Abstract
The role of antimony (Sb)—a non-essential trace metalloid—in physiological processes running in crops is still poorly understood. Present paper describes the effect of Sb tartrate (SbIII) on growth, Sb uptake, photosynthesis, photosynthetic pigments, and leaf tissue organization in young sunflower plants grown in hydroponics. We found that growth of below- and aboveground part was reduced with increasing concentration of Sb in the medium. Although Sb was mostly taken up by sunflower roots and only small part (1–2 %) was translocated to the shoots, decline in photosynthesis, transpiration, and decreased content of photosynthetic pigments were observed. This indicates that despite relatively low mobility of Sb in root-shoot system, Sb in shoot noticeably modifies physiological status and reduced plant growth. Additionally, leaf anatomical changes indicated that Sb reduced the size of intercellular spaces and made leaf tissue more compact.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antimony (Sb) is a trace element released into the environment mainly by mining, smelting of Sb ores, burning of fossil fuels, traffic, as well as industrial activity. Another important source of Sb input into the soils might represent shooting as Sb is used for hardening of bullets (Filella et al. 2002; Tschan et al. 2009a). Although Sb behaves differently from heavy metals, like cadmium, lead, zinc, or copper, this trace metalloid can be toxic for all living organisms, including human (Feng et al. 2013).
Antimony is a non-essential element relatively hardly soluble in the soil solution; however, it can be taken up by plants. Normal average concentration of Sb in aboveground plant parts was estimated to 0.05–0.1 mg kg−1 (Markert 1996; Reimann et al. 2010); however, relatively high concentration of Sb was found in the shoot of various plants growing in the vicinity of old mining sites and industrial areas (Bech et al. 2012; Jana et al. 2012; Levresse et al. 2012; Vaculík et al. 2013; Cidu et al. 2014). Antimony is mostly present in two oxidation states: antimonite (SbIII) and antimonate (SbV), and latter is considered to be more toxic for plants (Filella et al. 2002). Recently, there is an increase in knowledge about the uptake of Sb and its speciation in plants. SbIII is taken up by plants by transporters belonging to the NIP1 subgroup (Kamiya and Fujiwara 2009); however, the mechanisms of SbV uptake are still unclear. It was shown that plants are more efficient in taking up SbIII than SbV, although SbV was the predominant form of Sb in various plants, probably due to relative instability of trivalent Sb forms (Ren et al. 2014; Tisarum et al. 2014). Within the plants, Sb is mostly accumulated in roots (Feng et al. 2009; Shtangeeva et al. 2014; Tisarum et al. 2014); however, opposite was also observed (Pan et al. 2011). The sites of Sb accumulation within plant tissues are still not clear or may vary between the species; Feng et al. (2011) found most of Sb accumulated in cytosol, while Ren et al. (2014) found that in rice plants, most of Sb was accumulated in cell walls and less in organelles and cytosol.
It was found that Sb can increase the peroxidation of membrane lipids and stimulate defense antioxidant system in plants (Feng et al. 2009; Corrales et al. 2014; Vaculíková et al. 2014). However, there is still lack of knowledge about the uptake of Sb in agricultural important plants, and the role of Sb in physiological processes running in crops, especially photosynthesis, is still poorly understood. Therefore, the aim of this study was to investigate how Sb affected growth and processes related to the photosynthesis and leaf tissue structure of sunflower plants (Helianthus annuus L.).
Material and methods
Growth conditions and experimental set-up
Seeds of sunflower (Helianthus annuus L., cv. Codicap) were surface sterilized for 10 min in 3 % Savo (Sodium hypochlorite solution, Biochemie, Czech Republic) and washed carefully several times with distilled water before the germination. Thereafter, they were imbibed in water for 2 h at room temperature and germinated in rolls of wet filter paper for 72 h at 24 °C in a dark. Seedlings were transferred to 8 L plastic containers (14 plants per container) filled with modified Hoagland solution (Hoagland and Arnon 1950) with or without Sb. The solutions were changed two times per week. Three different treatments were applied:
-
1.
Control (C)—Hoagland solution without Sb
-
2.
Antimony 2.5 (Sb 2.5)—Hoagland solution with 2.5 mg L−1 Sb
-
3.
Antimony 5 (Sb 5)—Hoagland solution with 5 mg L−1 Sb
The pH of each cultivation solution was adjusted to 6.2 using HCl. Antimony was applied in the form of potassium antimony tartrate hemihydrate (SbIII). In total, the plants in each treatment were cultivated for 21 days in hydroponics in a growth chamber with a 12-h photoperiod, a temperature 25/23 °C (day/night), 60 % humidity, and 200 μmol m−2 s−1 PAR.
Evaluation of growth and biomass production
After 21 days, plants were divided into below- and aboveground parts, and roots were carefully washed in distilled water. Fresh weights of below- and aboveground parts of plants were determined. Root and shoot material was dried at 70 °C for 72 h, and the dry weights of below- and aboveground parts were determined.
Determination of Sb concentration in roots and shoots
The concentration of Sb was determined in finely ground-dried root and shoot tissues using atomic absorption spectrometry (AAS) in the Geoanalytical Laboratories of the Institute of Geomaterials, Faculty of Natural Sciences, Comenius University in Bratislava, Slovakia. Dried samples were double-step digested in HNO3 and H2O2, and for quality control, certified reference material was used. All measurements have been done in duplicates.
Determination of changes in photosynthesis and transpiration
Rates of net photosynthesis (A N), respiration (E), and stomatal conductance (Gs) were measured simultaneously on the second fully developed sunflower leaves with a CIRAS-2 (PP-Systems, Hitchin, UK) infrared gas analyzer. The actinic light of 50 μmol m−2 s−1 PAR was switched on for induction of photosynthesis. Then the light intensity was increased stepwise with irradiation periods of 3.5 min, until 1600 μmol m−2 s−1 PAR was reached. Then the actinic light was switched off, and after 10 min, the rate of respiration in the dark (RD) was recorded. The light was provided by blue (λ = 455 nm) and red (λ = 620 nm) LED diodes. A N was recorded at CO2 concentration 380 μmol mol−1, leaf temperature 25 ± 1 °C, and relative air humidity 65–70 %.
Analyses of chlorophylls and carotenoids
Approximately 50 mg of fresh leaf tissue samples from the midpart of the second fully developed sunflower leaves were homogenized in a mortar with small addition of sand and MgCO3, and photosynthetic pigments were extracted with chilled 80 % acetone. The suspension was centrifuged at 4 °C for 5 min at 5000×g. The concentration of chlorophylls (chla + chlb) and carotenoids (Car) in supernatant was determined using UV/Vis spectrophotometer (Jenway 6400, London, UK) with a maximum absorption of 664 and 646 nm for chla and chlb, respectively, and of 470 nm for Car, and the total content of chlorophyll a, b and carotenoids was calculated according to Lichtenthaler and Wellburn (1983).
Determination of quantitative changes in the leaf anatomy
For the investigation of anatomical differences of leaf tissues, tissue segments, approximately 2 × 2-mm large, were cut from the midpart of the second fully developed vital parts of the leaves and fixed in 2 % glutaraldehyde and 0.2 % osmium tetroxide. After dehydration with ethanol and propylene oxide, the samples were embedded in Spurr resin (Serva). Approximately 2-μm-thick semi-thin sections were prepared using microtome Tesla BS 490 and stained with 0.5 % toluidine blue and 0.1 % basic fuchsine according to Lux (1981). The sections were analyzed with Zeiss Axioskop 2 plus epifluorescence microscope (Zeiss, Germany), equipped with Olympus DP72 camera. Quantitative anatomical analysis of leaf tissues was performed with the image analysis software Lucia G 4.80 (LIM, Czech Republic). Leaf thickness, the thickness of adaxial and abaxial epidermal layers, the number of epidermal and mesophyll cells, the area of mesophyll cells and intercellular spaces, as well as the total area of selected cell layers were detected from the exact area (500 × 200 μm) of the cross section of embedded leaves.
Statistical analysis
Statistical significance was assessed using the Statgraphics Centurion XV v. 15.2.05 (StatPoint Technologies, Inc., Warrenton, VA, USA) and Excel (Microsoft Office 2010) programs, and a single-step multiple comparisons of means was performed via LSD test. A P value < 0.05 was defined as significant. Biomass data are from three different replicates. For determination of changes in photosynthesis, six different leaves from six different plants of each treatment were evaluated, and for pigment analysis, four different leaf samples from four different plants of each treatment were evaluated. For determination of changes in leaf anatomy, six different replicates from six different plants of each treatment were evaluated. For determination of Sb concentration, four different replicates were analyzed.
Results
Growth and biomass production affected by Sb
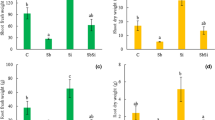
Plants exposed to antimony in our study showed typical symptoms of toxicity, including reduced growth of the bellow- and aboveground plant parts, and enhanced leaf chlorosis and necrosis (Fig. 1). The length of the primary root and number and length of lateral roots decreased with increasing concentration of Sb. Similarly, the length of the shoot decreased by about 42 % in Sb 5 when compared with control treatment. The fresh and dry weight of shoot biomass decreased with increasing concentration of Sb in the medium; shoot dry biomass of Sb 5 plants was reduced by about three times when compared with the control (Fig. 2a). Similarly, root biomass decreased with increasing concentration of Sb; however, no significant differences in root fresh and dry biomass were observed between control and lower Sb-treated plants (Sb 2.5). On the contrary, both fresh and dry root biomass of Sb 5 plants decreased by about 3.5 and 2 times, when compared with control plants, respectively (Fig. 2b).
Shoot (a) and root (b) fresh and dry biomass of sunflower plants exposed to SbIII. Abbreviations: C—control; Sb 2.5—2.5 mg kg−1 Sb; Sb 5—5 mg kg−1 Sb; FW—fresh weight; DW—dry weight. Different letters indicate significant differences among the treatments at P < 0.05 (n = 3). Data are shown as means ± SD
Concentration and uptake of Sb in roots and shoots
In general, the concentration of Sb was higher in roots than in shoots by about two orders of the magnitude, reaching a maximum of approximately 7700 and 50 mg kg−1 in roots and shoots of higher Sb treatment (Sb 5), respectively. The concentration of Sb in roots as well as in shoots increased with increasing concentration of Sb in the medium. Root Sb concentration was about 1.5 times higher in Sb 5 than in Sb 2.5 treatment, while shoot Sb concentration was more than three times higher in Sb 5 than in Sb 2.5 treatment (Fig. 3). Similarly, we found that most of the Sb taken up by plants is stored in the roots, and only small part of Sb is translocated to the shoots. However, total amount of Sb extracted by plants was not significantly different between Sb 2.5 and Sb 5 plants (Table 1).
Influence of Sb on photosynthesis, transpiration, and stomatal conductance
The rate of net photosynthesis (P N) in the young sunflower plants decreased with increasing Sb concentration in leaf tissues. In general, the differences between treatments were more obvious with increasing irradiance (Fig. 4a). At the highest level of irradiance (1600 μmol m−2 s−1), the P N of Sb 2.5 plants was about 60 % lower, and the P N of Sb 5 plants was even more lower (80 %) when compared with control plants (Fig. 4a). Similar results were observed when comparing the rate of the transpiration (E) between the treatments. Increasing concentration of Sb decreased E by about 50 % in Sb 2.5 and about 70 % in Sb 5 treatment when compared with the control (Fig. 4b). Stomatal conductance (G s) of sunflower leaves was also negatively influenced by Sb application, a decrease of G s by about 50 % in Sb 2.5 and about 70 % in Sb 5 plants was observed when compared with control plants, respectively (Fig. 4c).
Influence of Sb on the content of chlorophylls and carotenoids
Antimony at both applied concentrations negatively influenced the content of chlorophyll a (chla) and chlorophyll b (chlb), which resulted in the decrease of total chlorophylls content in sunflower leaves by about 14 and 64 % in Sb 2.5 and Sb 5 treatment, respectively, when compared with control plants. However, results were significantly different from the control only when a higher dose of Sb (Sb 5) was applied. Similar pattern of carotenoid concentration was observed between control and Sb-treated plants with a decrease by about 50 % in Sb 5 plants when compared to control (Table 2).
Effect of Sb on leaf anatomy of sunflower
Increasing concentration of Sb did not greatly modify the leaf tissue structure, although proportions of individual tissue layers in sunflower leaves were different between the treatments (Fig. 5). The leaf thickness decreased with increasing concentration of Sb in the medium; however, at lower applied Sb (Sb 2.5), the decrease was not significantly different when compared with the control (Table 3). When comparing the thickness of epidermal cells, we did not observe any differences between the treatments. Similarly, we did not observe any difference in the number of adaxial and abaxial epidermal cells between the treatments. On the other hand, we found that the number of mesophyll cells increased with increasing concentration of Sb in the medium, although the significant difference was observed only at Sb 5 treatment when compared with the control (Table 3).
Cross sections of sunflower leaves from control (a), Sb 2.5 (b), and Sb 5 (c) treatments. Abbreviations: C—control; Sb 2.5—2.5 mg kg−1 Sb; Sb 5—5 mg kg−1 Sb; ADE—adaxial epidermis; ABE—abaxial epidermis; PP—palisade parenchyma; SP—spongy parenchyma; IS—intercellular space; S—stomata; T—trichome. Scale bar 100 μm
When comparing the proportions of individual tissues on the cross sections of sunflower leaves, we found that the area of intercellular spaces, the area of mesophyll tissue (mesophyll cells including intercellular spaces), and the area of abaxial epidermal layer decreased with increasing concentration of Sb in the medium, although the area of adaxial epidermal layer was not influenced. On the other hand, we also compared the area of net mesophyll cells (mesophyll tissue without intercellular spaces) and found no differences between the treatments (Table 3).
Discussion
Antimony was reported as one of the hazardous substances and dangerous pollutants of the environment. Until now, several studies have shown that this element can be taken up from soil substrate into plants; however, available knowledge about the fate of Sb in plant bodies, and how it influences anatomical-morphological characteristics as well as physiological processes, is still limited. Our recent experiments clearly demonstrated the negative effect of Sb on sunflower plants that was mainly manifested by reduced growth of roots and shoots, decreased root and shoot biomass, and increased leaf chlorosis with relative pigment loss. Decreased growth and biomass production as a consequence of Sb have been already observed in sunflower (Tschan et al. 2009b), and also in some other important agricultural plants, like rice, wheat, or maize (He and Yang 1999; Pan et al. 2011; Shtangeeva et al. 2014; Vaculíková et al. 2014). The present study showed that relatively low Sb concentration in the medium (5 mg kg−1 Sb) greatly influenced root and shoot biomass. Partially, this might be the effect of the cultivar used in our experiments, and also form of Sb (Sb tartrate, SbIII), as it is known that the trivalent form of Sb is more toxic than the pentavalent Sb form (Filella et al. 2002). On the other hand, we did not investigate the speciation forms of Sb within plant tissues. It is possible that considerable amount of SbIII has been transformed to SbV in plants, as observed for example Ren et al. (2014) in rice. On the other hand, Okkenhaug et al. (2011) reported higher amount of SbIII in plants than in soil extracts and suggested that reduction of pentavalent to trivalent Sb form occurred after plant uptake.
Our recent experiments indicated that Sb was predominately accumulated in the roots of sunflower, which is in agreement with other studies describing the root as a main storage plant compartment for Sb (Vaculík et al. 2013; Ren et al. 2014; Tisarum et al. 2014; Corrales et al. 2014). Although only a small part of Sb (1–2 %) was translocated to the shoots, plants exposed to Sb exhibited decreased photosynthesis and transpiration. Photosynthesis has been shown as one of the processes that are sensitive to various kinds of stress and are being early influenced by element deficiency or toxicity (e.g., Ashraf and Harris 2013). Until know, knowledge about the influence of Sb on the photosynthesis and photosynthetic-related processes are very limited. It was only reported that Sb decreased chlorophyll concentration and reduced maximum photochemical efficiency in maize plants, although the tissue Sb concentration that was responsible for such changes was not provided (Pan et al. 2011). Our present study demonstrated that the net photosynthetic rate, photosynthetic pigment concentration, transpiration rate, as well as stomatal conductance were reduced correspondingly to the increasing concentration of Sb in the medium. We expected that reduced photosynthesis and corresponding lower shoot biomass production of sunflower is the consequence of Sb accumulated in leaf tissues. Although there is a big difference between Sb accumulation and translocation from roots to shoots, the roots seem to better cope with Sb toxicity than the shoots. It is evident that leaf tissue concentration of Sb over 10 mg kg−1 can significantly retard shoot growth, and leaf concentration over 50 mg kg−1 is for growth of sunflower plants highly toxic. Therefore, we assume that available Sb in agricultural soils that can be taken up by plants might negatively influence crop production and decrease yields.
It is known that heavy metals can affect root and leaf anatomy. For example, elevated concentration of Zn was shown to modify the leaf thickness and increase intercellular space area in poplar exposed to Zn excess (Todeschini et al. 2011; Stoláriková-Vaculíková et al. 2015). Similarly, Luković et al. (2012) observed reduction of stomatal size, adaxial and abaxial epidermis, and percentage of spongy tissue thickness in poplars and willows treated by Cd; and Zarinkamar et al. (2013) and Queiroz Alves et al. (2014) observed anatomical differences in leaves exposed to Pb. Therefore, to better understand the influence and role of Sb in plant tissues, we investigated leaf anatomy and compare tissue proportions between control and Sb-treated plants. We found that the thickness of sunflower leaves decreased with increasing concentration of Sb. The number of both abaxial and adaxial epidermal cells, as well as their thickness, was not influenced by Sb. However, the number of leaf mesophyll cells increased with increasing concentration of Sb. On the other hand, the area of intercellular spaces was the highest in control leaves, and decreased with increasing concentration of Sb. The decrease in intercellular space area negatively correlated with increase in number of mesophyll cells in Sb-treated plants. This means that due to the presence of Sb, the leaf thickness decreased, the area of intercellular spaces also decreased, but oppositely, the number of mesophyll cells increased. We observed no difference in the area of net mesophyll cells (mesophyll tissue without intercellular spaces) between control and Sb-treated plants. This further indicates that the net mesophyll cells cover the same area of the leaf cross section in control and Sb-treated leaves, although the number of cells is higher in Sb-treated plants. To summarize this, the Sb-treated leaves are thinner, their mesophyll tissue is more compact and dense, and intercellular spaces in the mesophyll tissue cover smaller area than in control plants. Therefore, these findings indicated that Sb reduced the size of intercellular spaces and have inhibitory effect on aerenchyma formation in sunflower leaves. We expected that reduced leaf aerenchyma is partially responsible for modified gas exchange parameters in Sb-treated plants. Although recent findings about aerenchyma reduction in leaves exposed to Sb are novel, additional investigation of exact role of Sb in these processes would be important.
We conclude that antimony is taken up by sunflower roots and only small part (1–2 %) is translocated to the shoots. Both roots and shoots exposed to Sb showed retarded growth and reduced biomass, although roots seem to better cope with the same level of Sb tissue concentration than shoots. Plants exposed to Sb exhibited decline in photosynthesis, transpiration, and decreased photosynthetic pigments. This might be partially also due to lower leaf aerenchyma formation, whereas Sb reduced the size of intercellular spaces and made leaf tissue more compact.
References
Ashraf M, Harris PJC (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51:163–190
Bech J, Corrales I, Tume P, Barceló J, Duran P, Roca N, Poschenrieder C (2012) Accumulation of antimony and other potentially toxic elements in plants around a former antimony mine located in the Ribes Valley (Eastern Pyrenees). J Geochem Explor 113:100–105
Cidu R, Biddau R, Dore E, Vacca A, Marini L (2014) Antimony in the soil–water–plant system at the Su sSuergiu abandoned mine (Sardinia, Italy): strategies to mitigate contamination. Sci Total Environ 497–498:319–331
Corrales I, Barceló J, Bech J, Poschenrieder C (2014) Antimony accumulation and toxicity tolerance mechanisms in Trifolium species. J Geochem Explor 147:167–172
Feng R, Wei C, Tu S, Wu F, Yang L (2009) Antimony accumulation and antioxidative responses in four fern plants. Plant Soil 317:93–101
Feng R, Wei Ch TS, Tang S, Wu F (2011) Simultaneous hyperaccumulation of arsenic and antimony in Cretan brake fern: evidence of plant uptake and subcellular distributions. Microchem J 97:38–43
Feng R, Wei C, Tu S, Ding Y, Wang R, Guo J (2013) The uptake and detoxification of antimony by plants: a review. Environ Exp Bot 96:28–34
Filella M, Belzile N, Chen YW (2002) Antimony in the environment: a review focused on natural waters I. Occurrence. Earth Sci Rev 57:125–176
He M, Yang J (1999) Effects of different forms of antimony on rice during the period of germination and growth and antimony concentration in rice tissue. Sci Total Environ 243–244:149–155
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circ. Univ. Calif. Agric. Exp. Station, Berkley, 347 pp
Jana U, Chassany V, Bertrand G et al (2012) Analysis of arsenic and antimony distribution within plants growing at an old mine site in Ouche (Cantal, France) and identification of species suitable for site revegetation. J Environ Manag 110:188–193
Kamiya T, Fujiwara T (2009) Arabidopsis NIP1;1 transports antimonite and determines antimonite sensitivity. Plant Cell Physiol 50:1977–1981
Levresse G, Lopez G, Tritlla J, Cardellach López E, Carrillo Chavez A, Mascunano Salvador E, Soler A, Corbella M, Hernández Sandoval LG, Corona-Esquivel R (2012) Phytoavailability of antimony and heavy metals in arid regions: the case of the Wadley Sb district (San Luis, Potosí, Mexico). Sci Total Environ 427–428:115–125
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Luković J, Merkulov L, Pajević S, Zorić L, Nikolić N, Borišev M, Karanović D (2012) Quantitative assessment of effects of cadmium on the histological structure of poplar and willow leaves. Water Air Soil Pollut 223:2979–2993
Lux A (1981) Rapid method for staining of semi-thin section from plant material. Biologia 36:753–757
Markert B (1996) Instrumental element and multi-element analysis of plant samples. Methods and applications. Wiley, New York
Okkenhaug G, Zhu YG, Luo L, Lei M, Li X, Mulder J (2011) Distribution, speciation and availability of antimony (Sb) in soils and terrestrial plants from an active Sb mining area. Environ Pollut 159:2427–2434
Pan X, Zhang D, Chen X, Bao A, Li L (2011) Antimony accumulation, growth performance, antioxidant defense system and photosynthesis of Zea mays in response to antimony pollution in soil. Water Air Soil Pollut 215:517–523
Queiroz Alves L, Mota de Jesus R, Furtado de Almeida AA, Lima Souza V, Oliveira Mangabeira PA (2014) Effects of lead on anatomy, ultrastructure and concentration of nutrients in plants Oxycaryum cubense (Poep. & Kunth) Palla: a species with phytoremediator potential in contaminated watersheds. Environ Sci Pollut Res 21:6558–6570
Reimann C, Matschullat J, Birke M, Salminen R (2010) Antimony in the environment: lessons from geochemical mapping. Appl Geochem 25:175–198
Ren JH, Ma LQ, Sun HJ, Cai F, Luo J (2014) Antimony uptake, translocation and speciation in rice plants exposed to antimonite and antimonate. Sci Total Environ 475:83–89
Shtangeeva I, Singh B, Bali R, Ayrault S, Timofeev S (2014) Antimony accumulation in wheat seedlings grown in soil and water. Commun Soil Sci Plant Anal 45:968–983
Stoláriková-Vaculíková M, Romeo S, Minnocci A, Luxová M, Vaculík M, Lux A, Sebastiani L (2015) Anatomical, biochemical and morphological responses of poplar Populus deltoides clone Lux to Zn excess. Environ Exp Bot 109:235–243
Tisarum R, Lessl JT, Dong X, De Oliveira LM, Rathinasabapathi B, Ma LQ (2014) Antimony uptake, efflux and speciation in arsenic hyperaccumulator Pteris vittata. Environ Pollut 186:110–114
Todeschini V, Lingua G, D’Agostino G, Carniato F, Roccotiello E, Berta G (2011) Effects of zinc concentration on poplar leaves: a morphological and biochemical study. Environ Exp Bot 71:50–56
Tschan M, Robinson B, Nodari M, Schulin R (2009a) Antimony uptake by different plant species from nutrient solution, agar and soil. Environ Chem 6:144–152
Tschan M, Robinson B, Schulin R (2009b) Antimony in the soil-plant system—a review. Environ Chem 6:106–115
Vaculík M, Jurkovič Ľ, Matejkovič P, Molnárová M, Lux A (2013) Potential risk of arsenic and antimony accumulation by medicinal plants naturally growing on old mining sites. Water Air Soil Pollut 224:1546
Vaculíková M, Vaculík M, Šimková L, Fialová I, Kochanová Z, Sedláková B, Luxová M (2014) Influence of silicon on maize roots exposed to antimony—growth and antioxidative response. Plant Physiol Biochem 83:279–284
Zarinkamar F, Ghelich S, Soleimanpour S (2013) Toxic effects of Pb on anatomy and hypericin content in Hypericum perforatum L. Biorem J 17:40–51
Acknowledgments
The authors appreciated valuable help of Dr. Andrej Pavlovič with photosynthesis measurement.
Funding
The work was financially supported by the Slovak Grant Agency VEGA No. VEGA 1/0817/12.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Vaculík, M., Mrázová, A. & Lux, A. Antimony (SbIII) reduces growth, declines photosynthesis, and modifies leaf tissue anatomy in sunflower (Helianthus annuus L.). Environ Sci Pollut Res 22, 18699–18706 (2015). https://doi.org/10.1007/s11356-015-5069-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5069-3