Abstract
For effective microbe-assisted bioremediation, metal-resistant plant growth-promoting bacteria (PGPB) must facilitate plant growth by restricting excess metal uptake in plants, leading to prevent its bio-amplification in the ecosystem. The aims of our study were to isolate and characterize copper (Cu)-resistant PGPB from waste water receiving contaminated soil. In addition, we investigated the phytotoxic effect of copper on the lentil plants inoculated with copper-resistant bacteria Providencia vermicola, grown in copper-contaminated soil. Copper-resistant P. vermicola showed multiple plant growth promoting characteristics, when used as a seed inoculant. It protected the lentil plants from copper toxicity with a considerable increase in root and shoot length, plant dry weight and leaf area. A notable increase in different gas exchange characteristics such as A, E, C i , g s , and A/E, as well as increase in N and P accumulation were also recorded in inoculated plants as compared to un-inoculated copper stressed plants. In addition, leaf chlorophyll content, root nodulation, number of pods, 1,000 seed weight were also higher in inoculated plants as compared with non-inoculated ones. Anti-oxidative defense mechanism improved significantly via elevated expression of reactive oxygen species -scavenging enzymes including ascorbate peroxidase, superoxide dismutase, catalase, and guaiacol peroxidase with alternate decrease in malondialdehyde and H2O2 contents, reduced electrolyte leakage, proline, and total phenolic contents suggesting that inoculation of P. vermicola triggered heavy metals stress-related defense pathways under copper stress. Overall, the results demonstrated that the P. vermicola seed inoculation confer heavy metal stress tolerance in lentil plant which can be used as a potent biotechnological tool to cope with the problems of copper pollution in crop plants for better yield.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution is widespread and is serious environmental problem due to its excessive use in industries and agriculture. The higher accumulation rate of heavy metals in water bodies as well as in agricultural lands is creating a serious threat to bio-ecosystems. Among heavy metals, copper (Cu) is a potential pollutant that accumulates in soils and sediments (Lamb et al. 2009). Copper acts as an essential micronutrient in low concentration and plays structural and catalytic role in plant growth and development (Demirevska-Kepova et al. 2004). However, its higher concentration adversely affects the functioning of photosynthetic machinery and photosynthetic process (González-Mendoza et al. 2013; Wang et al. 2014), nitrogen assimilation (Zhang et al. 2014), cell wall metabolism (Liu et al. 2014), mitochondrial electron transport chain, and root hair formation (Liu et al. 2014), leading to reduce plant biomass production (Cambroll et al. 2011). Cu toxicity in plant depends on the metal concentration and exposure duration (Gao et al. 2008). The physiological basis for phytotoxicity of Cu is still uncertain. Some plant species accumulate large amount of Cu (hyper accumulator) in roots and restrict its further translocation to shoot (Rascio and Navari-Izzo 2011). However, others accumulate it in the above ground parts, e.g., shoots and leaves (Janas et al. 2010; Kovacik and Backor 2008; Lin et al. 2003). It is well known that the higher concentrations of heavy metals including Cu cause oxidative stress that result in the production of reactive oxygen species (ROS) leading to increase membrane permeability due to lipid peroxidation (Kafel et al. 2010). Under such conditions, plants adjust their metabolism by inducing biochemical changes including the upregulation/downregulation of antioxidant defense system to protect themselves from the stress-induced oxidative injury (Demirevska-Kepova et al. 2004). Therefore, the induction of antioxidative enzymes, i.e., catalase (CAT), ascorbate peroxidase (APX), superoxide dismutase (SOD), and guaiacol peroxidase (GPX), is very important to determine the ability of plants to withstand metal-induced oxidative stress and adaptive response of the plants against metal toxicity (Gratão et al. 2005).
Plant growth-promoting bacteria (PGPB) can facilitate growth by improving plant resistance to a variety of environmental stresses (Gururani et al. 2013). The approach to utilize PGPB subordinates the plant stresses and is an alternative to traditional remediation methods that involve the addition of synthetic chemicals, which are time consuming and increase the cost of the final crop (Li et al. 2013). PGPB assist the plants to cope heavy metal-induced stress by improving the plant growth through different mechanism such as indole acetic acid production, phosphate solubilizaton, siderophore production, nitrogen fixation, and production of ACC deaminase (Ma et al. 2011; Rajkumar et al. 2009). Many strains of bacteria are able to tolerate considerable amounts of heavy metals by several resistant mechanisms, including active efflux system, complex formation with thiol-containing molecules, transformation of more toxic compounds into less toxic forms, extra or intracellular sequestration, and immobilization/mobilization of heavy metals (Bruins et al. 2000; Gibbons et al. 2011; Lima et al. 2006; Nies 2003). However, still little is known about the plant–microbe interactions under heavy metal stress (Nautiyal et al. 2008). Many Gram-positive and Gram-negative PGPB have been reported to colonize and confer beneficial effects by various direct and indirect mechanisms (Gururani et al. 2013). The beneficial plant–microbial interactions are very frequent in nature and help the plants to overcome various stresses. Microbial communities offer a potentially powerful opportunity for understanding these beneficial interactions. Accordingly, changes in the structure or function of microbial communities may have a major impact on ecosystem activities (Khan et al. 2011). Therefore, application of PGPB as an elicitor for tolerance to abiotic stresses, such as drought, salt, nutrient deficiency, and heavy metals in plant, and raising possibility for incorporation of microbial genes into plant and diverse microbial species are now being addressed and getting the interest of scientists in such studies (Yang et al. 2009).
In literature, there is diminutive information about the role of heavy metal-resistant PGPB inoculation, regarding their vast and varied functional properties, on the growth and alleviation of metal toxicity especially in lentil plants when grown on Cu-contaminated soils. The present study was aimed to isolate highly Cu-resistant PGPB strains and their effect on the lentil growth under Cu stress, in relation with nitrogen and phosphorus accumulation, alleviation of metal toxicity and oxidative stress, alterations in the antioxidative defense system, and metal uptake both in the presences and absences of Cu resistance bacteria.
Material and methods
Isolation and screening of copper-resistant bacteria
Thirty-five water samples were collected from Pharang drain (collecting industrial effluent which is further used for irrigation in peri-urban areas of Faisalabad, Pakistan). The bacterial isolation was carried out at the same day of sample collection by the spread plate method (Islam et al. 2014a). The 100 μL of 106 times serially diluted [1 mL industrial effluent + 9 mL saline (85 % NaCl)] effluent solution was spread on Luria–Bertani (LB) media plates and incubated for 24 h at 30 °C (McLellan et al. 2009). Colonies of different morphological appearances were selected and transferred to LB media plates. The quadrant streaking method was used to obtain isolated, independent bacterial colonies (Harley 2014).
Determination of minimum inhibitory concentration (MIC)
MIC of all the bacterial isolates was determined by gradual increase of copper concentration in LB media plates until the bacterial isolates failed to grow on plates over 7 days of incubation (Islam et al. 2014b). This concentration was considered as MIC of respective isolates.
Molecular identification
Among the isolated copper-resistant bacteria, the highly copper-resistant Cuc1 isolate (1,400 ppm; MIC) was identified and evaluated in a pot experiment to check its impact on lentil plants grown in Cu-contaminated soil. The bacterial isolate Cuc1 was identified to be Providencia vermicola by using standard morphological and biochemical tests including shape, color, gram staining, motility, methyl red, H2S production, HCN production, gelatin liquefaction, catalase, and oxidase production using the protocols of Bergey et al. (1994) and confirmed by 16 s rRNA gene analysis. Bacterial genomic DNA was extracted using QIAGEN genomic DNA isolation kit according to the manufacturer’s recommendations. The 16S rRNA gene was amplified using universal primers 27F (5′-AGA GTT TGA TCC TGG CTC AG-3′) and 1492R (5′- GGT TAC CTT GTT ACG ACT T-3′). The amplification reaction was carried out in a final volume of 25 μL containing 2.5 μL of Taq polymerase buffer, 200 μM of each dNTP, 1.5 mM of MgCl2, 0.5 μM of each primer, 0.625 U of Taq polymerase, and 1 μL of template solution. PCR was performed in a thermocycler (PTC 200 Gradient Cycler, MJ Research, Waltham, Massachusetts) according to the following program: one cycle of 4 min at 94 °C; 39 cycles of 1 min at 94 °C, 1 min at 55 °C, and 1.5 min at 72 °C; and one final cycle of 5 min at 72 °C. The amplified 16S rRNA products were purified by MinElute PCR Purification Kit following manufacturer’s instructions (QIAGEN). Sequencing was performed by using Big Dye terminator cycle sequencing kit (Applied BioSystems, USA). Sequence data of Cuc1 was compared with others in the GenBank databases using NCBI BLAST and submitted to GenBank with accession number KF471512.
Evaluation for plant growth promoting (PGP) characters
The production of indole acetic acid (IAA) with the addition of l-tryptophan was determined according to Salkowski’s method of Glickman and Dessaux (1995). The IAA concentration in the culture was determined using a calibration curve of pure IAA as a standard following linear regression analysis.
The siderophore production of isolated bacteria was determined by following the method of Alexander and Zuberer (1991) using chrome azurol S agar plates. The production of α-ketobutyrate (a-KB) by the enzymatic cleavage of ACC was determined by measuring its absorbance in bacterial culture at 540 nm and comparing with the absorbance of known concentration of pure α-ketobutyrate (a-KB) (Belimov et al. 2005). Total soluble protein concentration in bacterial culture was estimated by the method of Bradford (1976), and then the enzymatic activity was expressed as 1 M a-KB mg−1 h−1 (Huaidong et al. 2012; Jalili et al. 2009).
For the quantitative measurement of P solubilization, the freshly prepared bacterial culture (108 CFU mL−1) was inoculated in Pikovskaia’s broth containing 2.5 g of TCP. The culture was incubated at constant shaking of 200×g for 7 days at 30 °C. The supernatant of the culture was obtained by centrifugation at 6,000×g and used to determine the P-solubilization by a calorimetric method following Fiske and Subbarow (1925).
Pot experiment
Inoculums preparation
Bacterial culture was prepared by transferring pure single colony of P. vermicola to LB broth and incubated at 30 ± 1 °C for overnight. The bacterial cells were harvested by centrifugation at 6,000×g for 5 min. The pellets obtained were resuspended in sterilized distilled water. The optical density of the bacterial cultures was maintained at 107–108 CFU mL−1 using a UV visible spectrophotometer following the method of Sudisha et al. (2006).
Plant material, growth, and treatment conditions
For the pot experiment, soil was collected from the botanical garden of Government College University, Faisalabad Pakistan (31°24/N, 73°04/E) in April 2012. After drying, the soil was sieved (2 mm) and sterilized by autoclaving at 121 °C for 15 min at 15 psi. The pH of the soil was 7.4 with EC 2.3 dS m−1, organic mater 0.43 %, available P 8.3 mg kg−1, and Cu was about 0.9 mg kg−1. After sterilization, the soil was artificially contaminated with CuSO4 (1,000 ppm Cu2+) powder and thoroughly mixed. The contaminated and non-contaminated soils were packed in 2 kg plastic bags and left for approximately 160 days for metal stabilization (Islam et al. 2014a, b). During this period, the average soil moisture was maintained at 60 % field capacity.
Lentil seeds of cultivar Masoor-2006 were obtained from Nuclear Institute for Agriculture and Biology (NIAB), Faisalabad, Pakistan. Before inoculation, the seeds were surface sterilized with 1 % mercuric chloride solution for three minutes and then washed thoroughly with sterilized double distilled water. Lentil seeds were inoculated by seed dressing with P. vermicola culture of approximately 108 CFU mL−1. The seeds (8 seeds per pot) were sown in earthen pots lined with polythene layer containing 1.5 kg of autoclaved soil in each pot. All the pots were put under natural light (sunlight) with average 21 °C day and 6 °C night temperature, and the average humidity was 60 % during the whole study period. The pot experiment was laid out in a Complete Randomized Design (CRD) with four treatments as T0 (control contains non-contaminated soil), T1 (Cu contaminated soil), T2 (non-contaminated soil + P. vermicola), and T3 (Cu amended soil + P. vermicola). There were three replications for each treatment. For treatment T0 and T1, sterilized seeds were sown; while for treatment T2 and T3, inoculated seeds were sown. To maintain a sufficient community of bacterial isolates, when seedling emerged, 2 mL of bacterial inoculum (108 CFU mL−1) was pore along sides of emerging seedling.
Plant growth parameters and yield attributes
At maturity (103 days after sowing), three replicates from each treatment were harvested to measure different morphological attributes and biomass production. Plant roots were washed with deionized water and blot dried. Plant height, root length, and leaf area per plant were recorded while the numbers of nodules were counted at the flowering stage. Roots and shoots were separated, fresh weights were estimated, and dry weights were recorded after oven drying at 70 °C for 5 days. Yield and yield-contributing parameters were also taken from the remaining plants of each treatment.
Gas exchange attributes
Different gas exchange attributes such as stomatal conductance (g s ), water use efficiency (WUE), net photosynthesis rate (A), transpiration rate (E), and internal carbon dioxide concentration (C i ) were determined by using Infra-Red Gas Analyzer (IRGA; Analytical Development Company, Hoddesdon, England).
Biochemical studies
Leaf total chlorophyll contents
Leaf total chlorophyll contents were determined according to the method of Arnon (1949).
Antioxidant enzymes assay
Fresh leaves (0.5 g) were homogenized in 10 mL of ice cold potassium phosphate buffer (pH 7.0) in an ice bath by grinding with a mortar and pestle. The mixture was centrifuged at 4 °C for 20 min at 12,000×g. The supernatants were then stored at −20 °C and were used for the determination of various antioxidant enzymes. CAT activity was immediately determined in the supernatant according to Aebi (1974). GPX activity was determined as described by Rao et al. (1996), ascorbate peroxidase (APX; EC 1.11.1.11) activity was determined according to the method of Nakano and Asada (1981), and SOD activity was assayed as described by Dhindsa and Matowe (1981). One unit of enzyme activity was defined as an absorbance change of 0.01 units per minute and each enzyme’s activity was expressed as unit per milligram protein.
MDA and H2O2 contents
To find out the extent of Cu-induced oxidative damage, changes in lipid peroxidation in the form of malondialdehyde (MDA) content were estimated in the leaves of lentil plant. Malondialdehyde (MDA) content was estimated using thiobarbituric acid (TBA) following the method of Demiral and Türkan (2005). The amount of MDA was calculated from the difference in absorbance at 532 and 600 nm using an extinction coefficient of 155 mM−1 cm−1. For the estimation of H2O2, the same supernatant that was used for MDA content was used for H2O2 estimation. To 0.5 mL of the supernatant, 0.5 mL of phosphate buffer (pH = 7.0) and 1 mL of KI (1 M) were added. The mixture was vortexed and the absorbance was read at 390 nm (Velikova et al. 2000). H2O2 concentrations were calculated using a standard curve prepared with known concentrations of H2O2.
Non-enzymatic antioxidants
The total phenolic contents (TPC) were measured by adding 0.5 mL of methanolic extract in 2.5 mL of Folin–Ciocalteu reagent (10 % v/v) and 2 mL of Na2CO3 (7.5 %). The mixture was heated at 45 °C for 40 min, and the absorbance was measured at 765 nm. Different concentrations of the gallic acid were used as standards and the concentration of TPC were expressed as milligrams of gallic acid equivalent per gram extract (Duganath et al. 2010). The leaf proline content was measured following Bates et al. (1973).
Nitrogen and phosphorus contents
Oven-dried 100 mg plant material was taken into a heat-resistant glass conical flask and digested with nitric acid following the method ascribed by Allen et al. (1986). Measurement of total nitrogen in lentil plants was determined by Kjeldhal method as described by the Bremner and Mulvaney (1982), while phosphorous contents in lentil plants were determined by a vanado molybdate yellow color method using a spectrophotometer set at 440 nm (Jakson 1967) and Cu concentration in plants was measured using atomic absorbance spectrophotometer (AAS) in flame mode (air-acetylene).
Mineral composition of lentil grains
The grain samples of each treatment were incinerated in a muffle furnace for 12 h at 450 °C and then digested in a mixture of nitric acid and perchloric acid (2:1). Na+ and K+ contents were measured by flame photometer (Sherwood 410). Other minerals, such as Ca2+, Zn, Cu, and Fe, were determined through AAS. Whereas, P was determined through a calorimetric method following Jakson (1967).
Statistical analysis
All data were presented as mean values of three replicates. The data were analyzed using a statistical package, SPSS (Version 19.0). One-way analysis of variance was employed followed by Duncan’s multiple range test to determine the significant differences among means of the treatments at 5 % level of significance.
Results
Isolation of copper-resistant bacteria
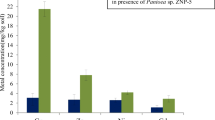
During the selection of copper-resistant bacteria, 367 bacterial colonies were isolated on LB media plates containing 10 ppm of Cu2+. As the copper concentration in media gradually increased from 10 to 1,400 ppm, the number of growing colonies decreased from 367 to 7 at 1,000 ppm and at 1,400 ppm of Cu2+ only four bacterial isolates succeeded to survive. These bacterial isolates were selected for further studies. To study the effect of Cu on the bacterial growth, bacterial cultures were grown in LB broth with different copper concentrations. In the absence of metal, bacterial isolates grew faster with minimum duration of lag phase as compared to the presence of copper in the medium. We found that out of four highly Cu-resistant bacterial strains, P. vermicola maintained higher relative growth as compared to other isolated bacteria (Fig. 1).
Plant growth promoting characteristics
The selected bacterial isolates were screened for IAA-production, siderophores, ACC deaminase, and phosphate solublization ability (data not shown except P. vermicola). The maximum plant growth promoting characters was observed in P. vermicola under the copper stressed and non-stressed conditions (Fig. 2). A significant decrease was recorded in IAA production (4-fold), ACC deaminase (1.5-fold), phosphate solubilization (1-fold), and siderophore activity (1.5-fold) under 1,000 ppm of copper stress as compared to control conditions.
Plant growth
Effect of copper and P. vermicola inoculation on the different growth attributes of lentil plants is shown in Table 1. All growth parameters such as shoot and root length, total dry weight and leaf area of the plants were significantly reduced in T1 and T3 plants compared to the T2 (P. vermicola) and control plants (T0). In comparison with T1 and T3, maximum decrease in plant growth was observed in T1 treatment. Visual copper toxicity symptoms, such as growth retardation and chlorosis of mature leaves, appeared only on copper stressed plants (T1). Moreover, P. vermicola inoculated plants, both under Cu stress or non-stressed conditions showed better growth and dry biomass accumulation than plants grown only under copper stress (T1).
Gas exchange attributes
Data presented in Table 2 demonstrates the variations in different gas exchange attributes of lentil plants driven by Cu stress and P. vermicola either in combination or alone. A significant decrease was observed in different gas exchange characteristics such as net transpiration rate (A), stomatal conductance (g s ), water use efficiency (WUE), and internal CO2 concentration (C i ) under copper stress, ultimately photosynthetic processes decreased under copper stress, showing serious damage to photosystem of lentil plants. However, this decrease in different gas exchange attributes due to copper stress was less in plants grown in soil inoculated with P. vermicola, showing positive effects of inoculation with P. vermicola in copper stress tolerance. Inoculation of P. vermicola was found effective not only under copper stress (T3), but also alone had a progressive effect on different gas exchange characteristics of lentil plants. Briefly, maximum inhibition of photosynthesis was recorded under copper stress (T1), while bacterial inoculation (T2 and T3) significantly improved the above mentioned photosynthetic parameters in both treatments, i.e., T3 (P. vermicola + Cu) and T2 (P. vermicola).
Leaf total chlorophyll, proline, and total phenolic contents (TPC)
Data presented in Fig. 3 shows that leaf total chlorophyll contents decreased significantly due to copper stress (T1). Inoculation of P. vermicola (T3) found effective in ameliorating the adverse effects of copper stress on leaf total chlorophyll contents. This increase in leaf chlorophyll contents due to P. vermicola inoculation not only found under copper stress, but also found under non-stressed conditions (T2).
Changes in the total cholrophyll, TPC, proline, MDA, H2O2, and electrolyte leakage in the leaves of lentil plants subjected to Cu stress and P. vermicola inoculation. Values are means ± S.E. (n = 3). Bars carrying different letters are significantly different at P ≤ 0.05 as determined by Duncan’s test
Under copper stress, proline and TPC accumulation in the shoots of lentil plants was found significantly higher in T1 (Cu amended soil) followed by T3 (P. vermicola + Cu) plants, while remained uneffected between treatment T0 and T2. P. vermicola inoculation significantly reduced the shoot proline contents in plants grown on Cu amended soil while TPC remained uneffected (Fig. 5).
Leaf MDA, H2O2 contents, and electrolyte leakage
The extent of oxidative stress and degree of damages caused by Cu was estimated in terms of leaf H2O2, MDA contents and electrolyte leakage as presented in Fig. 3. It was examined that Cu stress has posed considerable oxidative stress in the leaves of lentil plants, both in the presence or absence of P. vermicola inoculation. MDA and H2O2 contents of lentil plants increased in response to copper stress. However, P. vermicola inoculation significantly reduced the MDA and H2O2 contents in Cu stressed plants (T3). A significant increase in electrolyte leakages was also recorded under Cu stress, but was significantly reduced in P. vermicola inoculated plants (T2, T3) showing an improvement in the membrane stability as compared to the Cu stress treatment (T1).
Antioxidant enzyme activities
Antioxidative enzymes play a crucial role in the oxidative stress tolerance. Therefore, the studied enzymes were evaluated to find out the inoculation of copper-resistant P. vermicola on the enzymatic activities of Cu stressed lentil plants. The activities of antioxidant enzymes, catalase (CAT), guaiacol peroxidase (GPX), ascorbate peroxidase (APX), and superoxide dismutase (SOD) were significantly reduced by copper stress (Fig. 4), while bacterial inoculation appreciably raised the activities of these antioxidative enzymes. In P. vermicola inoculated copper stressed plants (T3), a significant increase in the activities of SOD (2-fold), CAT (1.8-fold), GPX (1.7-fold), and APX (1.8-fold) was recorded as compared to control (T0) and copper-stressed plants (T1) (Fig. 4).
Yield attributes
The response of lentil plants in term of yield attributes like number of pods per plant, 1,000 grains weight, and number of nodule per plant in applied conditions are shown in Table 3. Maximum number of pods per plant was recorded in T2 (P. vermicola) plants followed by T0 (control) and T3 (P. vermicola + Cu), but their interactions were non-significant. However, copper stressed (T1) lentil plants showed significant decrease in number of pods per plant. Regarding 1,000 grains weight of lentil plants, all treatments showed significant differences over control. Maximum decrease in 100 grains weight was observed in T1 (Cu) treatment, while highest weight was achieved in T2 (P. vermicola) followed by T3 (P. vermicola + Cu) plants. Number of nodules per plant at flowering stage was also significantly higher in T2 (P. vermicola) treatment. A significant decrease was observed in copper-stressed plants (T1); however, this decrease in number of nodules due to copper stress was less due to P. vermicola inoculation (Table 2).
Nitrogen and phosphorous contents of lentil plants
The effect of P. vermicola inoculation and Cu application on the N and P contents of lentil plants was presented in Fig. 5. A significant difference in the plants N and P contents grown in copper amended and inoculated soils were recorded. Reduced contents of N and P were recorded in plants grown in copper amended soil (T1) as compared with other treatments. However, this reduction in N and P uptake was compensated with the inoculation of P. vermicola. The enhanced uptake of N and P due to inoculation with P. vermicola was not only in the copper stressed plants (T3) but also was found under non-stressed conditions. Contents of N in copper stressed plants (T1) were 30.10 mg per plant, whereas the plants grown in T3 (P. vermicola + Cu) showed 36 mg N per plant. P. vermicola inoculated plants (T2) accumulated significantly higher values (46 mg per plant) of N in lentil plants. Similarly, application of Cu resulted a major reduction in P contents; however, P. vermicola inoculated lentil plants, both in the presence and absence of copper stress had higher values of P contents compared to other treatments. Significantly reduced P contents (10 mg per plant) were also measured in T1 (Cu-stressed plants; Fig. 3). The maximum P uptake was 30.90 mg per plant in T2 (P. vermicola), while copper stress also decreased the P uptake even in the presence of P. vermicola (T3; 17 mg per plant). From these results, it can be concluded that the inoculation of P. vermicola appeared to be more subsequent and tangible in reducing the inhibitory effects of Cu and also enhanced the nutrients uptake in both copper stressed and non-stressed plants (Fig. 5).
Copper accumulation
Copper accumulation in the roots and shoots of lentil plants grown in copper amended soil both in the presence and absence of P. vermicola inoculation is shown in Fig. 5. The results showed that bacterial inoculation considerably reduced the Cu uptake in P. vermicola inoculated lentil plants (T3) as compared to the copper stressed plants (T1).
Mineral composition of lentil grains
In parallel with the improvement in different yield attributes (presented above) due to P. vermicola inoculation, the grains mineral composition was also significantly improved. Different seed minerals such as Na, K, P, Ca, Fe, and Zn decreased significantly in plants grown under copper stress except for that of Cu that increased significantly. Inoculation of P. vermicola showed a significant effect on the seed mineral contents of lentil. The content of Na, K, P, Ca, Fe, and Zn increased, while that of Cu decreased due to inoculation of P. vermicola both under stressed conditions. Of different studied minerals, potassium is a major component of lentil grains, a significant increase in potassium contents was observed in the plants inoculated with P. vermicola (T2) both under stress and non-stressed conditions (Table 4).
Discussion
For effective microbe-assisted bioremediation, it is necessary to identify metal-resistant bacteria having plant growth-promoting characteristic that may facilitate plant growth and to restrict excess metal uptake from soil to plants. It has been reported by different researchers that inoculation of heavy metal-resistant PGPB may improve plant growth under artificially metal-contaminated soils due to their microbial activities in the rhizosphere (Tak et al. 2013; Gururani et al. 2012). In this study, the copper-resistant bacteria were isolated from the industrial effluent (that is used for the irrigation of agricultural fields due to the scarcity of fresh water) with an objective to investigate the effect of copper-resistant bacteria on the growth, yield, and amelioration of oxidative stress under copper contamination. Initially, 367 bacterial isolates were isolated but as the metal concentration was increased up to 1,400 ppm only four bacterial isolates with different bacterial colonies succeeded to survive. Out of them, only one P. vermicola was able to maintain higher biomass under 1,400 ppm of copper stress. However, longer lag phase was observed when bacteria were grown in the presence of copper. Prolonged growth phase is an adaptation towards heavy metal stress (Kardas et al. 2014). This type of phenomena was also observed in Bacillus circulans under Cd stress (Yilmaz 2003). Thus, the data presented clearly indicate that all isolated Cu-resistant bacteria exhibited tolerance and adaptations for survival under copper stress. The lower number of bacterial cells was observed in all four isolates when grown in the presence of Cu, suggested that the bacteria had reduced growth and likely alter their physiological mechanisms in response to Cu toxicity (Siripornadulsil and Siripornadulsil 2013).
In addition, it was found that bacterial strain P. vermicola produced indole acetic acid when tryptophan was added as a precursor of indole acetic acid. It was found that indole acetic acid production by bacterial isolates enhanced plant growth by stimulating cell division or by cell elongation (Gamalero et al. 2008). In addition to this, bacterial strains also showed positive siderophoric activity with the development of 2.9 cm orange holo zone under control condition and 1.9 cm under copper stress. Siderophores production by PGPR binds iron (Fe3+), thus, increasing its availability to plant (Arora et al. 2001). Sequestration and cleavage of ACC are other obvious features of PGPR to accelerate plant growth under heavy metal stress, which lowers the concentration of ethylene in plant tissues and promotes plant growth under stressful conditions (Glick 2005; Mayak et al. 2004). An additional important PGP mechanism is the solubilization of phosphorus by which bacteria enhance P availability to the inoculated plants (Zaidi et al. 2006). The ACC deaminase activity and phosphorous solubilization were also found in the studied P. vermicola. However, PGP characters significantly decreased under copper stress that might be due to the decreased number of bacteria under copper stress as we recorded a 65 % decrease in growth of P. vermicola at 1,000 ppm of copper compared to control.
In the present study, inoculation of P. vermicola significantly increased the root and shoot length, total dry weight and leaf area of lentil plants under the copper amended soil as compared to control and Cu stressed plants, respectively. A similar increase in mungbean plant growth was also observed under P. vermicola inoculation in another study (Akhtar and Ali 2011). The P. vermicola inoculated plant also contained high amounts of total chlorophyll and number of nodules per plants as compared with copper stress plants. All of these beneficial effects on the plant growth are due to the PGP abilities of P. vermicola. Plants grown under T3 (P. vermicola + Cu) resulted a significant increase in root lengths; this was probably due to the inhibition of ethylene production in the roots by the activity of ACC deaminase that utilizes the NH3 evolved from ACC as a source of nitrogen and thereby decreased ACC in the plants (Penrose and Glick 2003). In the present study, elevated level of Cu in soil also resulted in decreased uptake of phosphorus by lentil plants, but inoculation of P. vermicola compensated this deficiency that might be due to its phosphorus solubilization activity as shown in Fig. 2. This P solubilization activity of P. vermicola enhanced the levels of free P in the soil and as a result plant P uptake enhanced. Though we did not determine the N fixation ability of P. vermicola, the increased amount of nitrogen and proliferation of root nodules under bacterial inoculation may be linked to P. vermicola activity, which shows its involvement in the fixation of atmospheric nitrogen in root nodules.
The distribution, accumulation, and remobilization of metals in the plant tissue are important aspects to evaluate the role of plants in remediation of toxic metal-contaminated sites (Kumar et al. 1995). In the present investigation, the bacterial inoculation decreased the Cu uptake in lentil plants grown in Cu spiked soil as compared to control. However, higher accumulation of Cu found in the roots as compare to shoots, suggests the less translocation of Cu from root to shoot that is due to sequestration of most of the heavy metals in the vacuoles of root cells to render them non-toxic (Shanker et al. 2005). The specific response of plant tissues towards heavy metal accumulation under the influence of PGPR can be correlated with earlier studies on lentil plants where the uptake of Ni and Zn was more in the root as compared with shoot and grain (Wani et al. 2008). Similarly, in another study, it was found that inoculation with PGPB Brevi bacillus sp. reduced the Zn uptake in Trifolium repens (Vivas et al. 2006). The present study elucidates that P. vermicola facilitated copper immobilizing through the release of phosphorus from insoluble “P” compounds that resulted in less Cu uptake (Ma et al. 2013). Moreover, lower concentration of Cu in upper ground parts might be due to the removal of Cu through the adsorption/desorption mechanism of P. vermicola strain (Islam et al. 2014a, b). However, increased metal accumulation resulted in plants with the inoculation of PGPR. Overall view of the present and the previous research reports taken together suggests that besides the bacterial metal solubilization activity, the other factors such as soil nutrient level, pH, type of metals, and type of plant species also greatly influence the metal solubilization in soils which alter its uptake (Martínez-Alcalá et al. 2009; Rajkumar et al. 2013).
It is well known that the redox cycling between the two oxidation states of copper (Cu+ and Cu2+) catalyzes the formation of different types of ROS, which subsequently damage the cellular macromolecules (Halliwell and Gutteridge 1984). As during this process, hydrogen peroxide (H2O2) takes place by the dismutation of superoxide anion (O2−) by NADPH oxidases or via the Fenton reactions. Hydrogen peroxide act as a signaling molecule leading to the regulation of gene expression or it causes oxidative damaging of lipids through hydroxyl radical (OH·) formation (Opdenakker et al. 2012). Besides these, excess Cu indirectly leads to oxidative stress by disrupting the balance between ROS generation and detoxification (Møller et al. 2007). Under such conditions, scavenging of O2− by SOD and H2O2 decomposition by ascorbate peroxidase (APX) and catalase (CAT) are predominantly responsible for the maintenance of cellular redox state. In the present investigation, imposition of Cu stress (T1) induced a significant increase in MDA and H2O2 contents in lentil plants showing a role of excess Cu in oxidative stress. In contrast, a significant decrease in MDA and H2O2 contents was found in bacteria inoculated plants, which is the indication that better protective mechanism exists in bacterial inoculated plants.
Plants have developed different protective mechanisms to scavenge the free radicals and peroxides. These protective mechanisms include different antioxidative enzymes and non-enzymatic antioxidative compounds. The available literature describes both increasing and decreasing patterns in their activity, depending on the type of plant species, plant organ, type of metal and its concentration, duration of the stress, plant age, and plant growth medium (Gratão et al. 2005; Gill and Tuteja 2010). For example, in some earlier studies, it was found that the activities of CAT, SOD, APX, and GPX increased against lower concentrations of the metal and decreased under high metal concentrations as the high metal concentration disturbed the defense mechanism (Peng et al. 2006; Ke et al. 2007; Pinto et al. 2009). The increase in the activities of these enzymes under lower metal stress suggested that these enzymes have been activated or its expression is upregulated under stress. On the other hand, a decrease in the activity under higher metal stress conditions might be due to the drastic change in enzyme structure (Cohu and Pilon 2007). Similar to this, in Cu stressed plants (T1), CAT, SOD, APX, and GPX activities were decreased. This reduction in antioxidative enzymes activity in copper-stressed plants (T1) might be due to the severe oxidative stress that damaged the structure of antioxidant enzymes, and hence, the activity of enzymes was diminished (Mishra et al. 2006). Conversely, the P. vermicola inoculated plants under copper stress (T3) showed significant enhanced activities of antioxidative enzymes (CAT, APX, GPX, and SOD) as compared to control and copper-stressed plants. Earlier in lentil (Wani et al. 2008) and rice (Siripornadulsil and Siripornadulsil 2013), it was found that PGPB alleviated the metal stress by upregulating the antioxidative mechanism and enhancing plant growth. Similarly in another study, it was found that Pseudomonas aeruginosa OSG41 significantly reduced the toxicity of hexavalent chromium in chickpea and prevented the plant from the oxidative burst by the up regulation of antioxidative defense mechanism. In another study on wheat (Wang et al. 2013), it was found that inoculation of copper-resistant bacteria played an important role in the upregulation of antioxidative defense mechanism (increased activities of SOD, CAT and APX, and GPX) that eliminated the ROS and result in reduced MDA content. They also found that copper stress inhibited or destroyed protein synthesis/production, but bacterial inoculation prevented its degradation by lowering the metal toxicity. This might be due to the fact that bacterial inoculation activates the gene expression profile of metal detoxifying enzymes to cope the metal stress (Duponnois et al. 2006). Similarly, in potato, it was found on the basis of mRNA expression data that PGPR inoculation enhanced the activities of SOD, CAT, DHAR, GR, and APX under salt, water, and heavy metal stress that resulted in enhanced photosynthetic efficiency and ultimately plant growth (Gururani et al. 2012). These findings can be correlated with the present findings where inoculation with P. vermicola resulted in enhanced activities of antioxidative enzymes that might protect the photosynthetic machinery from oxidative damages, and finally, enhanced photosynthetic rate resulting increased biomass production and grain yield.
In parallel with enzymatic antioxidants, non-enzymatic antioxidants also play important role in protecting plant cells from oxidative damage by accommodating the uncontrolled oxidation as well as by scavenging ROS under metal stress (Gill and Tuteja 2010). A significant increase in the proline and TPC contents was found in copper stressed lentil plants, but this increase in proline and TPC contents in P. vermicola inoculated plants under stress conditions was less as compared with copper stressed plants without P. vermicola inoculation. The reduced contents of proline and TPC might be due to the stress mitigation abilities of P. vermicola strain that decreased the metal toxicity in inoculated plants as compared to copper stressed plants. Such decrease in the proline content was also found in chick pea plant under chromium stress due to inoculation with Pseudomonas strain (Oves et al. 2013). In view of earlier studies, till date, few or no report is available on the decrease of TPC in inoculated plants and why these non-enzymatic antioxidants (TPC and proline) diminished in inoculated plants under metal stress. However, the decreased contents of these antioxidants in lentil under copper stress might be due to some unknown activities of P. vermicola that are still to be explored.
Conclusion
Bacteria with multiple PGP characters will be helpful to increase crop productivity under stress conditions. The present investigation has shown that the copper-resistant bacterial strain (P. vermicola) with different PGP traits have facilitated lentil growth and protects the plants against adverse effects of metal stress. Our findings basically focus on the inoculation of bacteria that could be able to ameliorate the metal stress by the integration of several aspects including plant growth promotion through synthesis of the plant required hormone (IAA), P solubilization, siderophores production, and efficient ACC deaminase activity to reduce stress induced ethylene and better management/availability of N, P and Fe. Briefly, observed results in this study indicate that bacterial isolate P. vermicola Cuc1 could reduce damages caused by copper in the soil. Hence, the use of multifarious growth promoting bacteria with metal resistance properties hold a great potential to be used as biofertilizer in metal-contaminated soils.
Abbreviations
- PGPB:
-
plant growth promoting bacteria
- Cu:
-
copper
- PGP:
-
plant growth promoting
- A :
-
net photosynthetic rate
- E :
-
transpiration rate
- C i :
-
internal CO2 concentration
- g s :
-
stomatal conductance
- A/E :
-
water use efficiency
- SOD:
-
superoxide dismutase
- CAT:
-
catalase
- GPX:
-
guaiacol peroxidase
- MIC:
-
minimum inhibitory concentration
- MDA:
-
malondialdehyde
- H2O2 :
-
hydrogen peroxide
- TPC:
-
total phenolic contents
- N:
-
nitrogen
- P:
-
phosphorous
- P. vermicola :
-
Providencia vermicola
References
Aebi H (1974) Catalases. Methods Enzym Anal 2:673–684
Akhtar S, Ali B (2011) Evaluation of rhizobacteria as non-rhizobial inoculants for mung beans. Aust J Crop Sci 5:1723–1729
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fert Soils 12:39–45
Allen E, Grimshaw H, Parkinson J, Quamby C, Roberts J (1986) Chemical analysis. Methods plant ecology. Blackwell Scientific, London, pp 285–344
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Arora N, Kang S, Maheshwari D (2001) Isolation of siderophore producing strains of Rhizobium meliloti and their bio-control potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Curr Sci 81:673–677
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Belimov A, Safronova V, Demchinskaya S, Piluzza G, Bullitta S (2005) Cadmium-tolerant plant growth-promoting rhizobacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol Biochem 37:241–250
Bergey DH, Holt JG, Krieg NR, Sneath PHA (1994) Bergey's Manual of Determinative Bacteriology, 9th ed., (Breed RS, Murray EGD and Smith NR, eds.) Williams and Wilkims, Baltimore
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analy biochem 72: 248--254
Bremner J, Mulvaney C (1982) Nitrogen-total. Methods of soil analysis. Part 2. Chemical and Microbiological Properties 595–624
Bruins M, Kapil S, Oehme F (2000) Microbial resistance to metals in the environment. Ecot Environ Saf 45:198–207
Cambroll J, Mateos-Naranjo E, Redondo-Gomez S, Luque-Palomo M, Figueroa M (2011) Growth, reproductive and photosynthetic responses to copper in the yellow-horned poppy, Glaucium flavum Crantz. Environ Exp Bot 71:57–64
Cohu CM, Pilon M (2007) Regulation of superoxide dismutase expression by copper availability. Physiol Plant 129:747–755
Demiral T, Türkan İ (2005) Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot 53:247–257
Demirevska-Kepova K, Simova-Stoilova L, Stoyanova Z, Hölzer R, Feller U (2004) Biochemical changes in barley plant safter excessive supply of copper and manganese. Environ Exp Bot 52:253–266
Dhindsa SR, Matowe W (1981) Drought tolerance in two mosses: correlated with enzymatic defence against lipid peroxidation. J Exp Bot 32:79–91
Duganath N, Reddy KN, Nagasowjanya J, Sridhar S, Jayaveera KN (2010) Evaluation of phytochemical and in vitro antioxidant activity of Filicium decipiens. Ann Biol Res 1:134–140
Duponnois R, Kisa M, Assigbetse K, Prin Y, Thioulouse J, Issartel M, Moulin P, Lepage M (2006) Fluorescent pseudomonads occurring in Macrotermes subhyalinus mound structures decrease Cd toxicity and improve its accumulation in sorghum plants. Sci Total Environ 370:391–400
Fiske C, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Gamalero E, Berta G, Massa N, Glick BR, Lingua G (2008) Synergistic interactions between the ACC deaminase-producing bacterium Pseudomonas putida UW4 and the AM fungus Gigaspora rosea positively affect cucumber plant growth. FEMS Microbiol Ecol 64:459–467
Gao S, Yan R, Cao M, Yang W, Wang S, Chen F (2008) Effects of copper on growth, antioxidant enzymes and phenylalanine ammonia-lyase activities in Jatropha curcas L. seedling. Plant Soil Environ 54(3):117–122
Gibbons S, Feris K, McGuirl M, Morales S, Hynninen A, Ramsey P, Gannon J (2011) Use of microcalorimetry to determine the costs and benefits to Pseudomonas putida strain KT2440 of harboring cadmium efflux genes. Appl Environ Microbiol 77(1):108–113
Gill S, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Glick BR (2005) Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol Lett 251:1–7
Glickman E, Dessaux Y (1995) A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61:793–796
González-Mendoza D, Espadas y Gil F, Escoboza-Garcia F, Santamaría JM, Zapata-Perez O (2013) Copper stress on photosynthesis of black mangle (Avicennia germinans). An Acad Bras Cienc 85(2):665–670
Gratão PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Func Plant Biol 32:481–494
Gururani M, Upadhyaya C, Baskar V, Venkatesh J, Nookaraju A, Park S (2012) Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in solanum tuberosum through inducing changes in the expression of ros-scavenging enzymes and improved photosynthetic performance. J Plant Growth Regul 32:245–258
Gururani M, Upadhyaya C, Baskar V, Venkatesh J, Nookaraju A, Park S (2013) Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in solanum tuberosum through inducing changes in the expression of ros-scavenging enzymes and improved photosynthetic performance. J Plant Growth Regul 32(2):245–258
Halliwell B, Gutteridge J (1984) Oxygen toxicity, oxygen radical, transition metals and disease. Biochem Int J 219:1–14
Harley (2014) Laboratory resource guide laboratory exercises in microbiology. 9th edition. McGraw-Hill Education
Huaidong H, Zhihong Y, Danjing Y, Junlan Y, Xiao L, Zhong T, Yuan M, Cai X, Fang Z, Jing Y (2012) Characterization of endophytic Rahnella sp. JN6 from Polygonum pubescens and its potential in promoting growth and Cd, Pb, Zn uptake by Brassica napus. Chemosphere 90:1960–1965
Islam F, Yasmeen T, Ali Q, Ali S, Arif MS, Hussain S, Rizvi H (2014a) Influence of Pseudomonas aeruginosa as PGPR on oxidative stress tolerance in wheat under Zn stress. Ecotox Environ Safe 104:285–293
Islam F, Yasmeen T, Riaz M, Arif MS, Ali S, Raza SH (2014b) Proteus mirabilis alleviates zinc toxicity by preventing oxidative stress in maize (Zea mays) plants. Ecotox Environ Safe 110:143–152
Jakson M (1967) Soil chemical analysis. Prentice Hall of India Ltd, New Delhi
Jalili F, Khavazi K, Pazira E, Nejati A, Rahmani H, Sadaghiani H, Miransari M (2009) Isolation and characterization of ACC deaminase-producing fluorescent pseudomonads, to alleviate salinity stress on canola (Brassica napus L.) growth. J Plant Physiol 166:667–674
Janas K, Ska-Tomaszewska J, Rybaczek D, Maszewski J, Posmyk M, Amarowicz R, Kosińska A (2010) The impact of copper ions on growth, lipid peroxidation and phenolic compound accumulation and localization in lentil (Lens culinaris Medic.) seedlings. J Plant Physiol 167:270–276
Kafel A, Nadgórska-Socha A, Gospodarek J, Babczyńska A, Skowronek M, Kandziora M, Rozpędek K (2010) The effects of Aphis fabae infestation on the antioxidant response and heavy metal content in field grown Philadelphus coronarius plants. Sci Total Environ 408:1111–1119
Kardas M, Gozen AG, Severcan F (2014) FTIR spectroscopy offers hints towards widespread molecular changes in cobalt-acclimated freshwater bacteria. Aquat Toxicol 155:15–23
Ke W, Xiong Z, Xie M, Luo Q (2007) Accumulation, subcellular localization and ecophysiological responses to copper stress in two Daucus carota L. populations. Plant Soil 292:291–304
Khan N, Tuffin M, Stafford W, Cary C, Lacap DC, Pointing SB, Cowan D (2011) Hypolithicmicrobial communities of quartz rocks from Miers Valley, McMurdo Dry Valleys, Antarctica. Polar Biol 34:1657–1668
Kovacik J, Backor M (2008) Phenolic compounds composition and physiological attributes of Matricaria chamomilla grown in copper excess. Environ Exp Bot 62:145–152
Kumar P, Dushenkov V, Motto H, Raskin I (1995) Phytoextraction: The use of plants to remove heavy metals. Environ Sci Technol 29:1232–1238
Lamb DT, Ming H, Megharaj M, Naidu R (2009) Heavy metal (Cu, Zn, Cd, and Pb) partitioning and bioaccessibility in uncontaminated and long-term contaminated soils. J Harzad Mater 171:1150–1158
Li J, McConkey BJ, Cheng Z, Guo S, Glick BR (2013) Identification of plant growth-promoting bacteria-responsive proteins in cucumber roots under hypoxic stress using a proteomic approach. J Proteom 84:119–131
Lima A, Corticeiro S, Figueira E (2006) Glutathione-mediated cadmium sequestration in Rhizobium leguminosarum. Enzy Microb Technol 39:763–769
Lin J, Jiang W, Liu D (2003) Accumulation of copper by roots, hypocotyls, cotyledons and leaves of sunflower (Helianthus annuus L.). Biores Technol 86:151–159
Liu T, Shen C, Wang Y, Huang C, Shi J (2014) New insights into regulation of proteome and polysaccharide in cell wall of Elsholtzia splendens in response to copper stress. Plos One 9(10):1–13
Ma Y, Prasad M, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29:248–258
Ma Y, Rajkumar M, Luo Y, Freitas H (2013) Phytoextraction of heavy metal polluted soils using Sedum plumbizincicola inoculated with metal mobilizing Phyllobacterium myrsinacearum RC6b. Chemosphere, 93(7): 1386--1392
Martínez-Alcalá I, Clemente R, Bernal M (2009) Metal availability and chemical properties in the rhizosphere of Lupinus albus L. growing in a high-metal calcareous soil. Water Air Soil Poll 201:283–293
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42:565–572
McLellan T, Marr ES, Wondrack LM, Subashi TA, Aeed PA, Han S, Xu Z, Wang IK, Maguire BA (2009) A systematic study of 50S ribosomal subunit purification enabling robust crystallization. Acta Crystallo 65:1270–1282
Mishra S, Srivastava S, Tripathi R, Govindarajan R, Kuriakose S, Arasad M (2006) Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol Biochem 44:25–37
Møller I, Jensen P, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nautiyal C, Srivastava S, Chauhan P (2008) Rhizosphere colonization: molecular determinants from plant-microbe coexistence perspective. In: Nautiyal CS, Dion P (eds) Molecular mechanisms of plant, microbe coexistence, soil biology series. Springer, Berlin, pp 99–124
Nies D (2003) Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev 27:313–339
Opdenakker K, Remans T, Keunen E, Vangronsveld J, Cuypers A (2012) Exposure of Arabidopsis thaliana to Cd or Cu excess leads to oxidative stress mediated alterations in MAPKinase transcript levels. Environ Exp Bot 83:53–61
Oves M, Khan M, Zaidi A (2013) Chromium reducing and plant growth promoting novel strain Pseudomonas aeruginosa OSG41 enhance chickpea growth in chromium amended soils. Eur J Soil Biol 56:72–83
Peng H, Yang X, Yang M, Tian S (2006) Responses of antioxidant enzyme system to copper toxicity and copper detoxification in the leaves of Elsholtzia splendens. J Plant Nutr 29:1619–1635
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118:10–15
Pinto AP, Alves AS, Candeias AJ, Cardoso AI, de Varennes A, Martins LL, Mourato MP, Gonçalves ML, Mota AM (2009) Cadmium accumulation and antioxidative defences in Brassica juncea L. Czern, Nicotiana tobacum L. and Solanum nigrum L. Int J Environ 89:661–676
Rajkumar M, Prasad M, Freitas H, Ae N (2009) Biotechnological applications of serpentine soil bacteria for phytoremediation of trace metals. Crit Rev Biotechnol 29:120–130
Rajkumar M, Prasad M, Sandhya S, Freitas H (2013) Climate change driven plant-metal-microbe interactions. Environ Int 53:74–86
Rao L, Perez D, White E (1996) Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol 135:1441–1455
Rascio N, Navari-Izzo F (2011) Heavy metal hyper accumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180:169–181
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753
Siripornadulsil S, Siripornadulsil W (2013) Cadmium-tolerant bacteria reduce the uptake of cadmium in rice: potential for microbial bioremediation. Ecotoxicol Environ Safe 94:94–103
Sudisha J, Niranjana SR, Umesha S, Prakash HS, Shetty HS (2006) Transmission of seed-borne infection of muskmelon by Didymella bryoniae and effect of seed treatments on disease incidence and fruit yield. Biol Cont 37:196–205
Tak HI, Ahmad, Babalola OO (2013) Advances in the Application of plant growth-promoting rhizobacteria in phytoremediation of heavy metals. Rev Environ Contamin Toxicol In Whitacre, D M (Ed). IX, 147 p. 21 illus., 3 illus. Vol. 223
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Vivas A, Biro B, Ruiz-Lozano J, Barea J, Azcon R (2006) Two bacterial strains isolated from a Zn-polluted soil enhance plant growth and mycorrhizal efficiency under Zn-toxicity. Chemosphere 62:1523–1533
Wang H, Xu R, You L, Zhong G (2013) Characterization of Cu-tolerant bacteria and definition of their role in promotion of growth, Cu accumulation and reduction of Cu toxicity in Triticum aestivum L. Ecotoxicol Environ safety 94: 1--7
Wang L, Yang X, Ren Z, Hu X, Wang X (2014) Alleviation of photosynthetic inhibition in copper-stressed tomatoes through rebalance of ion content by exogenous nitric oxide. Turk J Bot 38:1312–1317
Wani P, Khan M, Zaidi A (2008) Effect of metal-tolerant plant growth-promoting Rhizobium on the performance of pea grown in metal-amended soil. Arch Environ Contam Toxicol Appl Pharmacol 55:33–42
Yang J, Kloepper J, Ryu C (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4
Yilmaz EI (2003) Metal tolerance and biosorption capacity of Bacillus circulans strain EB1. Res Microbiol 154:409–415
Zaidi S, Usmani S, Singh B, Musarrat J (2006) Significance of Bacillus subtilis strain SJ-101 as a bio-inoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 64:991–997
Zhang LL, He XJ, Chen M, An RD, An XL, Li J (2014) Responses of nitrogen metabolism to copper stress in Luffa cylindrica roots. J Soil Sci Plant Nutr 14(3):616–624
Acknowledgments
The authors thank the Higher Education Commission of Pakistan for the financial support under project No: PM-IPFP/HRD/HEC/2011/0582.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Islam, F., Yasmeen, T., Ali, Q. et al. Copper-resistant bacteria reduces oxidative stress and uptake of copper in lentil plants: potential for bacterial bioremediation. Environ Sci Pollut Res 23, 220–233 (2016). https://doi.org/10.1007/s11356-015-5354-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5354-1