Abstract
This study reports the potential of a soil bacterium, Bacillus subtilis strain SPB1, to produce lipopeptide biosurfactants. Firstly, the crude lipopeptide mixture was tested for its inhibitory activity against phytopathogenic fungi. A minimal inhibitory concentration (MIC), an inhibitory concentration at 50 % (IC50 %), and an inhibitory concentration at 90 % (IC90 %) values were determined to be 0.04, 0.012, and 0.02 mg/ml, respectively, for Rhizoctonia bataticola with a fungistatic mode of action. For Rhizoctonia solani, a MIC, an IC50 %, and IC90 % values were determined to be 4, 0.25, and 3.3 mg/ml, respectively, with a fungicidal mode of action. For both of the fungi, a loss of sclerotial integrity, granulation and fragmentation of hyphal mycelia, followed by hyphal shriveling and cell lysis were observed with the treatment with SPB1 biosurfactant fraction. After extraction, separation, and purification, different lipopeptide compounds were identified in the culture filtrate of strain SPB1. Mass spectroscopic analysis confirmed the presence of different lipopeptide compounds consisting of surfactin isoforms with molecular weights of 1007, 1021, and 1035 Da; iturin isoforms with molecular weights of 1028, 1042, and 1056 Da; and fengycin isoforms with molecular weights of 1432 and 1446 Da. Two new clusters of lipopeptide isoforms with molecular weights of 1410 and 1424 Da and 973 and 987 Da, respectively, were also detected. This study reported the ability of a B. subtilis strain to co-produce lipopeptide isoforms with potential use as antifungal compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Attacks by fungus can be disastrous to crops despite the preventive measures adopted to keep it in control. Management of fungus rot is generally a difficult challenge, and once initiated, epidemics are difficult to contain. Extensive use of chemicals to control plant diseases has disturbed the ecological balance of microbes inhabiting soil, leading to development of resistant strains of pathogens, groundwater contamination, and obvious health risks to humans. One of the biggest ecological challenges being faced by the microbiologists and plant pathologists in the future is the development of environmental-friendly alternatives to the currently used chemical pesticides for combating a variety of crop diseases. As a consequence of the recent demand for eco-friendly disease management, investigation of the antifungal activity of microbial derived products has become of major interest. As suggested by Fatima et al. (2009), biological control of plant diseases is gaining attention due to increased pollution concerns because of pesticides use for crop protection and development of pathogen resistance. The use of environmental-friendly microorganisms has proved useful in plant growth promotion and disease control in modern agriculture (Fatima et al. 2009). They are known to suppress soil-borne plant pathogens through the production of secondary metabolites including antibiotics and therefore improve the productivity of several crops. As reported by Okigbo (2005), Bacillus sp. and its related genera are reported for production of wide range of cyclic lipopeptides active against various fungal species.
Lipopeptides are among of the most popular and interesting class of microbial surfactants. They include mainly surfactin, fengycin, iturin, and lichenysin compounds which are amphiphilic membrane active peptide antibiotics with potent antimicrobial, antiadherent, antiinflammatory, immune modulator, anticancer, antifibrin clot formation, antiviral, antimycoplasma, and hypocholesterolemic activities for a large spectrum of application in medical and pharmaceutical fields (Mnif and Ghribi 2015). They are also of great interest in agricultural as biocontrol and insecticidal agents; bioremediation for their contaminant biodegradation and metal sequestering role; and in food processing industries for their emulsifying, foaming, and dispersing properties (Mnif and Ghribi 2015; Ongena and Jacques 2008).
Surfactin is the most known, interesting, and studied compound among the whole. It has been characterized for the first time in 1968 by Arima et al. (1968) with a primary structure described as a macrolide consisting of a heptapeptide sequence L-Glu(1)-L-Leu(2)-D-Leu(3)-L-Val(4)-L-Asp(5)-D-Leu(6)-L-Leu(7) linked to a ß-hydroxy fatty acid with 13, 14, or 15 carbon atoms (Arima et al. 1968). After that, surfactin production and structure elucidation have been reported in many studies (Ben Ayed et al. 2014; Kowall et al. 1998; Lin et al. 1994). In fact, a vast natural diversity occurs, giving rise to homologues or isomers differing from each other by the length (12 to 16 atoms of carbon) and the ramification of the fatty acid chain, and to isoforms, characterized by some differences in the peptidic sequence (Dufour et al. 2005; Leclère et al. 2005; Price et al. 2007; Pecci et al. 2010). Rather than being genetically determined, these variations depend on the specific Bacillus strain and environmental conditions (Kowall et al. 1998). Also, they can be related to alterations in nutritional culture conditions, especially feeding with some specific amino acid residues (Coronel-León et al. 2015). Loiseau et al. (2015) reported the co-production of two surfactin isoforms by B. subtilis. However, previous works noticed that surfactin can possess six isoforms (Kowall et al. 1998; Leclère et al. 2005). Bacon et al. (2012) reported the production of seven isomers with fatty acid chain ranging from C11 to C17, while Abdel-Mawgoud et al. (2008) reported the production of nine different isoforms. Among the produced lipopeptides, surfactin is the most recognized family. It can be produced mainly by B. subtilis species (Abdel-Mawgoud et al. 2008; Huang et al. 1993; Kowall et al. 1998; Liu et al. 2015; Sang et al. 2005; Willenbacher et al. 2014; Zeriouh et al. 2013), B. pumilis species (Morikawa et al. 1992; Seydlová and Svobodová 2008), B. licheniformis species (Li et al. 2008, 2010; Lin et al. 1994; Tendulkar et al. 2007), B. amyloliquefaciens species (Buensanteai et al. 2008; Horowitz and Griffin 1991; Sun et al. 2006), and B. mojavensis species (Ben Ayed et al. 2014). Similarly, B. subtilis species can produce other lipopeptide isoforms belonging to fengycin (Guo et al. 2013; Yánez-Mendizábal et al. 2012; Pathak et al. 2012; Tang et al. 2014; Yánez- Rebib et al. 2012), bacillomycin (Gong et al. 2014; Luo et al. 2014a), and iturin families (Jin et al. 2014; Yun-feng et al. 2012).
Lipopeptides are largely produced by microorganisms belonging to the Bacillus genus. In this work, we aimed to purify and identify lipopeptide compounds produced by B. subtilis strain SPB1, showing potent antifungal activities against phytopathogenic fungi. Lately, we have reported that B. subtilis strain SPB1 could produce lipopeptide biosurfactants with highly emulsification activity (Ghribi et al. 2012a). It was demonstrated as enhancer of hydrophobic compound bioavailability and biodegradability and could be widely applied in bioremediation technology (Mnif et al. 2013a, 2015a). Moreover, previous studies proved that SPB1 biosurfactant could be of a great interest in the bread-making industry (Mnif et al. 2012, 2013b). Here, we report the purification and identification of the biosurfactant produced by B. subtilis strain SPB1 along with potential antifungal activity against phytopathogenic fungi. Recently, we reported the effective use of the crude lipopeptide preparation of B. subtilis strain SPB1 as a natural fungicide for the control of Fusarium solani infestation in tomato and potato tubers (Mnif et al. 2015b).
Materials and methods
Microorganism
B. subtilis strain SPB1 (HQ392822), a biosurfactant producing bacterium, was isolated in our laboratory from a Tunisian soil contaminated by hydrocarbons. It was selected on the basis of the high hemolytic and emulsification activities of its biosurfactant and which exhibited also a broad spectrum of action, including insecticidal activity against lepidopteran larvae (Ghribi et al. 2011, 2012a, 2012c) and antimicrobial activity against microorganisms with multidrug-resistant profiles (Ghribi et al. 2012b). It was identified as B. subtilis by morphological, biochemical, and 16S rDNA sequence analysis (Ghribi et al. 2012b).
Lipopeptide biosurfactant extraction and purification
Culture conditions and a crude lipopeptide preparation were carried out as described by Mnif et al. (2015b). This serves as a crude lipopeptide to study the antifungal activity. For identification study, lipopeptide extraction and purification were performed as suggested by Coronel-León et al. (2015). The obtained crude lipopeptide was subjected to three extractions with an ethyl acetate-methanol mixture (2:1, v/v). The organic phases were combined, passed over anhydrous sodium sulfate, concentrated in a rotary vacuum evaporator (Büchi, Switzerland), and weighed. Lipopeptide compounds were chromatographed on a silica gel column. Elution was carried out with chloroform/methanol/ammonium hydroxide (65:35:5); the fractions were collected in vials. The process was monitored by thin layer chromatography with the same solvent of elution. They were revealed by ninhydrin specific for amino acid moiety and phosphomolybdic acid specific for fatty acid moiety. Fractions showing the presence of both amino acid and fatty acid parts were analyzed by tandem mass spectrometry (4800 Plus MALDI TOF/TOF, AB SCIEX, CA, USA).
Mass spectrometry
The molecular weight of the components of the surfactants was determined by negative- and positive-ion mode electrospray ionization (ESI) analyses (LC/MSD-TOF, Agilent Technologies, CA, USA) (Coronel-León et al. 2015). The capillary voltage were 4 and 3.5 kV for the positive and negative modes, respectively, with nitrogen as the nebulizing and drying gas. Tandem mass spectrometry (4800 Plus MALDI TOF/TOF, AB SCIEX, CA, USA) was used in the experiment. The full mass spectrum was acquired in the reflector positive-ion mode for the lipopeptide, using dihydroxybenzoic acid (DHB) as matrix.
Phytopathogen fungus
R. bataticola and R. solani were kindly provided by Dr. Mohamed Ali Triki (Olive Tree Institute of Tunisia). They were maintained at 4 °C in potato dextrose agar plates and at −20 °C in tryptone salt medium (tryptone 1 g, NaCl 8.5 g, Tween 20 1 %, glycerol 15 %, and distilled water 1 l).
Study of the antifungal activity of SPB1 biosurfactant
In vitro antifungal activity of SPB1 biosurfactant was checked initially by the disk diffusion method. After that, we studied the inhibition of radial growth of R. bataticola and R. solani. Both studies were performed as described by Mnif et al. (2015b). The mycelial growth inhibition was calculated according to the present formula:
MGI (%) = ((dc − dt)/dc) × 100, where dc and dt represent the mycelial growth diameter in control and treated Petri plates, respectively.
The minimal inhibitory concentration (MIC) defined as the smallest concentration that inhibits the fungal growth totally and the 50 % inhibitory concentration of the lipopeptide (IC50) values were determined.
The fungistatic–fungicidal nature of the SPB1 lipopeptide biosurfactant was tested by controlling revival of growth of the inhibited mycelia disk following its transfer to nontreated PDA (Mnif et al. 2015b). A fungicidal effect was where there was no growth, whereas a fungistatic effect was where temporary inhibition of fungal growth occurred. The agar disks of R. bataticola and R. solani, which failed to grow, were either transferred onto agar media without SPB1 biosurfactant. Petri plates were incubated for 5 days. The experiments were conducted in triplicates.
Results and discussion
Study of the antifungal activity of the B. subtilis SPB1 crude biosurfactant
Study of the antifungal activity of SPB1 biosurfactant against R. bataticola
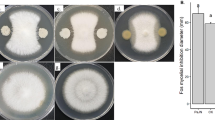
We examined the growth of R. bataticola during 3 days of incubation in presence of different biosurfactant concentrations. Growth patterns were observed in the presence of five increasing concentrations (0.02, 0.04, 0.06, 0.08, and 0.1 %) in comparison to a negative control without biosurfactant addition. As presented in Fig. 1, a total inhibition of almost 100 % was observed for concentrations equal and superior to 0.04 mg/ml. A very few growth was detected in the presence of 0.02 mg/ml of SPB1 biosurfactant, and the percentage of inhibition recorded is statistically significant from negative control and the other studied doses. Mean values were statistically significant according to Duncan test at p values <0.05. In fact, a MIC, an IC50, and IC90 % values were determined to be 0.04, 0.012, and 0.02 mg/ml, respectively. Hence, R. bataticola was shown very sensitive towards SPB1 biosurfactant.
In order to assess the nature of the antifungal potency of the lipopeptide fraction of B. subtilis strain SPB1 towards R. bataticola, an agar with mycelia of the studied fungi showing a total inhibition of growth (in the presence of 0.04 mg/ml biosurfactant) was placed on the center of a new Petri dish without biosurfactant addition and incubated at 25 °C for 5 days. Obtained result showed that the mycelium of R. bataticola was able to regain growth. So, we can assume that SPB1 biosurfactant had a fungistatic mode of action towards R. bataticola. Microscopic observations demonstrate that SPB1 biosurfactant reduced highly growth of R. bataticola and altered hypha and sclerotium morphologies. In fact, as presented in Fig. 2, bloomed sclerotia with teared mycelium were observed when R. bataticola was treated with 0.04 mg/ml SPB1 biosurfactant. Growing concentration accentuates the degree of alteration. In fact, a loss of sclerotial integrity, granulation and fragmentation of hyphal mycelia, followed by hyphal shriveling and cell lysis were observed when using 0.04 mg/ml SPB1 biosurfactant.
R. bataticola is a diverse omnipresent soil and seed-borne necrotrophic fungal pathogen. It has a global distribution and can infect more than 500 plant species including monocot and dicot plant hosts (Sharma et al. 2012). As reported by Sundravadana et al. (2011) and Sharma et al. (2012), high levels of pathogenic and genetic variations in R. bataticola (RB) from different parts of the world were described. This pathogen causes different types of diseases, viz., seedling blight, root rot, charcoal rot, wilt, stalk rot, stem blight, fruit rot, seedling decay, and leaf blight in crop plants, and can therefore cause up to 60 % yield loss in crop production (Sundravadana et al. 2011). In contrast to many pathogens favored by change to moisture conditions (Garrett et al. 2006), R. bataticola may become more problematic in agricultural areas, where climate change results in longer drought periods and higher temperatures when the crop is exposed to moisture stress conditions (Sharma et al. 2012). So, an urgent need for the control of these three disastrous fungi was developed.
Study of antifungal activity of SPB1 biosurfactant towards R. solani
Growth of R. solani was observed during 3 days of incubation in the presence of different biosurfactant concentrations. Growth patterns observed in the presence of increasing concentration of SPB1 biosurfactant ranging from 0.1 to 4 mg/ml show a weak inhibition of the mycelia growth of R. solani in comparison to a negative control without biosurfactant addition as observed in Fig. 3. In fact, mycelium growth of R. solani in the presence of 0.5 and 2 mg/ml were about 21.22 and 12.56 mm, respectively. At 4 mg/ml of biosurfactant, a total inhibition of the growth of the respective fungi was recorded. Hence, a MIC, IC50, and IC90 % values were determined to be 4, 0.25, and 3.3 mg/ml, respectively. An agar with mycelia of the R. solani fungi showing a total inhibition of growth (in the presence of 4 mg/ml biosurfactant) was placed on the center of a novel Petri dish without biosurfactant addition and incubated at 25 °C for 5 days. Result show that the respective fungus was not able to regain growth. With this, we can assume that SPB1 lipopeptide extract had a fungicidal activity towards R. solani.
Microscopic observations of the inhibited mycelium of R. solani by different concentrations of lipopeptide preparation demonstrate that SPB1 biosurfactant was able to lyses hyphae of the respective fungi (Fig. 4). In fact, the negative control shows normal articulate hyphae. Hyphal fragmentation and cell wall lysis indicated the fungicidal nature of the metabolite.
According to literature reviews and studies, fungus R. solani, which causes black scurf of potato tubers and makes its quality worsen, belong to commonly appearing potato pathogens (Kurzawinska and Mazur 2008). Sclerotia of the mentioned fungi occurring on sets can be the source of infection for plants and descendant tubers (Kurzawinska and Mazur 2008). However, many biocontrol strategies were developed to combat these phytopathogenic fungi. Many literature reviews described the use of biopreparation and chemical compounds to inhibit the growth of R. solani (Kurzawinska and Mazur 2008; Walters et al. 2004; Erper et al. 2011). Other studies reported the application of microbial isolates such as bacteria and fungi as biosystems to control the invasion of these fungi (Asaka and Shoda 1996; Montealegre et al. 2010; Naeimi et al. 2010). Here, we evaluated the efficiency of the biosurfactant fraction to control R. solani growth.
Present studies reported the efficiency of SPB1 lipopeptide preparation to inhibit R. bataticola and R. solani growth. Results are similar to those published by Senthilkumar et al. (2009), reporting the loss of sclerotial integrity, the granulation and fragmentation of hyphal mycelia, and cell lysis of the pathogenic fungi R. bataticola when treated by antifungal metabolite produced by Paenibacillus sp. Similar report describe the inhibition of R. solani sclerotium production when treated with many Trichoderma isolates (Naeimi et al. 2010). Therefore, iturin A produced by B. subtilis strain BS-99-H had inhibition potency towards Pestalotiopsis eugeniae presented by a swelling and a deformation of fungus hyphae leading to a fungicidal effect (Lin et al. 2010).
Identification of lipopeptide biosurfactants produced by B. subtilis strain SPB1
The key components of the use of emulsifiers are essentially the cost of their production and the ease of their recovery. Several techniques were developed to extract and purify lipopeptides. As described in the methodology part, lipopeptide fractions were extracted by a mixture of ethyl acetate/methanol (2:1) followed by an elution through silica gel (60) column chromatography.
Mass spectrum analyses of the purified fractions are presented in Fig. 5a, b. They showed five well-resolved clusters of peaks, the first at m/z values between 996 and 1010 Da (family A), the second at m/z values between 1030 and 1058 Da (family B), the third at m/z values between 1051 and 1079 Da (family C), the fourth at m/z values between 1433 and 1447 Da (family D), and the fifth at m/z values between 1455 and 1469 Da (family E). By comparing the mass with the mass numbers reported for the lipopeptide complexes from other Bacillus strains, each group of peaks could be attributed to different lipopeptide isoforms. Each isoform group can belong to the same family and have probably the same amino acid sequence with difference in the length of the fatty acid chain. Family B corresponds to the surfactin family with molecular weights of 1007, 1021, and 1035 Da (Kowall et al. 1998; Price et al. 2007; Chen et al. 2008; Pecci et al. 2010; Pyoung et al. 2010; Luo et al. 2014a, b; Ben Ayed et al. 2014); family C corresponds to the iturin family with molecular weights of 1028, 1042, and 1056 Da (Price et al. 2007; Chen et al. 2008; Pyoung et al. 2010); and family E corresponds to the fengycin family with molecular weights of 1432 and 1446 Da (Vater et al. 2002; Pathak et al. 2012; Guo et al. 2013; Luo et al. 2014b; Tang et al. 2014; Ben Ayed et al. 2014) (Table 1). However, the other two families, A and D, corresponded to two new clusters of lipopeptide families with molecular weights of 1410 and 1424 Da and 973 and 987 Da, respectively (Table 1).
a Mass spectroscopy (LC/MSD-TOF) spectra of molecular mass of SPB1 lipopeptide biosurfactants. Spectra of lipopeptides (a), surfactin (b), and iturin (c) produced by B. subtilis SPB1. b Mass spectroscopy (LC/MSD-TOF) spectra of molecular mass of SPB1 lipopeptide biosurfactants. Spectra of lipopeptides (d) and fengycin (e) produced by B. subtilis SPB1
Structurally, lipopeptides are amphiphilic anionic cyclic compounds that contain peptidic moieties linked to a ß-fatty acid tails (Mnif and Ghribi 2015). They consist of short linear chains or cyclic structures of amino acids, linked to a fatty acid via ester or amide bonds or both (Mnif and Ghribi 2015). A lactone bridge between the β-hydroxyl function of the acid and the carboxy-terminal function of the peptide confers a cyclic structure to this molecule (Mnif and Ghribi 2015). Lipopeptides constitute a diverse group of metabolites produced by various bacterial and fungal genera (Mnif and Ghribi 2015). Moreover, there is considerable structural diversity as a consequence of differences in the nature of the fatty acid component, as well as in the type, number, and configuration of the amino acids in the peptide chain (Fracchia et al. 2012). Cyclic lipopeptides belonging to surfactin, fengycin, iturin, and lichenysin families represent the four major classes of lipopeptide biosurfactant isoforms produced by Bacillus strains.
In the context of biocontrol of plant diseases, the three families of Bacillus lipopeptides—surfactins, iturins, and fengycins—were at first mostly studied for their appreciable antibacterial or antifungal properties (Ongena and Jacques 2008). Regarding many literature reviews and studies, microbial-derived lipopeptides were described as potential inhibitors of phytopathogen growth. As suggested by Raaijmakers et al. (2010), the main natural functions of lipopeptides from Bacillus species are believed to the control of other microorganisms, motility, and attachment to surfaces, although they may also have a signaling function to coordinated growth and differentiation. Canova et al. (2010) reported the suppression of R. solani by Paenibacillus sp. (IIRAC30) derived surfactin series. Moreover, lipopeptide biosurfactant remains a very interesting alternative to control R. solani invasion. To know, iturin A produced by B. amyloliquefaciens (Yu et al. 2002) and cyclic lipopeptides produced by fluorescent Pseudomonas spp. (Nielsen et al. 2002) were discussed to suppress R. solani. Mycelial growth of Fusarium moniliforme and F. graminearum and Sclerotinia sclerotiorum were effectively inhibited in vitro by B. subtilis B-FS01 derived fengycin (Hu et al. 2007) and bacillomycin and fengycin derived from Bacillus spp. (Ramarathnam et al. 2007), respectively. Also, iturin A produced by B. subtilis BS-99-H inhibits germination of P. eugeniae spores (Lin et al. 2010). In fact, fengycin-type lipopeptides were as suppressing agent of Fusarium wilt and foot rot (Rebib et al. 2012; Cao et al. 2012).
In the past decades, research on lipopeptides has been fueled by their antimicrobial, antitumor, immunosuppressant, and surfactant activities (Raaijmakers et al. 2010). Owing to their microbial origin, lipopeptide compounds are low or nontoxic, biodegradable, demonstrate excellent surface activity, possess high specificity, show effectiveness under extreme conditions, and can be reused through regeneration too as compared to synthetic surfactants (Lima et al. 2011; Sachdev and Cameotra 2013). Consequently, they are widely used in many fields such as environment, chemical, food and cosmetic industries, medical and pharmaceutical fields, and in agricultural field (Sachdev and Cameotra 2013).
In this study, we report the potential involvement of different lipopeptide isoforms in the biocontrol activity of B. subtilis SPB1 towards phytopathogenic fungi. In fact, cyclic lipopeptides (CLPs) produced by B. subtilis strains have been shown to protect host plants from a number of pathogens (Falardeau et al. 2013). Our findings are similar to those published by Li et al. (2014) and Waewthongrak et al. (2014) reporting the co-production of surfactin, iturin, and fengycin isoforms involved in the biocontrol of Plasmodiophora brassicae and F. solani and Penicilium digitatum, respectively. Also, Liu et al. (2014) reported that the involvement of three isoforms, surfactin, iturin, and fengycin, affected spore germination and membrane permeability of spores from four fungal plant pathogens (Alternaria solani, Fusarium sambucinum, Rhizopus stolonifer, and Verticillium dahliae). Similarly, Ben Slimene et al. (2012) showed the production of iturins, surfactins, and fengycins with long-chain fatty acids and other not yet identified compounds by spores of B. subtilis, an antagonist L194 strain against Phoma medicaginis pathogenic fungi. In a study published by Luo et al. (2014b), B. subtilis 916 co-produce not only the three families of well-known lipopeptides, surfactin, bacillomycin L (iturin family), and fengycin but also produce a new family of lipopeptide called locillomycin active against F. oxysporum. However, Cao et al. (2012) reported the involvement of B. subtilis SQR 9-derived fengycin and bacillomycin in the inhibition of mycelial growth and spore germination of F. oxysporum. Waewthongrak et al. (2014) suggested that fengycin and surfactin act as elicitors of defense-related gene expression in “Valencia” fruit following infection by P. digitatum.
According to literature reviews and studies, many essential oils were described as potent inhibitors of in vitro R. bataticola growth (Sharma et al. 2012; Beg and Ahmad 2002; Kundu et al. 2013). However, biocontrol using microbial-derived compounds remains the best alternative. Regarding previous studies, in vitro growth of R. bataticola was inhibited by antifungal metabolite secreted by the endophytic bacterium belonging to Bacillus and Paenibacillus strains (Senthilkumar et al. 2009) and Pseudomonas fluorescens (Sujatha and Ammani 2013).
Conclusion
The production of lipopeptides by B. subtilis SPB1 was confirmed by spectrometric analysis. Results demonstrated the ability of the strain to produce a mixture of lipopeptide isoforms. After extraction and purification, SPB1 biosurfactant was shown to be composed of different lipopeptide isoforms belonging to surfactin, iturin, and fengycin families in addition to two new un-identified lipopeptide clusters. The lipopeptide mixture exhibited strong antifungal activity against R. solani and R. bataticola. In vitro antifungal assay determined a minimal inhibitory concentration of 0.04 mg/ml with a fungistatic mode of action towards R. bataticola and 4 mg/ml with a fungicidal mode of action for R. solani. In conclusion, the present work shows that the SPB1 strain constitutes a promising biocontrol agent against plant diseases induced by phytopathogenic fungi. Its mode of action seems to involve synergism between various secreted lipopeptide antibiotics, some of which remain to be characterized.
References
Abdel-Mawgoud AM, Aboulwafa MM, Hassouna NA-H (2008) Characterization of surfactin produced by Bacillus subtilis isolate BS5. Appl Biochem Biotechnol 150:289–303
Arima K, Kakinuma A, Tamura G (1968) Surfactin, a crystalline peptide-lipid surfactant produced by Bacillus subtilis: isolation, characterization and its inhibition of fibrin clot formation. Biochem Biophys Res Communicat 31:488–494
Asaka O, Shoda M (1996) Biocontrol of Rhizoctonia solani damping-off of tomato with Bacillus subtilis RB14. Appl Environ Microbiol 62:4081–4085
Bacon CW, Hinton DM, Mitchell TR, Snook ME, Olubajo B (2012) Characterization of endophytic strains of Bacillus mojavensis and their production of surfactin isomers. Biol Control 62:1–9
Beg AZ, Ahmad I (2002) In vitro fungitoxicity of the essential oil of Syzygium aromaticum. World J Mirobiol Biotechnol 18:313–315
Ben Ayed H, Hmidet N, Béchet M, Chollet M, Chataigné G, Leclère V, Jacques P, Nasri M (2014) Identification and biochemical characteristics of lipopeptides from Bacillus mojavensis A21. Process Biochem. doi:10.1016/j.procbio.2014.07.001
Ben Slimene I, Tabbene O, Djebali N, Cosette P, Schmitter JM, Jouenne T, Urdaci M-C, Limam F (2012) Putative use of a Bacillus subtilis L194 strain for biocontrol of Phoma medicaginis in Medicago truncatula seedlings. Res Microbiol 163:388–397
Buensanteai N, Yuen GY, Prathuangwong S (2008) The biocontrol bacterium Bacillus amyloliquefaciens KPS46 produces auxin, surfactin and extracellular proteins for enhanced growth of soybean plant. Thai J Agricult Sci 41(3–4):101–116
Canova SP, Petta T, Reyes LF, Zucchi TD, Moraes LAB, Melo IS (2010) Characterization of lipopeptides from Paenibacillus sp. (IIRAC30) suppressing Rhizoctonia solani. World J Microbiol Biotechnol 26:2241–2247
Cao Y, Xu Z, Ling N, Yuan Y, Yang X, Chen L, Shen B, Shen QR (2012) Isolation and identification of lipopeptides produced by B. subtilis SQR 9 for suppressing Fusarium wilt of cucumber. Scientia Horticult 135:32–39
Chen H, Wang L, Su CX, Gong GH, Wang P, Yu ZL (2008) Isolation and characterization of lipopeptide antibiotics produced by Bacillus subtilis. Lett Appl Microbiol 47:180–186
Coronel-León J, de Grau G, Grau-Campistany A, Farfan M, Rabanal F, Manresa A, Marqués AM (2015) Biosurfactant production by AL 1.1, a Bacillus licheniformis strain isolated from Antarctica: production, chemical characterization and properties. Ann Microbiol. doi:10.1007/s13213-015-1045-x
Dufour S, Deleu M, Nott K, Wathelet B, Thonart P, Paquot M (2005) Hemolytic activity of new linear surfactin analogs in relation to their physico-chemical properties. Biochim Biophys Acta 1726:87–95
Erper I, Turkkan M, Karaca GH, Kılıc G (2011) Evaluation of in vitro antifungal activity of potassium bicarbonate on Rhizoctonia solani AG 4 HG-I, Sclerotinia sclerotiorum and Trichoderma sp. Afr J Biotechnol 10(43):8605–8612
Falardeau J, Wise C, Novitsky L, Avis TJ (2013) Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J Chem Ecol 39:869–878
Fatima Z, Saleemi M, Zia M, Sultan T, Aslam M, Rehman R-U, Chaudhary MF (2009) Antifungal activity of plant growth-promoting rhizobacteria isolates against Rhizoctonia solani in wheat. Afr J Biotechnol 8(2):219–225
Fracchia L, Cavallo M, Martinotti MG, Banat IM (2012) Biosurfactants and bioemulsifiers biomedical and related applications—present status and future potentials, Biomedical Science, Engineering and Technology, Prof. Dhanjoo N. Ghista (Ed.), ISBN: 978-953-307-471-9, InTech
Garrett KA, Dendy SP, Frank EE, Rouse MN, Travers SE (2006) Climate change effects on plant disease: genomes to ecosystems. Annual Rev Phytopathol 44:489–509
Ghribi D, Abdelkefi L, Boukadi H, Elleuch M, Ellouze-Chaabouni S, Tounsi S (2011) The impact of the Bacillus subtilis SPB1 biosurfactant on the midgut histology of Spodoptera littoralis (Lepidoptera: Noctuidae) and determination of its putative receptor. J Inver Pathol 109(2):183–186
Ghribi D, Abdelkefi-Mesrati L, Mnif I, Kammoun R, Ayadi I, Saadaoui I, Maktouf S, Chaabouni-Ellouze S (2012a) Investigation of antimicrobial activity and statistical optimization of Bacillus subtilis SPB1 biosurfactant production in solid-state fermentation. J Biomed Biotechnol. doi:10.1155/2012/373682
Ghribi D, Elleuch M, Abdelkefi LM, Ellouze-Chaabouni S (2012b) Evaluation of larvicidal potency of Bacillus subtilis SPB1 biosurfactant against Ephestia kuehniella (Lepidoptera: Pyralidae) larvae and influence of abiotic factors on its insecticidal activity. J Stored Prod Res 48:68–72
Ghribi D, Elleuch M, Abdelkefi-Mesrati L, Boukedi H, Ellouze Chaabouni S (2012c) Histopathological effects of Bacillus subtilis SPB1 biosurfactant in the midgut of Ephestia kuehniella (Lepidoptera: Pyralidae) and improvement of its insecticidal efficiency. J Plant Dis Protect 119(1):24–29
Gong Q, Zhang C, Lu F, Zhao H, Bie X, Lu Z (2014) Identification of bacillomycin D from Bacillus subtilis fmbJ and its inhibition effects against Aspergillus flavus. Food Control 36:8–14
Guo Q, Dong W, Li S, Lu X, Wang P, Zhang X, Wang Y, Ma P (2013) Fengycin produced by Bacillus subtilis NCD-2 plays a major role in biocontrol of cotton seedling damping-off disease. Microbiol Res. doi:10.1016/j.micres.2013.12.001
Horowitz S, Griffin WM (1991) Structural analysis of Bacillus licheniformis 86 surfactant. J Indust Microbiol Biotechnol 7:45–52
Hu LB, Shi ZQ, Zhang T, Yang ZM (2007) Fengycin antibiotics isolated from B-FS01 culture inhibit the growth of Fusarium moniliforme Sheldon ATCC 38932. FEMS Microbiol Lett 272:91–98
Huang C-C, Ano T, Shoda M (1993) Nucleotide sequence and characterization of the gene, lpa-14, responsible for biosynthesis of the lipopeptide antibiotics iturin A and surfactin from Bacillus subtilis RB14. J ferment Bioeng 76:445–450
Jin H, Zhang X, Li K, Niu Y, Guo M, Hu C, Wan X, Gong Y, Huang F (2014) Direct bio-utilization of untreated rapeseed meal for effective iturin A production by Bacillus subtilis in submerged fermentation. PLOS ONE. doi:10.1371/journal.pone.0111171
Kowall M, Vater J, Kluge B, Stein T, Franke P, Ziessow D (1998) Separation and characterization of surfactin isoforms produced by Bacillus subtilis OKB 105. J Colloid Interf Sci 204:1–8
Kundu A, Saha S, Walia S (2013) Antioxidant and antifungal properties of the essential oil of Anisomeles indica from India. J Med Plants Res 7(24):1774–1779
Kurzawinska H, Mazur S (2008) Biological control of potato against Rhizoctonia solani (Kühn). Scientific works of the Lithuanian Institute of Horticulture and Lithuanian University of Agriculture 27 (2)
Leclère V, Béchet M, Adam A, Guez J-S, Wathelet B, Ongena M, Thonart P, Gancel F, Chollet-Imbert M, Jacques P (2005) Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl Environ Microbiol 71:4577–4584
Li X-Y, Yang J-J, Mao Z-C, Ho H-H, Wu Y-X, He Y-Q (2014) Enhancement of biocontrol activities and cyclic lipopeptides production by chemical mutagenesis of Bacillus subtilis XF-1, a biocontrol agent of Plasmodiophora brassicae and Fusarium solani. Ind J Microbiol. doi:10.1007/s12088-014-0471-y
Li Y, Yang S, Mu B (2010) The surfactin and lichenysin isoforms produced by Bacillus licheniformis HSN 221. Analyt Lett 43:929–940
Li Y-M, Haddad NIA, Yang S-Z, Mu B-Z (2008) Variants of lipopeptides produced by Bacillus licheniformis HSN221 in different medium components evaluated by a rapid method ESI-MS. Springer Science+Business Media 14:229–235
Lima TM, Procópio LC, Brandão FD, Leão BA, Tótola MR, Borges AC (2011) Evaluation of bacterial surfactant toxicity towards petroleum degrading microorganisms. Bioresour Technol 102:2957–2964
Lin HF, Chen TH, Liu SD (2010) Bioactivity of antifungal substance iturin A produced by Bacillus subtilis strain BS-99-H against Pestalotiopsis eugeniae, a causal pathogen of wax apple fruit rot. Plant Pathol Bull 19:225–233
Lin S-C, Minton MA, Sharma MM, Georgiou G (1994) Structural and immunological characterization of a biosurfactant produced by Bacillus licheniformis JF-2. Appl Environ Microbiol 60:31–38
Liu Q, Lin J, Wang W, Huang H, Li S (2015) Production of surfactin isoforms by Bacillus subtilis BS-37 and its applicability to enhanced oil recovery under laboratory conditions. Biochem Eng J 93:31–37
Liu J, Hagberg I, Novitsky L, Hadj-Moussa H, Avis TJ (2014) Interaction of antimicrobial cyclic lipopeptides from Bacillus subtilis influences their effect on spore germination and membrane permeability in fungal plant pathogens. Fungal Biol. doi:10.1016/ j.funbio.2014.07.004
Loiseau C, Schlusselhuber M, Bigot R, Bertaux J, Berjeaud J-M, Verdon J (2015) Surfactin from Bacillus subtilis displays an unexpected anti-Legionella activity. Appl Microbiol Biotechnol. doi:10.1007/s00253-014-6317-z
Luo C, Liu X, Zhou H, Wang X, Chen Z (2014a) Identification of four NRPS gene clusters in Bacillus subtilis 916 for four families of lipopeptides biosynthesis and evaluation of their intricate functions to the typical phenotypic features. Appl Environ Microbiol. doi:10.1128/AEM.02921-14
Luo C, Zhou H, Zou J, Wang X, Zhang R, Xiang Y, Chen Z (2014b) Bacillomycin L and surfactin contribute synergistically to the phenotypic features of Bacillus subtilis 916 and the biocontrol of rice sheath blight induced by Rhizoctonia solani. Appl Microbiol Biotechnol. doi:10.1007/s00253-014-6195-4
Mnif I, Besbes S, Ellouze R, Chaabouni E S, Ghribi D (2012) Improvement of bread quality and bread shelf-life by Bacillus subtilis biosurfactant addition. Food Sci Biotechnol. doi:10.1007/s10068-012
Mnif I, Sahnoun R, Ellouze-Chaabouni S, Ghribi D (2013a) Evaluation of B. subtilis SPB1 biosurfactants’ potency for diesel-contaminated soil washing: optimization of oil desorption using Taguchi design. Environ Sci Pollut Res. doi:10.1007/s11356-013-1894-4
Mnif I, Besbes S, Ellouze-Ghorbel R, Ellouze-Chaabouni S, Ghribi D (2013b) Improvement of bread dough quality by Bacillus subtilis SPB1 biosurfactant addition: optimized extraction using response surface methodology. J Sci Food Agr 93:3055–3064
Mnif I, Ghribi D (2015) Lipopeptides biosurfactants, main classes and new insights for industrial, biomedical, and environmental applications. Bioplymers: Pept Sci. doi:10.1002/bip.22630
Mnif I, Mnif S, Sahnoun R, Ayedi Y, Ellouze-Chaabouni S, Ghribi D (2015a) Biodegradation of diesel oil by a novel microbial consortium: comparison between co-inoculation with biosurfactant-producing strain and exogenously added biosurfactants. Env Sci Pollut Res. doi:10.1007/s11356-015-4488-5
Mnif I, Hammami I, Triki MA, Azabou MC, Ellouze-Chaabouni S, Ghribi D (2015b) Antifungal efficiency of a lipopeptide biosurfactant derived from Bacillus subtilis SPB1 versus the phytopathogenic fungus, Fusarium solani. Env Sci Pollut Res (In press)
Montealegre J, Valderrama L, Sánchez S, Herrera R, Besoain X, Pérez LM (2010) Biological control of Rhizoctonia solani in tomatoes with Trichoderma harzianum mutants. Elect J Biotechnol 13
Morikawa M, Ito M, Imanaka T (1992) Isolation of a new surfactin producer Bacillus pumilus A-I, and cloning and nucleotide sequence of the regulator gene, psf. J Ferment Bioeng 74:255–261
Naeimi S, Okhovvat SO, Javan-Nikkhah M, Vagvolgyi C, Khosravi V, Kredics L (2010) Biological control of Rhizoctonia solani AG1-1A, the causal agent of rice sheath blight with Trichoderma strains. Phytopathol Mediterr 49:287–300
Nielsen TH, Sørensen D, Tobiasen C, Andersen JB, Christophersen C, Givskov M, Sørensen J (2002) Antibiotic and biosurfactant properties of cyclic lipopeptides produced by fluorescent Pseudomonas spp. from the sugar beet rhizosphere. Appl Environ Microbiol 68:3416
Okigbo RN (2005) Biological control of postharvest fungal rot of yam (Dioscorea spp.) with Bacillus subtilis. Mycopathol 159:307–314
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125
Pathak KV, Keharia H, Gupta K, Thakur SS, Balaram P (2012) Lipopeptides from the banyan endophyte, Bacillus subtilis K1: mass spectrometric characterization of a library of fengycins. Am Soc Mass Spectrom 23:1716–1728
Pecci Y, Rivardo F, Martinotti MG, Allegrone G (2010) LC/ESI-MS/MS characterisation of lipopeptide biosurfactants produced by the Bacillus licheniformis V9T14 strain. J Mass Spectrom 45:772–778
Price NPJ, Rooney AP, Swezey JL, Perry E, Cohan FM (2007) Mass spectrometric analysis of lipopeptides from Bacillus strains isolated from diverse geographical locations. FEMS Microbiol Lett 271:83–89
Pyoung K, Ryu J, Kim YH, ChI YT (2010) Production of biosurfactant lipopeptides iturin A, fengycin, and surfactin from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J Microbiol Biotechnol 20:138–145
Raaijmakers JM, de Bruijn I, Nybroe O, Ongena M (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiology 34:1037–1062
Ramarathnam R, Bo S, Chen Y, Dilantha Fernando WG, Xuewen G, de Kievit T (2007) Molecular and biochemical detection of fengycin- and bacillomycin D-producing Bacillus spp., antagonistic to fungal pathogens of canola and wheat. Canad J Microbiol 53:901–911
Rebib H, Hedi A, Roussent M, Boudabous A, Limam F, Sadfi-Zouaoui N (2012) Biological control of Fusarium foot rot of wheat using fengycin-producing Bacillus subtilis isolated from salty soil. Afr J Biotechnol 11:8464–8475
Sachdev DP, Cameotra SS (2013) Biosurfactants in agriculture. Appl Microbiol Biotechnol 97:1005–1016
Sang HY, Kim J-B, Lim Y-H, Hong S-R, Song J-K, Kim S-S, Kwon S-W, Park I-C, Kim S-J, Yeo Y-S, Koo B-S (2005) Isolation and characterization of three kinds of lipopeptides produced by Bacillus subtilis JKK238 from Jeot-Kal of Korean traditional fermented fishes. Kor J Microbiol Biotechnol 33:295–301
Senthilkumar M, Swarnalakshmi K, Govindasamy V, Lee YK, Annapurna K (2009) Biocontrol potential of soybean bacterial endophytes against charcoal rot fungus, Rhizoctonia bataticola. Curr Microbiol 58:288–293
Seydlová G, Svobodová J (2008) Review of surfactin chemical properties and the potential biomedical applications. Cent Eur J Med 3:123–133
Sharma P, Raina AP, Dureja P (2012) Evaluation of the antifungal and phytotoxic effects of various essential oils against Sclerotium rolfsii (Sacc) and Rhizoctonia bataticola (Taub). Afr J Microbiol Res 6:6653–6660
Sujatha N, Ammani K (2013) Siderophore production by the isolates of fluorescent Pseudomonads. J Cur Res Rev 5:1–7
Sun L, Lu Z, Bie X, Lu F, Yang S (2006) Isolation and characterization of a co-producer of fengycins and surfactins, endophytic Bacillus amyloliquefaciens ES-2, from Scutellaria baicalensis Georgi. World J Microbiol Biotechnol 22:1259–1266
Sundravadana S, Thirumurugan S, Alice D (2011) Exploration of molecular variability in Rhizoctonia Bataticola, the incitant of root rot disease of pulse crops. J Plant Protect Res 51:185–189
Tang Q, Bie X, Lu Z, Lv F, Tao Y, Qu X (2014) Effects of fengycin from Bacillus subtilis fmbJ on apoptosis and necrosis in Rhizopus stolonifer. J Microbiol 52:675–680
Tendulkar SR, Saikumari YK, Patel V, Raghotama S, Munshi TK, Balaram P, Chattoo BB (2007) Isolation, purification and characterization of an antifungal molecule produced by Bacillus licheniformis BC98, and its effect on phytopathogen Magnaporthe grisea. J Appl Microbiol 103:2331–2339
Vater J, Kablitz B, Wilde C, Franke P, Mehta N, Cameotra SS (2002) Matrix-assisted laser desorption ionization–time of flight mass spectrometry of lipopeptide biosurfactants in whole cells and culture filtrates of Bacillus subtilis C-1 isolated from petroleum sludge. Appl Environ Microbiol 68:6210–6219
Waewthongrak W, Leelasuphakul W, McCollum G (2014) Cyclic lipopeptides from Bacillus subtilis ABS–S14 elicit defense-related gene expression in citrus fruit. PLoS ONE. doi:10.1371/journal.pone.0109386
Walters D, Raynor L, Mitchell A, Walker R, Walker K (2004) Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathol 157:87–90
Willenbacher J, Syldatk C, Hausmann R (2014) Production of surfactin with Bacillus subtilis in a fermentation process with integrated foam fractionation. Chem Ing Tech 86:1593–1602
Yánez-Mendizábal V, Zeriouh H, Viñas I, Torres R, Usall J, de Vicente A, Pérez-García A, Teixidó N (2012) Biological control of peach brown rot (Monilinia spp.) by Bacillus subtilis CPA-8 is based on production of fengycin-like lipopeptides. Eur J Plant Pathol 132:609–619
Yu GY, Sinclair JB, Hartman GL, Bertagnolli BL (2002) Production of iturin A by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol Biochem 34:955–963
Yun-feng Y, Qi-qin L, Gang F, Gao-qing Y, Jian-hua M, Wei L (2012) Identification of antifungal substance (iturin A2) produced by Bacillus subtilis B47 and its effect on southern corn leaf blight. J Integr Agr 11:90–99
Zeriouh H, de Vicente A, Pérez-García A, Romero D (2013) Surfactin triggers biofilm formation of Bacillus subtilis in melon phylloplane and contributes to the biocontrol activity. Environ Microbiol. doi:10.1111/1462-2920.12271
Acknowledgments
This work has been supported by grants from “Tunisian Ministry of Higher Education, Scientific Research and Technology” and the “Tunisian Ministry of Agriculture.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest is declared.
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Mnif, I., Grau-Campistany, A., Coronel-León, J. et al. Purification and identification of Bacillus subtilis SPB1 lipopeptide biosurfactant exhibiting antifungal activity against Rhizoctonia bataticola and Rhizoctonia solani . Environ Sci Pollut Res 23, 6690–6699 (2016). https://doi.org/10.1007/s11356-015-5826-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5826-3