptera species are divided into the cased Trichoptera families Barbarochthonidae, Sericostomatidae, Glossosomatidae and Petrothrincidae, restricted to the rapid-flowing waters upstream, and the case-less Trichoptera families Ecnomidae and Hydropsychidae, found throughout the system, but in significantly higher numbers downstream. The larvae of the Coleoptera families Elmidae, Gyrinidae and Helodidae, adapted for harsh mountain streams, were in high abundance upstream. The families Hydraenidae and Hydrophilidae were most abundant downstream. Notonemouridae (Plecoptera) were highly abundant upstream and virtually absent downstream. Hemiptera numbers were evenly dispersed through the rapid-flowing, acidic areas upstream, and also the slower, less acidic areas downstream.
Feeding guilds were strongly affected by the dam. Shredders (Ephemeroptera, Plecoptera, Coleoptera and Crustacea) were more abundant where the leaf litter was high under dense natural canopy cover upstream. Grazers were highest where algal growth was highest, in slow-moving water downstream with little canopy cover. Filter feeders (Diptera and Trichoptera) need organic material to be broken down principally by shredders, and were thus more abundant farther downstream. Predator numbers (Odonata, Hemiptera and Megaloptera) in contrast, are dependent on the areas with the highest numbers of individual prey, making them indirectly dependent on the environmental conditions of their prey.
Significance of shade
The three shade categories (>66%, 33–66% and <33% canopy cover), mainly consisting of natural tree cover, showed peak water temperatures in summer and lows in winter. Overall, it was the <33% shaded areas that showed the highest means, and the >66% canopy cover category was the most variable.
Conductivity in all three shade categories peaked during February to mid-March period, with the lowest mean values in winter. Over the entire sampling time, the 33–66% division canopy cover showed the lowest mean conductivity, while the >66% cover showed the highest value in the first two sampling periods, and then shifted to the <33% cover for the remaining sampling periods.
Dissolved oxygen, both in mg l−1 and percentage, was similar for all three shade categories. The maximum mean values were in October, and minimum in March. Throughout the study period, the 33–66% canopy cover category showed the highest mean, with >66% showing the lowest mean, except for February, where the <33% cover division was the lowest.
The mean pH values for the three shade categories were much more variable than the other abiotic variables. Early March had the highest mean values for the >66% cover, as where both other categories peaked in May. The minimum mean pH for the >33% category was in October, while for both of the other categories this occurred in February.
In October, the 33–66% cover had the lowest mean acidity. In February, late March and May there was a decrease in acidity with decreasing percentage canopy cover. In early March, the >66% cover was the most acidic and the 33–66% cover was the least. Mid-March showed an increase in acidity with decrease of percentage cover, while June showed the <33% canopy to have the highest mean and the 33–66% cover the lowest.
Macroinvertebrate response to shade
The SASS5 adapted macroinvertebrate scores indicated a peak for >66% cover in May, while the other two shade categories peaked in early March. The lowest mean value was in mid March for >66% and 33–66%, while the <33% cover lowest mean was in October. The 33–66% cover category was the highest overall, except in February when the <33% category was the highest. The >66% category was the lowest in all the sample periods except for October and May. ASPT showed a similar pattern to macroinvertebrate scores, except for May, when the >66% cover was the lowest rather than the <33% category.
The mean number of individuals in the >66% category, showed a maximum in June and minimum in early March. The 33–66% category peaked during May with a minimum in early March. For the <33% category, the maximum was in October, and minimum during February. For October and early March there was an increase in abundance with a decrease of percentage canopy cover, while the rest showed greatest abundance in the 33–66% category. During all the sampling periods, the >66% cover category had least abundance, except for late-March and June.
Discussion
Disruption of the river continuum
The river system in this study provided an opportunity to determine the effects of a single, spatially explicit, albeit extreme, form of anthropogenic disturbance: the impact of a dam and associated disturbance factors such as a trout facility and alien trees. Any differences in biotic assemblages along the river at the point of the dam were a result of its impact. Any differences occurring upstream from the dam were from natural sources. Any difference between upstream and downstream sites, if it had not been observed in the upstream sites, can only be a direct or indirect result of the dam.
In river systems of the type studied here, biodiversity generally increases with decreasing elevation, because a greater variety of food is available and circumstances are less extreme (Davies and Day 1998). For most taxa in this study, this was the case, with an increase in diversity with declining elevation along the natural upstream reach, above the dam, between White bridge and the Concrete bridge sites.
Riparian vegetation plays an important role in the dynamics of the aquatic system and directly or indirectly influences the processes and the communities of the system (Vannote et al. 1980). Many macroinvertebrate species that are restricted to small rivers reflect the ecological characteristics of the surrounding terrestrial community. In such streams, conditions for the larvae are affected by shade, amount and periodicity of leaf-fall, and the distribution of local precipitation, all three of which are integrated with type of climax community occupying the general area (Williams and Feltmate 1992).
In a small montane river without human disturbance, the canopy cover which forms the riparian zone will create a variety of habitats, from areas with permanent shade, with less fluctuation in water temperature, to areas with direct sunlight that will in turn create hotspots in water temperature. This increase in habitat types, as well as the direct leaf-fall that increases nutrient levels, will normally equate to higher benthic invertebrate diversity.
In this study, the natural areas above the dam, shade was a very important driver of other environmental variables, especially temperature, pH and conductivity. In turn, these variables greatly interacted with season. Thus shade and season were probably likely to be major primary drivers of the great variance seen in the invertebrate assemblages above the dam (Figs. 2 and 5). The reservoir and dam, as well as other associated disturbances, greater affected these environmental variables (Fig. 4) and effectively homogenized the invertebrate assemblages, as seen by the reduced variances downstream of the dams (Figs. 2 and 5).
Dams impose a lentic habitat within a lotic system (Brittain and Saltveit 1989; Mackie 1998), and aquatic communities must adjust suddenly to the changes in physical, chemical and biological attributes of riverine systems to those of lacustrine systems (Armitage and Blackburn 1990), with some taxa decreasing or even disappearing when a lentic system is imposed upon them (e.g. Baetidae and Simuliidae) (Logan and Brooker 1983), while some others may increase (Steytler and Samways 1995). Here, a quantum decline was observed immediately below the dam, contrary to the otherwise natural situation, illustrating clearly that the dam was having a major impact on the aquatic fauna.
A dam causes elevated water temperature due to a reduction in water flow and the presence of a standing pool of water immediately above the dam wall. The warming of the water is also the result of reduced canopy cover from dieback of the natural overhanging vegetation and increased sunlight penetration. The increased sunlight also causes an increase in microbial and algal activities. These effects spill over and increase mean water temperature below the dam, as was recorded here. In Hawaii, canalisation and removal of the riparian canopy cover resulted in higher water temperatures, increased daily temperature fluctuations, increased siltation, and decreased substrate size (Brasher 2003).
Aquatic macroinvertebrates are well known to be strongly influenced by temperature (Nebeker 1971; Resh and Rosenberg 1984; Allan 1995). Increased sunlight also provides aquatic surface plants which decrease water flow and prevent oxygen enrichment of the water. Decreased levels of dissolved oxygen, in turn, influence benthic macroinvertebrate assemblages (Ward 1992). All these patterns were seen in this study. Dissolved oxygen values were lowest in the low-flow, downstream areas, where invertebrate diversity was also lowest. In the case of temperature, sensitive species of Ephemeroptera and Plecoptera were unable to survive in the area downstream from the dam, where the flow was reduced to only a small, slow flowing, sunlit stream.
Standing water also tends to become low in pH over time where there is no inflow of freshwater. However, the experimental cage-culture trout farm situated at the dam might also have offset this to some extent and caused a rise in pH of the river, by dilution due to the increase of water, but also by artificial feed input which is mainly alkaline.
Rivers do not usually become nutrient enriched, as they self-purify during periodic floods and peak flows (Madikizela et al. 2001). Nutrients normally occur in low concentrations in lotic systems (Chapman et al. 1992), except when there is an external input (Madikizela et al. 2001) through distribution of inorganic (e.g. phosphates) and organic (e.g. manure) nutrients from agricultural areas, some of which form part of the runoff during heavy precipitation (Mackie 1998). In this system, most of the water is impounded during the rainy winter season and also experiences nutrient enrichment from the cage-trout farm.
Usually, the pH of a stream declines from the headwaters to the lower reaches (Ward 1992). Most unpolluted streams have a pH value of 6.0–9.0 (Ward 1992). A pH below 6.0 is considered acidification of the stream, resulting in low diversity and low productivity of aquatic insects (Resh and Rosenberg 1984). Ward (1992) suggested that Ephemeroptera and Plecoptera species are incapable of surviving acidic levels lower than a pH of 6.0, but this study indicated otherwise. The mean pH values were below these levels, especially upstream from the reservoir, where both Ephemeroptera and Plecoptera numbers were high.
Water bodies suffering from industrial pollution are generally characterized by high densities of certain Chironomidae (Diptera), with an absence of Ephemeroptera and Plecoptera (Vigano et al. 2002). Furthermore, direct input of cattle manure results in heavy growths of blue-green surface algae, attached green algae and submerged macrophytes (Mackie 1998). In this study, Ephemeroptera dominated in the upstream zone, and Diptera in the disturbed area downstream from the dam, in keeping with other studies (Logan and Brooker 1983; Williams and Feltmate 1992). Furthermore, this study showed a major increase in algal activity just below the dam. These factors indicate that the impoundment has similar disturbing factor as industrial and agricultural pollution.
Ephemeroptera, Trichoptera and Plecoptera can be lost as soon as a dam is built, to be replaced by high densities of Diptera (Williams and Feltmate 1992). This was also the case here. Furthermore, the Notonemouridae (Plecoptera) being extremely sensitive to the impact of the dam, was abundant upstream yet virtually absent downstream.
A study of aquatic Coleoptera at a reservoir near Cape Town, indicated a negative effect on the aquatic fauna from the impoundment, reducing certain Cape endemic and internationally threatened species of Elmidae, Dryopidae and Hydraenidae by 73% (Turner 2000). This study illustrates a similar trend, with a 93% drop in both Elmidae and Hydraenidae.
Impoundments have been recorded as changing the species assemblage from one dominated by shredders and lotic filter feeders, grazers and predators to one of herbivores and lentic filter feeders and predators (Williams and Feltmate 1992). This was also seen in this study.
The macroinvertebrate scores (Figs. 2 and 5) were consistently lower immediately below the dam compared to above it. The dam therefore had a major ecologically cascading impact and impoverishing effect. This was related to various physical variables which were overall significantly different upstream versus downstream (Fig. 4). There was also a clear reduction in flow rate downstream, with a concurrent increase in sediment and silt, as well as an increase in algal growth downstream.
Dewatering of stream reaches can also inhibit downstream dispersal of larvae and upstream migration of postlarvae, which has a critical effect on the life cycles of many species (Brasher 2003). Also, the dam could have acted as a bottleneck that increased the densities of upstream migrating animals below the dam (March et al. 2003), although this was evident in this study.
The reduced Ephemeroptera, Plecoptera and Trichoptera taxa richness below the trout farm outfall compared to above it, indicated reduced water quality, in keeping with Loch et al. (1996). Pollution-tolerant taxa like Chironomidae, Simuliidae, Oligochaeta and Sphaeriidae have higher abundances just below the outfalls (Rosenberg et al. 1986; Loch et al. 1996) as was also the case in this study, suggesting that the trout farm adds to the adverse effects of the dam on the system.
In conclusion, the dam here clearly had a major impact on the character of the invertebrate assemblages. These results were similar to those in less biologically diverse areas, but the magnitude of the impact of the dam was extremely high on this clearly sensitive localized aquatic fauna.
References
Allan JD (1995) Stream ecology: structure and function of running waters. Chapman and Hall, London
Armitage PD, Blackburn JH (1990) Environmental stability and communities of Chironomidae (Diptera) in a regulated river. Regul Rivers Res Manage 5:319–328. doi:10.1002/rrr.3450050404
Barow ME, McIntosh MD, Burky AJ, Way CM (2005) The influence of stream flow reduction on the energetics of Hawaiian torrenticolous aquatic insects, Telmatogeton Schiner and Pocanace Hendel. J Insect Conserv 9:175–185. doi:10.1007/s10841-005-4831-0
Basson MS, Van Niekerk PH, Van Rooyen JA (1997) Overview of water resources availability and utilisation in South Africa. DWAF Report No. PRSA/00/0197. Department of Water Affairs and Forestry and BKS (Pty) Ltd, Pretoria, South Africa
Bednarek A (2001) Undamming rivers: a review of the ecological impacts of dam removal. Environ Manage (N Y) 27:803–814. doi:10.1007/s002670010189
Born SM, Genskow KD, Filbert TL, Hernandez-Mora N, Keefer ML, White KA (1998) Socioeconomic and institutional dimensions of dam removals: the Wisconsin experience. Environ Manage (N Y) 22:359–370. doi:10.1007/s002679900111
Brasher AMD (2003) Impacts of human disturbances on biotic communities in Hawaiian streams. Bioscience 53:1052–1060. doi:10.1641/0006-3568(2003)053[1052:IOHDOB]2.0.CO;2
Brittain JE, Saltveit SJ (1989) A review of the effect of river regulation on mayflies (Ephemeroptera). Regul Rivers Res Manage 3:191–204. doi:10.1002/rrr.3450030119
Brown CA, Dallas HF (1995) Eerste River, Western Cape: situation assessment of the riverine ecosystem. Final report, June 1995. Southern Waters Ecological Research and Consultancy, CSIR, Stellenbosch, South Africa
Chapman PM, Power EA, Burton GA Jr (1992) Integrative assessments in aquatic ecosystems. In: Burton GA Jr (ed) Sediment toxicity assessment. Lewis Boca Raton, Florida, pp 313–340
Chutter FM (1994) The rapid biological assessment of streams and rivers water quality by means of macroinvertebrate community in South Africa. Progress Report to the Water Research Commission, Pretoria, South Africa
Davies B, Day J (1998) Vanishing waters. University of Cape Town Press, Cape Town, South Africa
Dickens CWS, Graham PM (2002) The South African Scoring System (SASS5) Version 5 rapid bioassessment method for rivers. Afr J Aquat Sci 27:1–10
Doeg TJ, Koehn JD (1994) Effects of draining and desilting a small weir on downstream fish and macroinvertebrates. Regul Rivers Res Manage 9:263–277. doi:10.1002/rrr.3450090407
Drinkwater KF, Frank KT (1994) Effects of river regulation and diversion on marine fish and invertebrates. Aquat Conserv Freshw Mar Ecosyst 4:135–151. doi:10.1002/aqc.3270040205
Kevan PG (1999) Pollinators as bioindicators of the state of the environment: species, activity and diversity. Agric Ecosyst Environ 74:373–393. doi:10.1016/S0167-8809(99)00044-4
King JM, Schael DM (2001) Assessing the ecological relevance of a spatially-nested geomorphological hierarchy for river management. WRC Report No 754/1/01 Freshwater Research Unit, University of Cape Town, Cape Town, South Africa
Kondolf GM (1997) Hungry water: effects of dams and gravel mining on river channels. Environ Manage (N Y) 21:533–551. doi:10.1007/s002679900048
Ligon FK, Dietrich WE, Trush WJ (1995) Downstream ecological effects of dams. Bioscience 45:183–192. doi:10.2307/1312557
Loch DD, West JL, Perlmutter DG (1996) The effect of trout farm effluent on the taxa richness of benthic macroinvertebrates. Aquaculture 147:5–10. doi:10.1016/S0044-8486(96)01394-4
Logan P, Brooker MP (1983) The macroinvertebrate faunas of riffles and pools. Water Res 17:263–270. doi:10.1016/0043-1354(83)90179-3
Mackie GL (1998) Applied aquatic ecosystem concepts. University of Guelph Custom Coursepack
Madikizela BR, Dye AH, O’Keeffe JH (2001) Water quality and faunal studies in the Umzimvubu Catchment, Eastern Cape with particular emphasis on species as indicators of environmental change. WRC Report No.716/1/01. Freshwater Research Unit, University of Cape Town, Cape Town, South Africa
Malmqvist B, Englund G (1996) Effects of hydropower-induced flow perturbations on Mayfly (Ephemeroptera) richness and abundance in North Swedish river rapids. Hydrobiologia 341:145–158. doi:10.1007/BF00018118
March JG, Benstead JP, Pringle CM, Scatena FN (2003) Damming tropical island streams: problems, solutions, and alternatives. Bioscience 53:1069–1078. doi:10.1641/0006-3568(2003)053[1069:DTISPS]2.0.CO;2
McIntosh MD, Benbow ME, Burkey AJ (2002) Effects of stream diversion on riffle macroinvertebrate communities in a Maui, Hawaii, stream. River Res Appl 18:569–581. doi:10.1002/rra.694
Metcalf-Smith JL (1994) Biological water-quality assessment of rivers: use of macroinvertebrate communities. In: Calow P, Petts GE (eds) The rivers handbook, hydrological and ecological principles, vol 2. Blackwell Scientific Publications, Oxford, pp 144–170
Mittermeier RA, Gil PR, Hoffmann M, Pilgrim J, Brooks T, Mittermeier CG et al (2004) Hotspots revisited. Cemex, Mexico City
Myers N, Mittermeier CG, Da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. doi:10.1038/35002501
Nebeker AV (1971) Effect of high winter water temperatures on adult emergence of aquatic insects. Water Res 5:777–778. doi:10.1016/0043-1354(71)90100-X
Petts GE (1984) Impounded rivers: perspectives for ecological management. Wiley, Chichester, p 322
Poff NL, Allan JD, Bain MB, Karr JR, Prestegaard KL, Richter BD et al (1997) The natural flow regime. Bioscience 47:769–784. doi:10.2307/1313099
Quinn JM, Hickey CW (1990) Magnitude of effects of substrate particle size, recent flooding, and catchment development on benthic invertebrates. N Z J Mar Freshw Res 24:411–427
Resh VH (1995) The use of bentic macroinvertebrates and rapid assessment procedures for water quality monitoring in developing and newly industrialized countries. In: Davis WS, Simon T (eds) Biological assessment and criteria: tools for water resource planning and decision making. Lewis Publishers, Chelsea, pp 159–194
Resh VH, Rosenberg DM (1984) The ecology of aquatic insects. Praeger, New York
Roque FO, Trivinho-Strixino S, Strixino G, Agostinho RC, Fogo JC (2002) Benthic macroinvertebrates in streams of the Jarague State Park (Southeast of Brazil) considering multiple spatial scales. J Insect Conserv 7:63–72. doi:10.1023/A:1025505323668
Rosenberg DM (1998) A national aquatic ecosystem health program for Canada: we should go against the flow. Bull Entomol Soc Can 30:144–152
Rosenberg DM, Resh VH (1993) Freshwater biomonitoring and benthic macroinvertebrates. Chapman and Hall, New York
Rosenberg DM, Danks HV, Lehmkuhl DM (1986) Importance of insects in environmental impact assessment. Environ Manage (N Y) 10:773–783. doi:10.1007/BF01867730
Samways MJ, Taylor S (2004) Impacts of invasive alien plants on Red-listed South African dragonflies (Odonata). S Afr J Sci 100:78–80
Smith J, Samways MJ, Taylor S (2007) Assessing riparian quality using two complementary sets of bioindicators. Biodivers Conserv 16:2695–2713. doi:10.1007/s10531-006-9081-2
Stanford JA, Ward JV, Liss WJ, Frissell CA, Williams RN, Lichatowich JA et al (1996) A general protocol for restoration of regulated rivers. Regul Rivers Res Manage 12:391–413. doi:10.1002/(SICI)1099-1646(199607)12:4/5<391::AID-RRR436>3.0.CO;2-4
Steytler NS, Samways MJ (1995) Biotope selection by adult male dragonflies (Odonata) at an artificial lake created for insect conservation in South Africa. Biol Conserv 72:381–386. doi:10.1016/0006-3207(94)00052-R
Turner CR (2000) A contribution to the study of the Table Mountain water beetles (Coleoptera) with special reference to the environmental impact of reservoirs. City Engineers Department, Cape Town
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137
Vigano L, Arillo A, Buffagni A, Camusso M, Ciannarella R, Crosa G et al (2002) Quality assessment of bed sediments of the Po River (Italy). Water Res 37:501–518. doi:10.1016/S0043-1354(02)00109-4
Ward JV (1992) Aquatic insect ecology: biology and habitat. Wiley, New York
Ward JV (1998) Riverine landscapes: biodiversity patterns, disturbance regimes, and aquatic conservation. Biol Conserv 83:269–278. doi:10.1016/S0006-3207(97)00083-9
Ward JV, Stanford JA (1995) Ecological connectivity in alluvial river ecosystems and its disruption by flow regulation. Regul Rivers Res Manage 11:105–119. doi:10.1002/rrr.3450110109
Williams DD, Feltmate BW (1992) Aquatic insects. CAB International, Redwood Press, Melksham
Winston MR, Taylor CM, Pigg J (1991) Upstream extirpation of four minnow species due to damming of a prairie stream. Trans Am Fish Soc 120:98–105. doi:10.1577/1548-8659(1991)120<0098:UEOFMS>2.3.CO;2
Yeager BL (1994) Impacts of reservoirs on the aquatic environment of regulated rivers. Tennessee Valley Authority, Water Resources, Aquatic Biology Department, TVA/WR/AB-93/1, Norris, Tennessee, USA
Zwick P (1992) Stream habitat fragmentation—a threat to biodiversity. Biodivers Conserv 1:80–97. doi:10.1007/BF00731036
Acknowledgements
Adam Johnson kindly assisted with fieldwork. Financial support was from the National Research Foundation (NRF) and the University of Stellenbosch.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bredenhand, E., Samways, M.J. Impact of a dam on benthic macroinvertebrates in a small river in a biodiversity hotspot: Cape Floristic Region, South Africa. J Insect Conserv 13, 297–307 (2009). https://doi.org/10.1007/s10841-008-9173-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-008-9173-2