Abstract
The major challenges for the plants growing in metal-contaminated soils are deficiency of nutrients, biomass reduction, and severe oxidative damages in the presence of heavy metals. In this regard, our aim was to overcome these challenges through the use of efficient microbial strains in metal-polluted soils and to assess its/their physiological and biochemical effects. In the current study, a copper (Cu)-resistant bacterium was isolated from the rhizospheric soil of ‘Ziziphus nummularia’ and evaluated for its ability to promote the wheat growth under the gradient stress of copper. Based on 16S rRNA gene sequencing, the isolate was identified as Pantoea sp. Among the plant growth promoting tests, the isolate showed the production of indole acetic acid, solubilization of inorganic phosphate, and ACC deaminase activity. Also, the isolate showed resistance to many heavy metals and antibiotics and increased the water-soluble copper in solution. The results of pot studies showed that bacterial application promoted various growth parameters of wheat plants and also enhanced the Cu uptake of wheat from the Cu-amended soil. The results showed that enhancement of Cu stress (100 to 300 mg kg−1) resulted in a decrease in various compatible solutes such as proline, total soluble sugars, and total protein content, and increase in the level of malondialdehyde (MDA), latter of which is the indicator of oxidative stress. Bacterial treatment markedly increased the proline, soluble sugar, total protein content, and decreased the MDA content under Cu stress. In addition, bacterial inoculation significantly alleviated the harmful effect of metal toxicity by decreasing the activation of ROS molecules including superoxide (O2−) and hydrogen peroxide (H2O2). The activation of various antioxidative enzymes such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) was noted following bacterial inoculation under Cu stress. Therefore, the present study demonstrates the potential of the isolate Pantoea sp. ZNP-5 to improve the growth and phytoextraction of metal from the metal-polluted soil through the polyphasic mechanism of action.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution in soils imposes a great threat to the environmental and human health. It can decrease not only the soil microbial activity and plant yield, but also may spread to the surrounding areas (Fellet et al. 2011; Mclaughlin et al. 1999). An elevated level of metal toxicity resulting from the use of agrochemicals, industrial activities, and deposition of sewage sludge pose a threat to the human health by altering the food chain. Among other heavy metals, Cu is a potent pollutant that accumulates in soils and sediments (Lamb et al. 2009). The copper (Cu) is a trace element for all life forms on earth; however, at high concentrations, it becomes toxic (Ouzounidou 1995). Its accumulation in the soil also adversely affects the microbial community, inhibits plant growth, causes leaf chlorosis, and alters the photosynthetic apparatus. Therefore, there is an urgent need to develop the remediation strategy that can effectively remove the toxicity of metals in soil for ecological conservation and safety of human health. Previous reports have shown the importance of metal-accumulating plants that are commonly used in the removal of metal toxicity from soils. However, most of the hyper-accumulators are not efficient in accumulating metals at field level due to their slow growth as well as unavailability of metal for root uptake by field-grown plants (Belimov et al. 2005; Rajkumar et al. 2006). Also, low bioavailability of metal in soils also limits the efficiency of phytoremediation (Chen et al. 2005; Sheng and Xia 2006).
The abnormal upsurge of reactive oxygen species (ROS), such as superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH−), is a rapid response to excessive metal stress exposure. The continuous generation of ROS can be extremely harmful as it oxidizes protein, lipid, nucleic acids, and carbohydrates and causes alteration of metabolic pathways (Mittler 2002).Therefore, the increased level of metal proportion in soil impairs the plants metabolic activity, physiological, and developmental response and severely affects the growth. Antioxidative defense mechanisms play a key role to enhance plant oxidative stress tolerance. The important antioxidative enzymes include superoxide dismutase (SOD), catalase (CAT), and peroxidase (POX). SOD plays a critical role in the first line of defense by catalyzing the dismutation of O2− into O2. At the same time, the concerted action of CAT and POX decreases the level of toxic H2O2 by degrading it to water and molecular oxygen. However, with the increase in the severity of stressors, the sufficient antioxidant capacity may not be available to overcome the deleterious effects of oxidative injury with the balance of ROS shifting, which in turn lead to metabolic abnormalities, membrane damage, loss of pigments, and inactivation of enzymes (Roychoudhury et al. 2012).
Under the circumstances mentioned above, the certain rhizospheric microorganism can augment the growth of associated plants and its tolerance to heavy metal, significantly improving the phytoremediation efficiency (Glick 2003). It has been shown in previous studies that inoculation of plants with metal resistant/mobilizing bacteria significantly improves the metal phytoremediation process (Ma et al. 2011; Hrynkiewicz and Baum 2013). Thus, the use of metal-solubilizing rhizospheric bacteria could be used as a promising approach to increase the metal bioavailability in soils as well as to decrease its effect on host plants in heavy metal amended soils. The rhizosphere constitutes the dynamic and complex microenvironment where the microorganisms form the unique communities for detoxification of potentially lethal heavy metals. Microbial population under such environments affects the metal mobility through various mechanisms like acidification, redox behavior and release of chelators (De Souza et al. 1999; Smith and Read 2010).
Plant growths under metal polluted soils are severely affected by the accumulation of metal-induced ‘stress ethylene’ level. Toxic effect of stress ethylene is manifested by various symptoms like chlorosis, wilting, senescence and growth inhibition. Microorganism with ACC deaminase (1-aminocyclopropane-1-carboxylate) activity, breaks down the precursor of ‘stress ethylene’ ACC (1-aminocyclopropane-1-carboxylic acid) to α-ketobutyrate (KB) and ammonia and thus help plants to combat with metal stress (Glick et al. 1995; Rajkumar et al. 2006). In addition to ACC deaminase activity, microbes able to solubilize the inorganic phosphate and produce the indole acetic acid that may improve plant competitiveness and its physiological response to external stress factors (Egamberdiyeva and Höflich 2004; Rajkumar et al. 2013a). The presence of these beneficial features prevents stress ethylene-mediated reduction of plant growth and biomass under stressful conditions (Grichko and Glick 2001; Penrose and Glick 2003). Increase in growth of Canola plant ‘Brassica napus’ and alleviation of metal stress was observed after inoculation with a metal-resistant ACC utilizing bacterium Rahnella sp. JN6 (He et al. 2013). Similarly, Cicer aritenum grown in arsenic polluted soil in the presence of metal immobilizing plant growth promoting bacterium Acinetobacter sp. nbri 05 showed better growth and higher phytostabilization potential. Plant growth-promoting Acinetobacter sp. CC30 significantly enhanced the plant biomass and increased the phytoextraction of Cu by sunflower (Helianthus annus).
Use of plant growth-promoting bacteria helps plants to combat the metal stress (Sessitsch et al. 2013). However, little is known about the role of metal solubilizing PGPR on the phytoextraction of Cu from the metal-polluted soils. Since Cu is one of the most widespread contaminants in the environment, it is essential to expand our understanding towards its possible detoxification mechanisms. To the best of our knowledge, few studies have been conducted on the effect of PGPR. Therefore, the present study aimed to characterize metal resistant and mobilizing bacteria to increase the plant biomass, chlorophyll content, and Cu uptake under metal stress conditions as well as to improve the efficiency of phytoextraction of Cu-polluted soils. Moreover, the effect of selected PGPR on the biochemical parameters, antioxidant activities, and oxidative damages was also investigated. The present work can be helpful to evaluate the microbe-mediated detoxification mechanism adopted by the wheat plant in Cu-polluted soils.
Material and methods
Isolation of a Cu-tolerant strain
The rhizospheric soil of ‘Ziziphus nummularia’ growing in the Shekhawati region of Rajasthan, India (28.13°N, 75.4° East) was collected and brought to the laboratory for study. ‘Ziziphus nummularia’ locally known as ‘Jharber’ is a shrub, which grows primarily in an arid region, and is well adapted to stressed environments. Serially diluted (up to 10−9) soil sample was spread over sucrose minimal salt low-phosphate (SLP) medium amended with different concentrations of Cu (CuSO4, 100 to 300 mg l−1). The plates were incubated for 48 h at 30 °C in an incubator. The appearance of metal-resistant colonies was further streaked and tested for their ACC utilization ability by sub-culturing on solid DF-salt minimal medium amended with 3 mM ACC (Sigma-Aldrich, USA) and incubated for 48 to 72 h at 30 °C. The isolate ZNP-5 was selected based on its Cu tolerance as well as successive growth on the DF-ACC plate. To check its metal mobilizing activity, the isolated organism was tested for its ability to increase the water-soluble Cu, Zn, Ni, and Cd in soils. A mixture of clay and sandy soil (3:1) collected from a non-fertilized farm was supplemented with 200 mg kg−1 concentration of each heavy metal and mixed thoroughly. The metal-supplemented soils were allowed to equilibrate for a period of 1 month in the greenhouse. The test isolate was grown in LB medium, and optical density (OD) was adjusted to 1.5. Afterward, the cultures were centrifuged, washed in phosphate buffer (pH 7.0), and re-suspended in sterile water. The bacterial culture (1 ml) was added to the 20 g of metal-contaminated soils in the 250-ml Erlenmeyer flasks and incubated at 30 °C for 1 week. The same amount of boiled culture was added to the 20 g of sterilized soil to be used as a control. For each set of treatments, three replicates were used. The concentration of metal in the filtrate was determined by atomic absorption spectrophotometer.
Microorganism identification and phylogenetic analysis
The luxuriant growth of the test isolate illustrated its resistance to Cu. For identification of the selected Cu-tolerant ZNP-5, 16S rRNA gene sequence was amplified, sequenced, and analyzed. For this, total genomic DNA was extracted using a genomic DNA extraction kit (Quiagen, USA) and the 16S rRNA gene was amplified using universal primer 27 F1 (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1494 Rc (5′-TACGGCTACCTTGTTACGAC-3′). The preparation of the reaction mixture and thermal condition of PCR (polymerase chain reaction) was followed as per previous standardized protocol (Singh et al. 2015). The 1.5 kb size of PCR amplicon was sequenced at Xcelris Genomics Labs Ltd. (Xcelris, Ahmedabad) India, and obtained nucleotides were matched against GenBank database and deposited in the NCBI database. The sequence was aligned using CLUSTAL-X, and a phylogenetic tree was constructed by the neighbor-joining method (bootstrap value 1000) by using Software MEGA6.0.
Heavy metal resistance of the isolate
Metal resistance ability of isolate was tested by growing in increasing concentration of metal cations added to SLP medium. The various metal ions used in the experiment were Cu as CuSO4, Ni as NiSO4, Cd as CdSO4, and Zn as ZnSO4. Stock solution in the range of 200–1200 g L−1 of each metal salts was prepared separately. The selected isolate was streaked on SLP-agar plate supplemented with different concentration of metals. Plates were incubated at 30 °C for 5 days in triplicate sets. The plate without metal ions was used as a control. Further, the growth behavior of test isolate in the metal-contaminated medium was also determined based on their minimum inhibitory concentration (MIC). Culture flasks containing 20-ml LB medium supplemented with heavy metals at the concentration of 250 mg l−1 were inoculated with test organism (OD 1.5) and incubated on a rotary shaker at 200 rpm for 40 h at 30 °C. The growth was measured at every 5 h at 600 nm in a UV-Vis spectrophotometer (Jasco Corporation, Japan).
Test for plant growth-promoting traits
The selected isolate was tested for the presence of plant growth-promoting features like ACC deaminase activity, phosphate solubilization, production of indole acetic acid (IAA), ammonia, and siderophore. For estimating ACC deaminase activity, the selected isolate was cultured in a nutrient medium, and the resulting biomass was suspended in a minimal medium (DF, Dworkin and Foster 1958) with the induction of ACC (3 mM). The ACC deaminase activity was performed as per the protocol described by Singh et al. (2015). The isolate was screened for its solubilization of inorganic phosphate by growing it on NBRIP medium following the method of Mehta and Nautiyal (2001). The ability to produce phytohormone IAA was estimated spectrophotometrically by Salkowsky’s reagent assay (Gordon and Weber 1951). The ability to produce ammonia was qualitatively evaluated by Nessler’s reagent method (Cappuccino and Sherman 2002). Further, the isolate was tested for arginine decarboxylase production by growing it MDAM (Moeller’s-decarboxylase agar medium) supplemented with L-arginine-monohydrochloride (1 g l−1) and phenol red (0.02 g l−1) used as the pH dye indicator. The ability of the bacterium to produce siderophore was tested by spot inoculating the test organism (1 μl) on chrome azurole S (CAS) agar plates. After growth on CAS-agar plate, it was observed for the presence of yellow or orange halo zone around the bacterial growth.
Morphological and biochemical characterization
The test isolate was screened for basic biochemical and microbiological tests as per the method described by Harley and Prescott (2002). The carbohydrate utilization ability was tested by carbohydrate utilization test kit (KB 009, Himedia). Test for its resistance to standard antibiotics namely gentamicin (30 μg), ampicillin (10 μg), erythromycin (10 μg), kanamycin (5 μg), tetracycline (10 μg), streptomycin (25 μg), and chloramphenicol (10 μg) was assayed by the antibiotic disks (HTM 002, Himedia).
Test of plant growth under Cu stress
The strain ZNP-5 was tested for its ability to promote the plant growth and metal (Cu) uptake in wheat under gradient metal (Cu) stress. The wheat (Triticum aestivum L) seeds were properly sterilized with the bleaching solution (NaOCl) and washed with Milli-Q water to remove any traces of bleach solution. For the pot experiment, a mixture of clay sandy soil (3:1) was used. The basic soil properties were: electric conductivity 0.61 ± 0.19 dSm−1, pH 6.27 ± 0.08, organic matter 11.30 ± 0.17%, cationic interchange coefficient 7.10 ± 1.08 c mol kg−, total phosphorus 10.8 ± 0.35 mg kg−1, total sulfur 9.5 ± 0.8 mg kg−1, total iron 31.4 ± 4.7 mg kg−1, and total copper 1.90 ± 0.06 mg kg−1. After sterilization, the soil was mixed with an aqueous solution of CuSO4 to achieve the desired concentration of 100, 200, and 300 mg kg−1 and left for 7 days for metal stabilization. Bacterial inoculum was adjusted to 2 × 108 cells ml−1 in phosphate buffer (pH 7.2). Sterilized seeds were soaked in bacterial suspension for 1 h under dark condition. Following bacterization, seeds were sown in plastic pots filled with 400 g of soil in a controlled environment of growth chamber with 16/8 light/dark regimes at 24 ± 2 °C with a humidity of 65–70%. The experiment was conducted for 21 days. Each experiment was performed in triplicate sets.
Quantification of Cu accumulation and biomass
After 21 days of Cu treatment, plant samples were washed with de-ionized water and were rinsed with 5 mM CaCl2 to remove absorbed metals, if any. Plant tissue was oven dried at 80 °C, grounded, and digested in a mixture of HNO3 and HClO4 (3:1, v/v). The powdered dry samples (0.5 g) were digested with 4 ml of HNO3/HClO4 (3:1, v/v), suspended in 10 ml of 0.1 M HNO3, and diluted to 10 ml with distilled water. The Cu contents were determined by using atomic absorption spectrophotometer at the National Horticultural Research and Development Foundation (Nashik, India). Each sample was analyzed in triplicate for accuracy.
Determination of photosynthetic pigment
For chlorophyll (a and b) estimation, 0.5 g leaf tissue was homogenized in 10 ml of 95% ethanol in the dark and spun at 5000 g for 15 min. The absorbance of the supernatant was measured at 450, 645, and 663 nm in a UV-Visible spectrophotometer (Jasco, Japan), as described by Arnon (1949) and concentrations were expressed as mg g−1 fresh weight (Chl a = 12.7A663–2.59A645, Chl b = 22.9A645−4.67A663).
Antioxidative enzyme assay
A 0.5 g plant leaves were crushed homogenously in 5 ml of 50 mM phosphate buffer (pH 7.0) containing 1% polyvinylpyrrolidone for SOD and POX and in 0.1 mM EDTA and 12.5 mM H2O2 for CAT assay. The crude macerate was centrifuged at 10,000g for 15 min at 4 °C, and the obtained supernatant was used for the antioxidant assay. For superoxide dismutase assay, the reaction mixture containing 100 μl enzyme extract, 50 mM phosphate buffer (pH 7.8), 13 mM methionine, 75 μM NBT, 2 μM riboflavin, 0.1 mM EDTA was incubated to observe for the development of purple colorformazan, which was then measured at 560 nm against the blank.
The peroxidase (POX) mixture consisted of 100 μl of enzyme extract with 0.1 M phosphate buffer, 0.1 mM pyrogallol, and 5 mM H2O2. The assay mixture was incubated for 5 min at 25 °C. The absorbance of indigo color formed was read at 420 nm against blank. Catalase mixture (3 ml) consisted of 100 μl enzyme extract with 50 mM phosphate buffer (pH 7.8), 0.1 mM EDTA and 12.5 mM H2O2. Calculation of CAT activity was based on an extinction coefficient of 0.04 mM−1 at 240 nm. A unit of enzyme activity was defined as a variation of 0.01 in the absorbance at 240 nm per min.
Biochemical analysis of plants treated with bacterium under Cu-stress
Lipid peroxidation
The malondialdehyde (MDA) content represents the degree of membrane damage under metal stress regarding lipid peroxidation. The extent of lipid peroxidation was determined by estimating the malondialdehyde (MDA) content produced by the thiobarbituric acid (TBA) method with minor modification (Hodges et al. 1999). Briefly, leaf tissues (0.5 g) were extracted in 80% ethanol and 1 ml of alcoholic extract was mixed with 1 ml of 0.5% (w/v) thiobarbituric acid (TBA) containing 20% (w/v) trichloroacetic acid (TCA). The mixture was incubated at 95°C for 30 min and cooled in an ice-bath. After cooling, the sample was centrifuged at 4500g for 15 min, and the absorbance was measured at 400, 532, and 600 nm. The MDA concentration was determined by its molar extinction coefficient (155 nm−1 cm−1) after subtracting the nonspecific absorbance.
Proline estimation
For proline analysis, fresh leaves of 0.5 g were homogenized in 3 ml of 5% (w/v) sulfosalicylic acid and centrifuged at 8500g for 10 min. The resulting supernatant of 500 μl was mixed with water and 2% ninhydr in 1:2 ratios. After incubating at 100 °C for 30 min, an equal volume of toluene (2 ml) was added to the mixture, and upper aqueous phase was used for taking absorbance at 520 nm in a spectrophotometer (Jasco Corporation, Japan). The proline content was measured by comparing the absorbance with a standard curve plotted from the defined concentration of L-proline (Bates et al. 1973).
Total soluble sugar and protein content
The total soluble sugar (TSS) content was determined using anthrone as per the method of Irigoyen et al. (1992), and the values were calculated using glucose as a standard (20–400 μg ml−1) to quantify soluble sugar synthesized in plants. A 0.1 ml of alcoholic leaf extract was mixed with 3 ml freshly prepared anthrone reagent and placed in a boiling water bath for 10 min. The absorbance of the resultant sample was measured at 620 nm in a UV-Vis spectrophotometer (Jasco, Japan).
The total protein content was quantified by the Bradford method (Bradford 1976).Plant tissue of 0.5 g was homogenized in 3 ml of extraction buffer containing 50 mM Tris-HCl (pH 8.3), 1 mM EDTA, 3 mM DTT, 0.08% ascorbic acid, 1 mM PMSF (phenylmethanesulfonyl fluoride) in a pre-chilled mortar. After centrifugation at 10,000g at 4 °C for 20 min, the absorbance of the supernatant was measured at 595 nm to evaluate the total protein content present in each treatment.
Measurement of metal-induced oxidative damage
Detection of hydrogen peroxide (H2O2)
The content of H2O2 in control and bacterium-treated plants was measured under different concentrations of Cu stress (Jana and Choudhuri 1981). Leaf tissue (0.2 g) was macerated homogenously in 50 mM phosphate buffer (pH 6.5), and 3 ml of the solution was then mixed with 1 ml of 0.1% titanium sulfate prepared in 20% H2SO4. The development of yellow color indicated a positive test for H2O2, whose intensity was measured at 410 nm. An extinction coefficient of 0.28 μM−1 cm−1 was used to estimate the amount of H2O2 and expressed as μmol g−1 fresh weight.
Estimation of membrane stability index
For measurement of MSI, leaf tissues were heated in two sets in 10 cm3 of double distilled water (DDW), one at 40 °C for 30 min while the other set at 100 °C for 10 min. The electrical conductivity was measured (C1 and C2) on a conductivity meter, and MSI was calculated using the formula:
Determination of the formation rate of O2 −
To measure the O2− content, leaf tissue (0.5 g) from different treatments was ground in liquid nitrogen. The powdered material was suspended in phosphate-buffered saline (PBS) buffer (50 mM, pH 7.8) and centrifuged at 10,000g for 15 min. The obtained supernatant was used for O2− content measurements.
Statistical analysis
The data were analyzed by standard statistical software (SPSS 12.0), and values were presented as the mean ± SD of three replications. The mean difference comparison between the control group and experimental group was analyzed by analysis of variance (ANOVA) and subsequently by Duncan’s multiple range test (p = 0.05).
Results
Identification and PGP features of test isolate
During isolation, 21 native bacterial strains were isolated from the rhizospheric soil of ‘Ziziphus nummularia.’ Among these, a potential bacterial strain ZNP-5 was found to tolerate high concentration of Cu. Compared to uninoculated control, ZNP-5 inoculation significantly increased the concentrations of water-soluble Cu, Zn, Ni, and Cd by 10.5-, 4.3-, 2.60-, and 2.10-fold, respectively (Fig. 1). Based on 16S rRNA gene sequence, the isolate was identified as Pantoea sp. The obtained sequence of the isolate was submitted to GenBank database under the accession number KJ950706. Phylogenetic analysis revealed that ZNP-5 is closely related to other Pantoea sp. and Pantoea ananatis (Suppl. Fig. 1). The test strain ZNP-5 showed luxuriant growth on the ACC utilizing plate. The colorimetric assay of the ACC deaminase activity demonstrated production of 135.20 ± 0.010 EU nmol of α-KB mg−1Pr.h−1. Quantitative estimation of phosphate solubilization was carried out in NBRIP medium, which showed phosphate solubilization of 13.35 ± 3.05 μg ml−1 after 72-h incubation. The isolate showed IAA production of 0.270 ± 0.036 μg ml−1 in the tryptophan-supplemented medium. The appearance of the orange-colored zone on the streaked CAS agar plate illustrated the siderophore production ability of strain ZNP-5. The isolate was also observed positive for ammonia production and argininedecarboxylase test.
Biochemical analysis of Pantoea sp. ZNP-5
Based on the biochemical analysis, it was found as Gram-negative bacteria and positive for catalase, Voges-Proskauer (VP), citrate utilization, lipase, urease, and nitrate reductase. Among the other tested features, it was found negative for indole, methyl red (MR), and amylase tests. The isolate was found to utilize various carbon sources, and it has been summarized in Suppl. Table 1. Isolate was found to be resistant to ampicillin, vancomycin, streptomycin, kanamycin, gentamicin and sensitive to tetracycline, chloramphenicol (Suppl. Table 2).
Metal resistance profile of Pantoea sp. ZNP-5
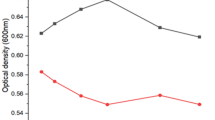
The test isolate showed a varied degree of resistance to heavy metals. The strain showed resistance against 700 mg Zn l−1, 500 mg Ni l−1, 900 mg Cu l−1, and 400 mg Cdl−1. Among the heavy metals, Cu seems to be less toxic to the strain, followed by Zn and Ni. The order of toxicity of the metals to the strains was found to be Cd > Ni > Zn > Cu. Further, the growth behavior of test strain in the metal supplemented medium was determined to evaluate its capacity to survive in an unfavorable condition. It is evident from Fig. 2 that ZNP-5 exposed to 250 mg l−1Cu showed the growth pattern as compared to control. A decrease in the overall growth of ZNP-5 was observed under other metal stress exposure (Zn, Ni, Cd).
Effect of Pantoea sp. ZNP-5 on plant growth under Cu stress
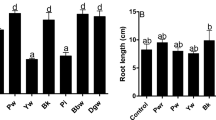
After growth for 21 days, significant differences in plant biomass were in different treatments. The addition of a different level of Cu (100–300 mg kg−1) metal inhibited the plant biomass. However, the addition of bacterial inoculum ZNP-5 significantly (p = 0.05) improved the biomass. The increase in biomass regarding fresh weight was 34, 59, 74, and 51% at 0, 100, 200, and 300 mg kg−1, respectively (Table 1). The results demonstrated that the addition of strain to the soil increased the dry biomass in the range of 27–80% under gradient (100–300 mg kg−1 Cu) metal stress. Furthermore, the effect of Cu stress and a bacterial addition was also monitored on the chlorophyll a and b content. It is evident from Table 1 that increases in Cu level from 100 to 300 mg kg−1soil, decreases the chlorophyll a and b in the range of 13–123% and 24–169%, respectively. The application of strain ZNP-5 significantly increased (p = 0.05) the chlorophyll a content of 41, 31, 98, and 64% at 0, 100, 200, and 300 mg kg−1 of Cu in the soil as compared to respective control plants (Table 1). Similarly, the increase in chlorophyll b was 48, 47, 80, and 74% at 0, 100, 200, and 300 mg kg−1 of Cu stress in bacteria-inoculated plants (Table 1). The effect of bacterial inoculation on shoot/root length has been summarized in Suppl. Table 2.
Accumulation of Cu and distribution in plant
The bacterial treatment significantly improved the accumulation of Cu in the wheat plant tissue to the different degrees. As seen in Table 2, the accumulation of Cu was more in the shoots as compared to roots. The observed results illustrated that bacterial application mobilizes the metal content in the shoot tissue as compared to root. There was no any significant change in the shoot-Cu content at 0 mg kg−1 Cu. However, bacterial inoculation significantly improved the Cu content in shoot and root of wheat plant treated with other Cu concentrations (100, 200, and 300 mg kg−1 Cu). The highest significant (p = 0.05) accumulation of Cu in the shoot was 87% at 100 mg kg−1 Cu, followed by 73 and 57% at 300 mg kg−1 Cu and 200 mg kg−1 Cu, respectively. The accumulation of Cu in the roots of wheat plant increased in the range of 21 to 64% in bacterial-primed plants. The highest significant (p = 0.05) accumulation of Cu content in the root was 64% at 200 mg kg−1 Cu, followed by 60, 56, and 21% at 300, 100, and 0 mg kg−1Cu as compared to respective control plants (Tables 2 and 3).
Antioxidant activities
The activity of various antioxidant enzymes in wheat plants was evaluated in the presence of bacterial inoculum to monitor the ROS level under metal stress. It can be seen from Fig. 3 that metal stress induced the production of tested antioxidant enzymes; however, its level was more prominent in the presence of bacterial inoculums. The ZNP-5 inoculation significantly (p = 0.05) increased the SOD activity by 26, 41, and 39% at 0, 100, and 200 mg kg−1 of Cu stress, respectively, as compared to their control (Fig. 3a). However, there was no significant change in SOD activity at 300 mg kg−1. Considering the peroxidase (POX) activity, it is evident from Fig. 3b that at 100 and 200 mg kg−1, the increase in activity was 42 and 39% (p = 0.05) in ZNP-5-treated plants as compared to corresponding control. There was no significant change observed at 0 and 300 mg kg−1. Similarly, the bacterial primed plants showed higher catalase activity as compared to control plants. The significant (p = 0.05) increase in catalase (CAT) activity was 30, 35, 28, and 25% at 0, 100, 200, and 300 mg kg−1 (Fig. 3c).
Biochemical analysis
The change in certain compatible solutes namely proline, malondialdehyde (MDA), total soluble sugar (TSS), and total protein content (TPC) known to play important protective roles during stress condition. Differential productions of the above components were detected in bacterially treated and untreated plants under Cu stress condition. The proline content in ZNP-5 inoculated plants significantly (p = 0.05) enhanced by 35% as compared to a respective control without Cu stress. The significant (p = 0.05) increase in proline content (37%) was observed at 100 mg kg−1of Cu, followed by 33 and 27% at 300 and 200 mg kg−1 of Cu stress, when compared to un-inoculated control treated with respective metal stress (Fig. 4a).
It is evident from Fig. 4b, which with the increase in Cu stress MDA content increased by 65%, however, bacterial application significantly (p = 0.05) decreased the MDA content by 49%. Also, MDA contents significantly decreased in the leaves of the wheat plant with different levels of Cu stress in ZNP-5-inoculated plants. The highest (p = 0.05) decrease of 41% was observed at 300 mg kg−1, followed by 34, 30, and 28% at 200, 100, and 0 mg kg−1, respectively, as compared to the corresponding control plants.
The results of total soluble sugar estimation revealed that bacterial inoculation significantly (p = 0.05) increased its content by 32, 43, and 36% at 100, 200, and 300 mg kg−1 of Cu in soil (Fig. 4c). A significant difference was found for total protein content in wheat leaves stressed with different metal stress. From Fig. 4d, it can be seen that bacterial inoculation significantly (p = 0.05) increased the total protein content by 48, 46, and 25% at 100, 200, and 300 mg kg−1 of Cu stress as compared to respective control plants.
Oxidative stress measurement
Bacterial inoculation protected the wheat plants from the Cu-induced oxidative damages. It was monitored by evaluating the H2O2 and O2− content as well as by measuring membrane stability index. The increase in the Cu content in the soil from 100 to 300 mg kg−1 increased the H2O2 content from 29 to 107%. However, its content decreased significantly in the presence of isolate ZNP-5. The highest (p = 0.05) decrease with 42% was recorded at 200 mg kg−1, followed by 30, 23, and 20% at 100, 0, and 300 mg kg−1 of Cu, respectively, as compared to corresponding control (Fig. 5a). Decrease in the MSI was noted in the range of 22 to 110% under increased Cu stress; however, stability was significantly (p = 0.05) elevated to 50, 33, 16, and 15% at 200, 100, 0, and 300 mg kg−1 of Cu stress, respectively, over the control (Fig. 5b). Exposure to Cu stress caused a substantial increase to the O2− content in wheat plants. The effect was found to increase concerning the gradual increase of Cu content in the soil. However, a decrease in the O2− content was noticed in response to bacterial inoculation. The highest (p = 0.05) decrease 52% was found to be at 200 mg kg−1 Cu concentration, followed by 36, 24, and 21% at 100, 0, and 300 mg kg−1 Cu concentration (Fig. 5c).
Discussion
The present study aimed to explore a potent microbial strain that can alleviate the toxicity of heavy metal stress in host plants. The strain was specifically chosen for further studies due to its high metal solubilization ability in the soil. Previous studies (Jiang et al. 2008; Rajkumar et al. 2008) also demonstrated that metal mobilizing bacteria increases the water-soluble fraction of heavy metals. This behavior might be attributed to microbe-mediated alteration of soil pH, organic acid release, and oxidation/reduction reactions (Rajkumar et al. 2013). The present study is the first report of Pantoea sp. to protect the wheat plant from Cu-stress and its ability to alleviate the metal stress through polyphasic mechanisms.
It is evident from the previous studies that metal-resistant bacteria can enhance the metal uptake by the plants (De Souza et al. 1999; Whiting et al. 2001). Our results of experiments too demonstrated that the heavy metal-resistant bacterium ZNP-5 significantly enhanced Cu uptake by wheat plants. It was noticed that in the presence of ZNP-5, the copper accumulation in the roots and shoots of wheat was enhanced. However, the presence of bacterial isolate caused a lesser inhibitory effect on biomass production. The accumulation of metal (Cu) in plant tissues after ZNP-5 inoculation indicates its potential to tolerate, survive, and express plant beneficial traits under metal stress conditions. Although, role of bacteria in plant growth and heavy metal accumulation has been documented in several reports (Abou-Shanab et al. 2003; Idris et al. 2004; Sheng and Xia 2006), to the best of our knowledge, present study is the first to report role of metal-resistant PGPR Pantoea sp. in Cu mobilization with concurrent promotion of plant growth by plant growth study.
Cellular antioxidants play a vital role in protecting plants from the attack from metal stress-induced free radicals. ROS-scavenging enzymes including SOD, POX, and CAT belong to the first group of antioxidant enzymes which are involved in the defense system (Smeets et al. 2005). During exposure to Cu stress, there was no obvious variation in SOD, POX, and CAT activity. In contrary, application of bacterium increased the antioxidant activity in the Cu-stressed plants, indicating the modulation of the expression profile of metal-detoxifying enzymes to counteract the metal stress (Duponnois et al. 2006). The up-regulation of the activity of antioxidant enzymes depicts their role in detoxification of ROS. The enhanced activity of SOD suggests the efficient decomposition of O2− into H2O2 at a very fast rate, thereby decreasing the risk of formation of hydroxyl free radicals. The increased activity of SOD could have resulted from the action of heavy metal ions and an in direct effect of increased level of superoxide radicals (Chongpraditnun et al. 1992). The induction of SOD encoding genes leading to the de novo synthesis of enzyme protein (Verma and Dubey 2003) might be associated with superoxide-mediated signal transduction (Fatima and Ahmad 2005). POX and CAT activity remove the H2O2 produced by dismutation activity of SOD. The higher POX and CAT activity could be responsible for quenching of H2O2 in metal-stressed conditions (Kaur et al. 2012).
Under heavy metal treatment, the accumulation of proline was decreased. However, its concentration was higher in ZNP-5-inoculated plants. The accumulation of proline suggests its role as an osmotic adjustment in Cu stress, which was also reported in earlier studies (Thounaojam et al. 2012; Yan and Tam 2013). Further, one of the most prominent symptoms of oxidative stress in the biological membrane is exhibited through lipid peroxidation, which can be observed from the increase in the MDA levels. We observed a statistically significant increase in MDA concentration for all the concentrations (100, 200, and 300 mg kg−1 Cu) concerningcontrol. However, the level of MDA in the bacteria-treated plant was noted to decrease significantly (p < 0.01), which indicated the bacterial-mediated attenuated effect of metal stress on plants.
Soluble sugar and proteins, which includes a vast array of metabolic products, are widely known for their cellular metabolic stability. Accumulations of such essential metabolites are positively correlated with tolerance to oxidative stress in plants. In addition to directly scavenging ROS, soluble sugar also induces expression of genes encoding antioxidative enzymes (Sun et al. 2012). Cu stress can lead to the oxidative damage of molecular structure and enhances proteolytic activity which was evident from decreased protein content in Cu-stressed plants (Szollosi et al. 2012). It can be assumed that bacterial application serves as a stabilizer of protein and sugar structure and thus allows the wheat seedlings to maintain normal intracellular metabolism.
Exposure to heavy metals in soil enhances the content of stress markers such as H2O2, O2− and lead to loss of membrane stability. This may be attributed to the increased acclimatization of Cu in plant tissues. In the previous studies, increased electrolyte leakage in wheat seedlings was observed on exposure to Ni and Pb stress (Gajewska et al. 2012; Kaur et al. 2012). This may be correlated with the increased accumulation of ROS that induces oxidative stress on exposure to metal stress. Oxidative stress leads to reduced metabolism and greater accumulation of H2O2 in plant tissues. On the other hand, bacterial inoculation gradually decreased the content of free radicals (O2−) hydrogen peroxide (H2O2) and maintained the membrane stability.
In the metal-stressed environment, rhizobacterium with PGP features could help to reduce copper stress and play an essential role in plant growth. The morphological, physiological, and functional traits of the selected bacterial isolate tested so that it can be utilized for bioremediation of Cu metal. Ability of the test isolate to utilize ACC as a nitrogen source owes due to the presence of ACC deaminase, which hydrolyzes ACC and enhances plant root and plant growth (Glick 2012). The test isolate was found to solubilize the inorganic phosphate which is one of the important attributes of plant growth-promoting bacteria to support plant growth (Jiang et al. 2008). The test isolate also exhibited production of siderophore which provides Fe nutrition, play important role in inhibiting pathogenic microorganisms, and help to reduce copper toxicity by increasing iron supply to the plant (Ali and Vidhale 2013). The overall plant growth-promoting attributes of the test isolate suggest its potential to alleviate copper toxicity and promote plant growth under copper stress.
There was significant plant growth promotion effect after bacterial inoculation in the Cu-contaminated soil. Pantoea sp. is well known for its plant growth-promoting features and improve crop yield in the field as well (De Maayer et al. 2014). Better plant growth following bacterial inoculation suggests the synergistic effect of plant growth-promoting features of the test isolate. In the previous study, it was observed that rhizobacterial inoculation significantly increases the metal tolerance in plants (Saleh and Saleh 2006). Similarly, the previous study has also indicated that inoculation with PGPR possessing the beneficial PGP traits enhances the growth of inoculated plants primarily through enhancing the nutrient uptake under heavy metal stress conditions (Ma et al. 2011). A decrease in the level of chlorophyll a/b was observed in uninoculated Cu-stressed plants, which could be due to the inhibition of enzymes about Calvin cycles, peroxidation of thylakoid membrane, or substitution of Mg2+ located in the tetrapyrrole ring of chlorophyll molecules (Yılmaz and Parlak 2011). Application of bacterial isolate increased the amount of the photosynthetic pigment, which suggested that wheat seedlings can tolerate Cu stress without notable leaf necrosis and chlorosis during the given time span. Furthermore, the colonization behavior should be tested in future studies under normal and stress condition to reveal the endophytic nature of the test isolate. Therefore, in the present study, microbial inoculant enhanced the capacity of plants to extract Cu from soil via the mechanisms discussed above, which are the increased heavy metal availability, increased plant tolerance, and growth and uptake.
Conclusion
Plant-microbe interaction can be exploited to enhance phytoextraction efficiency of soil contaminated with heavy metal pollutants. The benefits of metal resistant and plant growth-promoting bacteria resulted in faster growth, better access to nutrients, and sustainable biomass production through enhanced remediation of metal-contaminated soils. In the present work, Pantoea sp. ZNP-5 was tested for the first time regarding its ability to alleviate the Cu-toxicity. Evoked antioxidative response (CAT, SOD, POX) following bacterial inoculation clearly revealed the effective plant mechanism to suppress ROS under high Cu concentrations. The stimulate production of various compatible solutes in response to the bacterium is likely to protect the plant from oxidative stress, induced by an elevated level of Cu in the soil. Thus, Pantoea sp. has emerged as a promising candidate for future to mitigate Cu from polluted soils. Further, the future studies are needed to screenout the genes responsible for metal tolerance and expression analysis in response to metal stress.
References
Abou-Shanab R, Angle J, Delorme T, Chaney R, Van Berkum P, Moawad H, Ghanem K, Ghozlan H (2003) Rhizobacterial effects on nickel extraction from soil and uptake by Alyssum murale. New Phytol 158:219–224
Ali SS, Vidhale N (2013) Bacterial siderophore and their application: a review. Int J Curr Microbiol App Sci 2:303–312
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Belimov A, Hontzeas N, Safronova V, Demchinskaya S, Piluzza G, Bullitta S, Glick B (2005) Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol Biochem 37:241–250
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cappuccino J, Sherman N (2002) A laboratory manual, 6th edn. Benjamin Cummings Publishing Co., San Francisco
Chen X, Wu C, Tang J, Hu S (2005) Arbuscular mycorrhizae enhance metal lead uptake and growth of host plants under a sand culture experiment. Chemosphere 60:665–671
Chongpraditnun P, Mori S, Chino M (1992) Excess copper induces a cytosolic Cu, Zn-superoxide dismutase in soybean root. Plant Cell Physiol 33:239–244
De Maayer P, Chan WY, Rubagotti E, Venter SN, Toth IK, Birch PR, Coutinho TA (2014) Analysis of the Pantoea ananatis pan-genome reveals factors underlying its ability to colonize and interact with plant, insect and vertebrate hosts. BMC Genomics 15:404
De Souza M, Huang C, Chee N, Terry N (1999) Rhizosphere bacteria enhance the accumulation of selenium and mercury in wetland plants. Planta 209:259–263
Duponnois R, Kisa M, Assigbetse K, Prin Y, Thioulouse J, Issartel M, Moulin P, Lepage M (2006) Fluorescent pseudomonads occuring in Macrotermes subhyalinus mound structures decrease cd toxicity and improve its accumulation in sorghum plants. Sci Total Environ 370:391–400
Dworken M, Foster J (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:592–601
Egamberdiyeva D, Höflich G (2004) Effect of plant growth-promoting bacteria on growth and nutrient uptake of cotton and pea in a semi-arid region of Uzbekistan. J Arid Environ 56:293–301
Fatima RA, Ahmad M (2005) Certain antioxidant enzymes of Allium cepa as biomarkers for the detection of toxic heavy metals in wastewater. Sci Total Environ 346:256–273
Fellet G, Marchiol L, Delle Vedove G, Peressotti A (2011) Application of biochar on mine tailings: effects and perspectives for land reclamation. Chemosphere 83:1262–1267
Gajewska E, Bernat P, Długoński J, Skłodowska M (2012) Effect of nickel on membrane integrity, lipid peroxidation and fatty acid composition in wheat seedlings. J Agron Crop Sci 198:286–294
Glick BR (2003) Phytoremediation: synergistic use of plants and bacteria to clean up the environment. Biotechnol Adv 21:383–393
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012:1–15
Glick BR, Karaturovíc DM, Newell PC (1995) A novel procedure for rapid isolation of plant growth promoting pseudomonads. Can J Microbiol 41:533–536
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26:192–195
Grichko VP, Glick BR (2001) Amelioration of flooding stress by ACC deaminase-containingplant growth-promoting bacteria. Plant Physiol Biochem 39:11–17
Harley J, Prescott L (2002) Laboratory exercises in microbiology, 5th edn. The McGraw-Hill Companies, Texas
He H, Ye Z, Yang D, Yan J, Xiao L, Zhong T, Yuan M, Cai X, Fang Z, Jing Y (2013) Characterization of endophytic Rahnella sp. JN6 from Polygonum pubescens and its potential in promoting growth and Cd, Pb, Zn uptake by Brassica napus. Chemosphere 90:1960–1965
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hrynkiewicz K, Baum C (2013) Selection of ectomycorrhizal willow genotype in phytoextraction of heavy metals. Environ Technol 34:225–230
Idris R, Trifonova R, Puschenreiter M, Wenzel WW, Sessitsch A (2004) Bacterial communities associated with flowering plants of the Ni hyperaccumulator Thlaspi goesingense. Appl Environ Microbiol 70:2667–2677
Irigoyen J, Einerich D, Sánchez-Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol Plant 84:55–60
Jana S, Choudhuri MA (1981) Glycolate metabolism of three submersed aquatic angiosperms: effect of heavy metals. Aquat Bot 11:67–77
Jiang C-Y, Sheng X-F, Qian M, Wang Q-y (2008) Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere 72:157–164
Kaur G, Singh HP, Batish DR, Kohli RK (2012) A time course assessment of changes in reactive oxygen species generation and antioxidant defense in hydroponically grown wheat in response to lead ions (Pb2+). Protoplasma 249:1091–1100
Lamb DT, Ming H, Megharaj M, Naidu R (2009) Heavy metal (Cu, Zn, Cd and Pb) partitioning and bioaccessibility in uncontaminated and long-term contaminated soils. J Hazard Mater 171:1150–1158
Ma Y, Prasad MNV, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29:248–258
McLaughlin MJ, Parker D, Clarke J (1999) Metals and micronutrients–food safety issues. Field Crop Res 60:143–163
Mehta S, Nautiyal CS (2001) An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr Microbiol 43:51–56
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Ouzounidou G (1995) Effect of copper on germination and seedling growth ofMinuartia, Silene, Alyssum and Thlaspi. Biol Plant 37:411–416
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118:10–15
Rajkumar M, Nagendran R, Lee KJ, Lee WH, Kim SZ (2006) Influence of plant growth promoting bacteria and Cr 6+ on the growth of Indian mustard. Chemosphere 62:741–748
Rajkumar M, Ma Y, Freitas H (2008) Characterization of metal- resistant plant-growth promoting Bacillus weihenstephanensis isolated from serpentine soil in Portugal. J Basic Microbiol 48:500–508
Rajkumar M, Prasad MNV, Swaminathan S, Freitas H (2013) Climate change driven plant–metal–microbe interactions. Environ Int 53:74–86
Roychoudhury A, Basu S, Sengupta DN (2012) Antioxidants and stress-related metabolites in the seedlings of two indica rice varieties exposed to cadmium chloride toxicity. Acta Physiol Plant 34:835–847
Saleh M, Saleh Al-Garni (2006) Increased heavy metal tolerance of cowpea plants by dual inoculation of arbuscular mycorrhizal fungi and nitrogen-fixer Rhizobium bacterium. Afer J Biotechnol 5:132–144
Sessitsch A, Kuffner M, Kidd P, Vangronsveld J, Wenzel WW, Fallmann K, Puschenreiter M (2013) The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem 60:182–194
Sheng X-F, Xia J-J (2006) Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere 64:1036–1042
Singh RP, Jha P, Jha PN (2015) The plant-growth-promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J Plant Physiol 184:57–67
Smeets K, Cuypers A, Lambrechts A, Semane B, Hoet P, Van Laere A, Vangronsveld J (2005) Induction of oxidative stress and antioxidative mechanisms in Phaseolus vulgaris after cd application. Plant Physiol Biochem 43:437–444
Smith SE, Read DJ (2010) Mycorrhizal symbiosis. Academic press, London
Sun W-J, Nie Y-X, Gao Y, Dai A-H, Bai J-G (2012) Exogenous cinnamic acid regulates antioxidant enzyme activity and reduces lipid peroxidation in drought-stressed cucumber leaves. Acta Physiol Plant 34:641–655
Szollosi R, Varga IS, Erdei L, Mihalik E (2012) Cadmium-induced oxidative stress and antioxidative mechanisms in germinating Indian mustard (Brassica juncea L.) seeds. Ecotoxicol Environ Saf 72:1337–1342
Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma G, Sahoo L, Sanjib P (2012) Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem 53:33–39
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Whiting SN, de Souza MP, Terry N (2001) Rhizosphere bacteria mobilize Zn for hyperaccumulation by Thlaspi caerulescens. Environ Sci Technol 35:3144–3150
Yan Z, Tam NFY (2013) Effects of lead stress on anti-oxidative enzymes and stress-related hormones in seedlings of Excoecaria agallocha Linn. Plant Soil 367:327–338
Yılmaz DD, Parlak KU (2011) Changes in proline accumulation and antioxidative enzyme activities in Groenlandia densa under cadmium stress. Ecol Indic 11:417–423
Funding
This research was financially supported by Department of Biotechnology (Grant No. BT/PR14527/AGR/21/326/2010), Govt. of India, New Delhi to PNJ.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Singh, R.P., Jha, P.N. Priming with ACC-utilizing bacterium attenuated copper toxicity, improved oxidative stress tolerance, and increased phytoextraction capacity in wheat. Environ Sci Pollut Res 25, 33755–33767 (2018). https://doi.org/10.1007/s11356-018-3022-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3022-y