Abstract
In the present study, in vitro toxicity as well as biopersistence and photopersistence of four artificial sweeteners (acesulfame, cyclamate, saccharine, and sucralose) and five antibiotics (levofloxacin, lincomycin, linezolid, marbofloxacin, and sarafloxacin) and of their phototransformation products (PTPs) were investigated. Furthermore, antibiotic activity was evaluated after UV irradiation and after exposure to inocula of a sewage treatment plant. The study reveals that most of the tested compounds and their PTPs were neither readily nor inherently biodegradable in the Organisation for Economic Co-operation and Development (OECD)-biodegradability tests. The study further demonstrates that PTPs are formed upon irradiation with an Hg lamp (UV light) and, to a lesser extent, upon irradiation with a Xe lamp (mimics sunlight). Comparing the nonirradiated with the corresponding irradiated solutions, a higher chronic toxicity against bacteria was found for the irradiated solutions of linezolid. Neither cytotoxicity nor genotoxicity was found in human cervical (HeLa) and liver (Hep-G2) cells for any of the investigated compounds or their PTPs. Antimicrobial activity of the tested fluoroquinolones was reduced after UV treatment, but it was not reduced after a 28-day exposure to inocula of a sewage treatment plant. This comparative study shows that PTPs can be formed as a result of UV treatment. The study further demonstrated that UV irradiation can be effective in reducing the antimicrobial activity of antibiotics, and consequently may help to reduce antimicrobial resistance in wastewaters. Nevertheless, the study also highlights that some PTPs may exhibit a higher ecotoxicity than the respective parent compounds. Consequently, UV treatment does not transform all micropollutants into harmless compounds and may not be a large-scale effluent treatment option.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Artificial sweeteners and antibiotics are used worldwide in remarkably large amounts and, during the last few years, have attracted the attention of environmental scientists (e.g., Yan and Song 2014).
The extensive worldwide use of antibiotics in human and veterinary medicine has promoted the development of antibiotic resistance in pathogens (Kemper 2008), and concerns have been raised regarding the resulting disadvantages in their therapeutic use. In addition, this wide-ranging use led to the presence of antibiotics as micropollutants in the aquatic environment (Michael et al. 2013). For example, the widely prescribed group of antibacterial agents, the fluoroquinolones, have been found in aquatic environments in various parts of the world (Table 1, Sukul and Spiteller 2007).
Artificial low-calorie sweeteners also represent a new class of micropollutants that give raise to additional concern. Sweeteners are regularly added to foods and beverages in considerable quantities. After ingestion, they pass through the human body, where they are largely unaffected by the metabolism, and reach the aquatic environment through domestic wastewater (Rodero et al. 2009). They have been repeatedly detected in wastewater, groundwater, seawater, and tap water (Buerge et al. 2009; Mead et al. 2009; Van Stempvoort et al. 2011). Because of their frequent occurrence in the aquatic environment (Table 1), artificial sweeteners are even used as anthropogenic wastewater markers (Buerge et al. 2009; Oppenheimer et al. 2012). Their unintended presence in different aquatic compartments is a matter of concern, even though they undergo comprehensive toxicological testing prior to their use in consumer products (e.g., Grice and Goldsmith 2000; Weihrauch and Diehl 2004). In the 1980s, the sweetener saccharine was prohibited as a sugar substitute in Canada because of a possible risk of bladder cancer, but today, it is no longer regarded as a potential cancer-inducing agent (Weihrauch and Diehl 2004). Ecotoxicological test results on acute toxicity also suggest a rather low risk (Soh et al. 2011; Hjorth et al. 2010). Nevertheless, it is largely unknown whether or not artificial sweeteners exert long-term (chronic) effects on aquatic communities (Huggett and Stoddard 2011). Recently, an ecotoxicity study on crustaceans found toxic effects of sweeteners when new sensible, but nonstandardized parameters were tested (Wiklund et al. 2012).
Both antibiotics and sweeteners that reach the aquatic environment are prone to biotic and abiotic degradation processes. Numerous biotransformation products and phototransformation products (PTPs) may be formed during theses processes. In terms of biotransformation products, their formation is limited due to the fact that biochemical pathways are governed by enzymes (Fatta-Kassinos et al. 2011). However, myriads of PTPs are often formed during phototransformation processes since nonselective radicals are involved in these photoprocesses (Fatta-Kassinos et al. 2011).
In the last few years, new technical approaches have been tested to continually improve water quality, including ozonolysis, photolysis, and UV irradiation (Khetan and Collins 2007; Pütmann et al. 2008). These methods, which involve advanced oxidation processes (AOP), have often proven to be very effective in removing parent compounds. It is well known, however, that the disappearance of the parent compound does not imply a complete degradation of the compound, but rather, transformation products (TP) are being formed. These TPs may be even more persistent and/or toxic than the respective parent compound (Bergheim et al. 2014). The number of studies dedicated to the structural elucidation of such TPs is increasing, but toxicological studies on TPs are still lacking.
In order to fill this data gap, we investigated the biodegradability and photodegradability of four sweeteners and five antibiotic compounds as well as the (eco)toxicity of the concomitantly formed TPs. For this purpose, a detailed study on their biodegradability was performed with three widely used Organisation for Economic Co-operation and Development (OECD)-standardized biodegradation tests. Photodegradation was tested using an Hg lamp and a Xe lamp to simulate photochemical reactions during AOP processes and natural sunlight, respectively. A first toxicity screening of the generated PTPs was performed using various in vitro ecotoxicity and human toxicity assays.
Materials and methods
Chemicals
Four artificial sweeteners (acesulfame, cyclamate, saccharine, and sucralose) were tested in this study. They are all commonly used to sweeten products such as food and drinks. Furthermore, five antibiotics (levofloxacin, lincomycin, linezolid, marbofloxacin, and sarafloxacin) were also investigated here. Levofloxacin, marbofloxacin, and sarafloxacin are all broad spectrum antibiotics of the fluoroquinolone drug class. They primarily exert their antibacterial effect by preventing bacterial DNA from unwinding and duplicating. Lincomycin is a lincosamide antibiotic with a narrow spectrum and primarily targets gram-positive bacteria. Linezolid is a member of the oxazolidinone class of antibiotics and is used for the treatment of infections caused by gram-positive bacteria that are resistant to several other antibiotics.
All test compounds were at least of analytical grade. They were purchased from Sigma-Aldrich (Steinheim, Germany), with the exception of linezolid, which was purchased from Pharmacia Corporation (Kalamazoo, USA).
All solutions were prepared using ultrapure water, obtained from a Milli-Q Millipore Reagent-Water-System (Eschborn, Germany).
Biodegradation tests
In order to evaluate the biological degradability of all test compounds, three biodegradation tests were applied in accordance with the 1992 OECD test guidelines described in detail elsewhere (Bergheim et al. 2012). The standard test period for all biodegradation experiments was 28 days, and all test series were run as duplicate.
Closed bottle test (CBT)
The first test for assessing whether or not organic compounds are readily biodegradable in the aquatic environment is the CBT (OECD 301D), as recommended by the OECD. We performed the CBT with a low bacterial density, a low nutrient content, and with a low concentration of the test compound, which corresponded to a theoretical oxygen demand (ThOD) of 5 mg/L (without nitrification). According to the OECD guideline, a test compound is readily biodegradable if biodegradation (expressed as percentage of the oxygen consumed in the test vessel) exceeds 60 % within a period of 10 days after oxygen consumption reached 10 %.
Aerobic biodegradation was monitored daily by determining the oxygen concentration in the test vessels with an optode oxygen sensor system (Fibox 3 PreSens, Regensburg, Germany). In order to test the biodegradability of the PTPs, additional test series were run with aliquots from the irradiation experiments.
Manometric respirometry test (MRT)
A second test for assessing whether or not organic compounds are readily biodegradable in the aquatic environment is the MRT (OECD 301F), as recommended by the OECD. The MRT was performed with a medium bacterial density, a medium nutrient content, and a medium concentration of test compounds, which corresponded to a ThOD of 30 mg/L. In analogy to the CBT, a test compound was classified as readily biodegradable if biodegradation exceeded 60 % within a period of 10 days after oxygen consumption reached 10 %.
Aerobic biodegradation was measured daily with an automatic analyzer (System OxiTop® OC100, WTW, Weilheim, Germany), which quantifies the microbial oxygen consumption by measuring CO2 production through determination of pressure.
Zahn-Wellens test (ZWT)
The OECD recommends the ZWT (OECD 302B), a tier-2 biodegradability test, for the assessment of the inherent biodegradability of organic compounds, e.g., during sewage treatment. Commonly, a high nutrient content and a high bacterial diversity are used for this assessment. With test compound concentrations equivalent to 50 mg of dissolved organic carbon (DOC) per liter (85 up to 212 mg/L, see Table 2), the compounds were added to the test containers as the only source of carbon. A test compound is classified as inherently biodegradable if the DOC concentration was reduced by more than 70 %.
Aerobic biodegradation was monitored at specific time intervals by measuring DOC loss in the test vessels with a TOC 5000 (Shimadzu GmbH, Duisburg, Germany) total organic carbon (TOC) analyzer. In the case of lincomycin only, the antibiotic was not tested in the ZWT because lincomycin is used in veterinary medicine and will therefore not enter sewage treatment plants via domestic wastewater.
DOC measurement
The DOC content was determined to monitor the progress of both aerobic and anaerobic biodegradation as well as of the photochemical and photolytic degradation. The latter was determined in three replicates according to European standard procedure EN 1484 by using a TOC 5000 analyzer (Shimadzu GmbH, Duisburg, Germany). Samples of the biodegradation test were first filtered (cutoff 0.45 μm, Sartorius, Goettingen, Germany) in order to meet the conditions for DOC measurements, and then measured continually over the course of the 28-day test period. Samples of the irradiation experiments were measured for fixed time periods (2, 4, 8, 16, 32, 64, and 128 min) subsequent to the irradiation.
LC-UV-MS analysis
A high-performance liquid chromatography (LC) system (Agilent Technologies, Waldbronn, Germany, LC 1100 series) consisting of two G1312A binary pumps, an ALS G1329A + ALS Therm G1330B sampler, a G1316A column oven (temperature set at 40 °C), and a G1322A degasser (Agilent, Germany) was used. Chromatographic separation was performed on an RP-18 column (CC length 70/ID 3 mm NUCLEODUR 100–3 (particle size 3 μm) C18 ec, Macherey and Nagel, Dueren, Germany), protected by a guard column (CC length 8 mm/ID 4 mm NUCLEODUR 100–5 (particle size 5 μm) C18 ec; Macherey and Nagel, Dueren, Germany). For elution, 0.1 % formic acid in water (HCOOH, solution A) and 100 % acetonitrile (CH3CN, solution B) were used by applying the following linear gradient: 0 min 1 % B, 20 min 45 % B, 22.3 min 55 % B, 25 min 80 % B, 26 min 1 % B, 30 min 1 % B. The sample injection volume was 20 μL, and the flow rate was set to 0.5 mL/min. Total run time was 30 min. Test compounds at concentrations of 1, 2.5, 5, 7.5, 10, 15 mg/L, and up to 20 and 30 mg/L for cyclamate and acesulfame, were used to establish the corresponding standard calibration curves. Quality controls at 10 mg/L were included in each run and were within ±20 %. The limit of detection and quantification was below all concentrations measured. The protonated molecule of each compound was monitored for quantification. Samples were either directly analyzed or stored at −80 °C for subsequent analysis.
Quantification and detection were performed on a Bruker Daltonic Esquire 6000 plus ion trap mass spectrometer (IT-MS) equipped with a Bruker data analysis system and an atmospheric pressure electrospray ionization (API-ESI) interface (Bruker Daltonic GmbH, Bremen, Germany). The scan range was set to mass to charge (m/z) values varying between 50 and 1000, and the scan time was 200 ms. For UV detection, a UV/Vis detector (Agilent G1314 A) was used, and absorbance maxima were measured at 210, 260, 275, 310, and 350 nm. Fluorescence was assessed with an Agilent G1321 A fluorescence detector (excitation 278 nm, emission 445 nm). For further details, see Bergheim et al. (2014).
Irradiation experiments and absorbance spectra
Irradiation experiments were performed using a TXE 150 W xenon lamp and a TQ 150 W medium-pressure mercury lamp (UV-Consulting Peschl, Mainz, Germany) with stock solutions of the test compounds at 10 mg/L in ultrapure water. The Hg lamp emits a low-intensity polychromatic radiation spectrum from 200 to 600 nm, with some higher intensities at 254, 265, 302, 313, 366, 405/408, 436, 546, and 577/579 nm. The Xe lamp has a lower total photon flux and a continuous spectrum of radiation from 300 to 800 nm. For further details, see Bergheim et al. (2014).
Upon irradiation, the temperature of the irradiated stock solutions was maintained at 20 ± 2 °C. Aliquots were taken at fixed time intervals (0, 2, 4, 8, 16, 32, 64, and 128 min) for further evaluation in terms of DOC, LC-UV-MS experiments as well as for the growth inhibition test. Immediately following irradiation, the absorbance spectra (Perking Elmer Instruments, USA) as well as the DOC (see “DOC measurement” section) were measured.
Bacterial toxicity bioassays
Growth inhibition test (EN ISO 10712:1995)
The growth inhibition test was performed according to the EN ISO 10712 test guideline (1995) in order to investigate the effects of the irradiated and nonirradiated samples on bacterial growth (for details, see Bergheim et al. 2012). Briefly, a monoculture strain of Pseudomonas putida (ATCC 50026), obtained from the German collection of microorganisms and cell cultures (DSMZ, Braunschweig, Germany), was used as inoculum. The toxicity of the test compounds was determined by comparing bacterial growth in samples from the test vessels with those of the blanks and without test compounds. This procedure was applied for the irradiated and nonirradiated (parent compounds) solutions as well as for the filtered (cutoff 0.22 μm) samples from the ZWT (day 28).
Bioluminescence assay
Bacterial toxicity was further assessed with the bioluminescence assay (for details, see Bergheim et al. 2014). Briefly, aliquots of the nonirradiated (0 min) and the irradiated (128 min) samples were spotted band-wise (4 mm) on a thin-layer-chromatography (TLC) plate (10 × 10 cm) with fluorescent dye using a DESAGA AS 30 device. For solvent and positive control, 2 and 10 μL of distilled water and 3,5-dichlorophenol (conc. 50 mg/L), respectively, were spotted onto the TLC plate.
After dipping the plate into a prepared bacteria-containing suspension, the plate was directly placed below a light-sensitive camera (ST-1603ME CCD camera with 1.56 megapixel, Santa Barbara Instrument Group, Santa Barbara USA) at a distance of 30 cm measuring the luminescence for 10 min. A video-densitometric quantification method was used to evaluate the degree of inhibition of the bacterial illumination (Seigel et al. 2011).
In vitro viability and genotoxicity assays
Cell viability: WST-1 assay and NR-uptake assay
To determine the cytotoxicity of the various PTPs to human cells, the water-soluble tetrazolium (WST-1) assay and the neutral red (NR)-uptake assay were used. Both WST-1 and NR-uptake assays were performed with Hep-G2 cells (hepatocellular carcinoma). In addition, toxicity of the PTPs to HeLa cells (human cervical cancer) was also tested, but only with the NR-uptake assay.
For the WST-1 experiment, 5 × 105 Hep-G2 cells were seeded in each well of a 96-well microplate and incubated for 24 h. Subsequently, the medium was replaced with fresh medium (200 μL per well) for the control sample, with medium containing the test solution (1:10) for the test wells, with medium and distilled water (1:10) for the solvent controls, and with medium containing 0.01 % Triton X (Merck, Germany) for the positive control. The microplate was incubated under standard culture conditions for 48 h. Cells were then washed with PBS (PAA, Austria), and a 5 vol.% WST-1 solution in a phenol red-free RPMI medium (Invitrogen, Karlsruhe, Germany) was added to each well. After 1-h incubation at 37 °C, absorbance was measured at 435 nm using a microplate reader (Tecan, Crailsheim, Germany).
For the NR assay, the cells were seeded and treated as described above for the WST-1 assay. HeLa cells and Hep-G2 were seeded into each well at a density of 4 × 103 and 5 × 105 cells per well, respectively. After an exposure period of 48 h, cells were washed with PBS, and 200-μL fresh medium containing 0.5 mg/L NR solution (stock solution was prepared with 4 mg/L of NR) was added into each well. After 3-h incubation at standard culture conditions, the cells again were washed twice with 100 μL PBS, and then 200 μL of a destaining solution (ethanol 99 %, formic acid 99 %, distilled water, v/v 50:1:49) was added. After shaking the plate for 20 min at 300 rpm, absorbance was measured at 540 nm using a microplate reader (Tecan, Crailsheim, Germany).
Genotoxicity: fluorimetric detection of alkaline DNA unwinding (FADU assay)
Genotoxic effects of the PTPs were assessed by performing the FADU assay with HeLa cells as described in detail elsewhere (Bergheim et al. 2014; Debiak et al. 2011; Moreno-Villanueva et al. 2009). The cells were cultivated and treated as described above (NR-uptake assay).
In brief, genotoxic effects were identified by quantification of DNA integrity after an induced and incomplete unwinding of double-stranded DNA (dsDNA). Controlled, partial unwinding of dsDNA in the cell lysate, starting from chromosome ends and internal DNA breaks, was achieved through alkaline conditions and stopped via a neutralization step. Subsequently, Sybr-Green was added in order to quantify the remaining dsDNA through fluorescence measurement. In principle, the more DNA double- and single-strand breaks are induced by a genotoxic substance, the faster the unwinding and the less remaining dsDNA is observed.
As a positive control, cells were irradiated with X-rays (X-ray generator: CHF Müller, Germany, 70 keV energy) on ice in a 96-well plate.
Results
Biodegradability
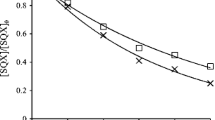
The results of the three biodegradation tests are summarized in Table 2 and exemplarily presented for linezolid in Fig. 1. All validity criteria of the OECD test guideline were met, and none of the tested compounds was found to be toxic to the inocula. Since biodegradability is related to the blind vessels and expressed as a percentage, the outcome of biodegradability results can have negative values. For visual clarity, these are set to zero in Table 2.
Biodegradability shown exemplarily for nonirradiated and irradiated linezolid, monitored as oxygen consumption in the closed bottle test (CBT) and the manometric respirometry test (MRT); also monitored as loss of dissolved organic carbon (DOC) in the Zahn-Wellens test (ZWT) over the OECD-standardized test period of 28 days
With respect to the oxygen consumption and the stringent OECD criteria, no test compounds could be classified as readily biodegradable in the CBT. However, in the MRT, the two sweeteners cyclamate and saccharine could be classified as readily biodegradable as they have exceeded 60 % biodegradation within the 10-day window. Biodegradability was additionally tested in the CBT for the irradiated samples of all compounds that were transformed into PTPs (acesulfame, linezolid, and the three fluoroquinolones). As for the parent compounds, none of these irradiated solutions could be classified as readily biodegradable (data exemplarily shown for linezolid; see Fig. 1).
In terms of DOC loss in the ZWT, none of the tested compounds were classified as inherently biodegradable.
Formation of PTPs
The variations of the DOC as well as the concentration of the parent compounds as a function of irradiation time are presented in Fig. 2.
Variations of DOC
Irradiation with the Hg lamp for 128 min led to a reduction in DOC to a level of 73 % for the sweetener acesulfame, and to 85 and 83 % for the fluoroquinolones levofloxacin and sarafloxacin, respectively, but it did not affect the DOC contents of the other compounds. Irradiation with the Xe lamp, on the other hand, did not lead to a notable decrease of the DOC contents for any of the compounds tested.
Concentrations of parent compounds
Irradiation with the Hg lamp led to a considerable decrease in the concentration of most parent compounds. The concentrations of cyclamate, lincomycin, and sucralose decreased as well, albeit to a lesser extent. Irradiation with the Xe lamp also led to a general decrease in the concentrations of the parent compounds, but the reductions were only minor except for the three fluoroquinolones (levofloxacin, marbofloxacin, sarafloxacin) and for linezolid (Fig. 2).
Absorbance spectra
Figure 3 shows the absorbance intensity of all nonirradiated and irradiated samples as a function of wavelength and during the irradiation experiments with the Hg lamp. The absorbance spectra of the solutions containing the fluoroquinolones, the sweetener acesulfame, and linezolid were clearly modified during irradiation. No changes were observed for the other compounds. For the irradiation experiments that were carried out with the Xe lamp, no variations of the absorbance spectra were found for any of the compounds except for linezolid (data not presented).
LC-UV-MS
The formation of PTPs was further investigated by means of LC-UV-MS analysis. The intensities in the extracted ion chromatograms (EIC) in terms of signal height and UV absorbencies were compared in each irradiated sample (data exemplarily shown for linezolid in Online Resource (Online Resource 1). After irradiation, newly formed signals (masses) or newly formed UV absorbencies may both represent the presence of PTPs. Such newly formed signals (masses) or newly formed UV absorbencies were found for the test compounds acesulfame, linezolid, and the three fluoroquinolones.
Toxicity against P. putida and V. fischeri
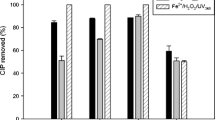
As a first screening for bacterial toxicity, all nonirradiated (parent compounds) as well as irradiated samples from the experiments with the Hg-light source were tested using the widespread bacterial species P. putida. Additionally, and for linezolid only, the irradiated samples from the experiments using the Xe lamp were also tested. The screening tests were undertaken at the relatively high concentration of 8 mg/L. Figure 4 shows the bacterial toxicity as growth inhibition of P. putida.
Bacterial toxicity of all nonirradiated (black) and irradiated samples (light gray, 128 min, dark gray, 8 min for sarafloxacin, 128 min for linezolid (Xe lamp)) using the Hg lamp, relative to solvent control (sterile-filtered ultrapure water) and monitored as growth inhibition of P. putida following 16-h exposure. Positive control (+): 3,5-dichlorophenole (40 mg/L). Each bar represents the mean ± SEM of at least three independent experiments; *p < 0.05; **p < 0.01 v. nonirradiated sample (student’s t test)
When comparing nonirradiated and irradiated samples, toxicity was significantly lower in the 128-min irradiated samples of marbofloxacin and sarafloxacin (p < 0.01). A considerable decrease in toxicity was also found for levofloxacin. On the other hand, an increase in toxicity after irradiation was observed for the compound linezolid, but only for the sample irradiated with the Hg lamp.
Furthermore, the growth inhibition test with P. putida was also used for an evaluation of antimicrobial activity of samples from the ZWT (day 28). The antibacterial activity as growth inhibition of P. putida was tested for the three fluoroquinolones (Fig. 5). None of the fluoroquinolones had lost their antibacterial activity after incubation with inocula in the ZWT and after a test period of 28 days.
Bacterial toxicity of solutions of parent compounds (black) and the 28-day samples from the Zahn-Wellens test (gray) relative to solvent control (sterile-filtered ultrapure water) and monitored as growth inhibition of P. putida following 16-h exposure. Positive control (+): 3,5-dichlorophenole (40 mg/L). Each bar represents the mean ± SEM of four independent experiments
Further evaluation of bacterial toxicity was undertaken by using V. fischeri bioluminescence bacteria. Toxicity was tested for all compounds that were not biodegradable and for all compounds that were considerably transformed into PTPs during irradiation. Results are shown in Fig. 6, where inhibition of bioluminescence is plotted against concentration (6 and 0.4 mg/L due to limited solubility) and for different irradiation times. Toxicity was neither found for the nonirradiated nor irradiated samples of the tested compounds.
Bacterial toxicity of all nonirradiated (black) and irradiated samples (light gray, 128 min, dark gray, 8 and 16 min for marbofloxacin and sarafloxacin) using the Hg lamp and for linezolid also the Xe lamp (dark gray, 128 min), and monitored as inhibition of V. fischeri bioluminescence. Solvent control (−): sterile-filtered ultrapure wate; positive control (+): 3,5-dichlorophenole (0.1 μg). Each bar represents the mean ± SEM of at least four independent experiments
Cell viability and DNA integrity of HepG-2 and HeLa cells
Toxicity was further investigated for the same irradiation endpoints by means of two cell viability tests (WST-1 and NR assays) and one genotoxicity test (FADU assay) using human Hep-G2 and/ or HeLa cells. The cell viability and DNA integrity of Hep-G2 and/ or HeLa cells were not affected by any of the sweetener or antibiotic compounds tested. Comparing nonirradiated and irradiated samples, toxicity was neither enhanced nor reduced in the environmentally relevant concentrations tested. Figure 7 shows an exemplary result for linezolid.
Cell viability of HepG-2 cells and DNA-integrity of HeLa cells of the nonirradiated (black) and the irradiated samples (light gray, 128 min) of linezolid using the Hg lamp; monitored by the water-soluble tetrazolium (WST-1) and the neutral red (NR)-uptake assay (following 48-h exposure) and the fluorimetric detection of alkaline DNA unwinding (FADU) assay (following 1- and 24-h exposure). Solvent control (−): sterile-filtered ultrapure water; positive control for the cell viability assays (+): triton X-100 (0.01 %). Positive controls for FADU assay (data not shown): irradiation from 0.5, 1, 1.5, 2, 3, and 4 Gy. *p < 0.05; **p < 0.01; ***p < 0.001 v. nonirradiated sample (one-way ANNOVA test with Dunnet posttreatment). Each bar represents the mean ± SEM of at three independent experiments
Discussion
In this study, as a first step, the persistence from emerging aquatic micropollutants was evaluated with three biodegradation tests in order to assess their environmental relevance. The compounds identified here as persistent were further evaluated in more detail with two irradiation experiments in order to test for the possible formation of PTPs. Subsequently, toxicity was assessed in a series of ecotoxicity and human toxicity tests.
Biodegradation
Biodegradability is a key parameter in conventional environmental risk analysis. Therefore, this study focuses on biodegradability of the test compounds by applying three widely accepted and standardized OECD-biodegradability tests. The results demonstrate a relatively high persistence of the tested artificial sweeteners and antibiotics. Accordingly, these compounds may accumulate in the aquatic environment. Furthermore, persistent antibiotic compounds may contribute to the development of bacterial resistance, a feature that has already been described in various environmental compartments (Kümmerer 2009).
Surprisingly, the two sweeteners cyclamate and saccharine could be classified as readily biodegradable although they have been detected in different aquatic media (Van Stempvoort et al. 2011; Scheurer et al. 2009; Buerge et al. 2009). This apparent persistence, or pseudopersistence, clearly demonstrates that cyclamate and saccharine are discharged via wastewater at remarkably high quantities. This finding implies that degradation tests should be accompanied by monitoring programs and by measurement of the actual concentration of these compounds in the aquatic environment in order to accurately predict the fate of chemical compounds in environmental compartments.
Microbial metabolism is a major process in the environment, which is involved in the degradation of not only parent compounds but also PTPs. In order to assess the environmental relevance of PTPs, we therefore also tested the biodegradability of the PTPs and found that none of the studied PTPs were readily biodegradable. As already suggested for the parent compounds, these persistent PTPs may also accumulate in the environment, and ecotoxicological concerns may arise.
Phototransformation
In addition to biological degradation, we also studied abiotic transformation via photoinduced processes. In these photodegradation experiments, we were able to generate PTPs that were subsequently subjected to different in vitro toxicity tests to assess their environmental relevance.
In two different test series and with two different light sources, we were able to document that PTPs can be formed by UV light (Hg lamp) and by simulated sunlight (Xe lamp). However, the extent of PTP formation was very different depending on the light source. Generation of PTPs was pronounced upon irradiation with the Hg lamp, whereas in terms of sunlight irradiation, PTPs were only formed to a lesser extent.
Combining the results on DOC reduction with those of parent compound elimination, our findings reveal that PTPs have been formed especially during irradiation with the Hg lamp and in the solutions containing the test compounds acesulfame, saccharine, linezolid, and the three fluoroquinolones. Here, the distinct reduction of the parent compounds and the variations of the absorbance spectra (except for saccharine) as well as the constant DOC values strongly indicate the formation of PTPs, consistent with the results obtained through the LC-UV-MS analysis. In contrast, reduction of the parent compound concentration and variations of the absorbance spectra are much less pronounced for all other compounds, or when exposed to the Xe light source, implying that PTPs were formed to a lesser extent. However, for linezolid only, the formation of PTPs became also apparent when exposed to the Xe lamp.
In agreement with the results presented here, photoinduced transformation has already been described for linezolid and acesulfame (Agrawal et al. 2003; Fasani et al. 2008; Coiffard et al. 1999). Furthermore, Fasani et al. (2008) expected linezolid as well as all drugs bearing an aminofluorophenyl substituent to be photolabile and possibly phototoxic in a similar manner to fluoroquionolones. In the case of the antibacterial class of fluoroquinolones, photodegradation processes have also been described previously (e.g., Garcia-Käufer et al. 2012; Ge et al. 2010; Prabhakaran et al. 2009; Sturini et al. 2012). These authors, however, did not study the environmental relevance nor the (eco)toxicity of the PTPs.
Toxicity
The reported relatively low concentrations in the environment of the chemical compounds tested here are unlikely to cause acute toxic effects in aquatic organisms. However, it has not yet been reported whether or not toxic effects on aquatic life or human beings may arise, or even may be enhanced, as a result of photoinduced transformation. We therefore initiated first studies on the toxicity of the observed PTPs using different in vitro screening assays with two human cell lines and two bacterial toxicity tests with two different bacterial strains. V. fischeri bacteria are commonly used in monitoring and ecotoxicity studies (Seigel et al. 2011); however, as a marine bacterium, they are not common in surface waters. Therefore, we also performed the growth inhibition test with P. putida, which are common bacteria in different environmental compartments.
The results of these tests showed that UV irradiation of the three fluoroquinolones led to a reduction of the toxic effect on P. putida. Their antibiotic activity was significantly reduced after high-energetic UV irradiation, and thus, the use of UV irradiation as a technology in water purification seems promising for these pharmaceuticals.
However, we found that the PTPs created by UV irradiation of linezolid are more toxic to P. putida than the parent compound. This is surprising since it is known that linezolid possesses a significant activity against gram-positive pathogens, but not against gram-negative bacteria, such as pseudomonads. Marchese and Schito (2001) assume that membrane-located efflux pumps in gram-negative bacteria may effectively transport active compounds out of the cell. We assume that photoinduced transformation of linezolid resulted in PTPs that somehow overcome this efflux effect. Further mechanistic studies are necessary to understand these toxifying reactions.
In conclusion, UV irradiation is not always effective to completely degrade or transform hazardous micropollutants into harmless compounds. Our study demonstrates that irradiation can either reduce or enhance toxicity of the irradiated compounds.
Up to now, environmental risks due to formation of toxic PTPs through irradiation of linezolid are probably low because the environmental concentrations are still expected to be low. Linezolid is yet prescribed in individual cases only and is kept as a reserve antibiotic. Furthermore, as yet, we were unable to identify a risk to humans when performing in vitro toxicity tests with human cell lines. Once vancomycin resistance is increasing and the antibiotic is more routinely prescribed, however, higher environmental concentrations are very likely. We therefore recommend to monitor and identify the occurrence and toxicity of linezolid and its TPs in an early stage.
In the present study, we could further demonstrate that the inocula of STPs were not able to reduce the antibiotic activity of any of the tested fluoroquinolones. Our findings make it obvious that, apart from abiotic sorption to sludge (Li and Zhang 2010), STPs are not capable of biotically reducing the antibiotic activity of fluoroquinolones. To overcome bacterial resistance in the aquatic environment, new technologies or less persistent antibiotics are therefore strongly needed.
The ecotoxicity test with V. fisheri as well as the in vitro studies with human cell lines did not reveal an increased or reduced toxicity after irradiation. To our knowledge, there has been no previous study dealing with the toxicity of the PTPs from the compounds tested in this study.
In view of the large amounts of chemicals used, it can be expected that various TPs will be formed in the aquatic environment. Nevertheless, extensive and costly analyses cannot be conducted for all compounds and for all TPs. Screening tests on toxicity help to prioritize PTPs that have a higher environmental risk potential. Based on our finding, we therefore recommend further studies on the formation of PTPs as well as more detailed toxicity and/or mechanistic studies.
Summary and conclusions
-
Only two of the tested compounds (cyclamate and saccharine) could be classified as readily biodegradable in the MRT. All other compounds were neither classified as readily biodegradable nor inherently biodegradable (ZWT). Hence, micropollutants like the antibiotic class of fluoroqinolones as well as sweeteners can be very persistent to biological degradation and therefore may accumulate in the aquatic environment.
-
During 128-min irradiation with an Hg lamp, the formation of PTPs was particularly pronounced for the compounds acesulfame, levofloxacin, linezolid, marbofloxacin, and sarafloxacin. During 128-min irradiation with a Xe lamp, PTPs were generated to a significant extent only from linezolid. It can be concluded that PTPs are likely to be formed from poorly biodegradable compounds during high-energy UV-water treatment processes and, to a lesser extent, also by sunlight.
-
Bacterial toxicity was not significantly increased for any of the irradiated samples in comparison to their respective nonirradiated samples. Only for linezolid, bacterial toxicity to P. putida was slightly enhanced after irradiation with the Hg lamp. However, bacterial toxicity was reduced through irradiation for the three fluoroquinolones (levofloacin, marbofloxacin, sarafloxacin). Hence, in this study, UV treatment has shown to reduce antimicrobial activity of all fluoroquinolones, whereas UV treatment of linezolid solutions has resulted in a higher toxicity to P. putida. Water treatment with UV may therefore help to reduce antimicrobial resistance, but does not transform all micropollutants into harmless compounds.
-
Antibacterial activity of the three fluoroquinolones (levofloacin, marbofloxacin, sarafloxacin) did not change after 28-day exposure to inocula in the ZWT, and thus, biological treatment in a conventional sewage treatment plant is not capable in reducing antimicrobial activity of the fluoroquinolones tested.
-
None of the nonirradiated or irradiated samples were found to be cytotoxic or genotoxic to human Hep-G2- and/or HeLa-cells. Thus and as yet, we could not identify a risk for humans.
References
Agrawal H, Mahadik KR, Paradkar AR, Kaul N (2003) Stability indicating HPTLC determination of linezolid as bulk drug and in pharmaceutical dosage form. Drug Dev Ind Pharm 29:1119–1126
Bergheim M, Gieré R, Kümmerer K (2012) Biodegradability and ecotoxicitiy of tramadol, ranitidine, and their photoderivatives in the aquatic environment. Env Sci Pol Res 19:72–85
Bergheim M, Gminski R, Spangenberg B, Debiak M, Bürkle A, Mersch-Sundermann V, Kümmerer K, Gieré R (2014) Recalcitrant pharmaceuticals in the aquatic environment: a comparative screening study on their occurrence, formation of phototransformation products and their in-vitro toxicity. Environ Chem 11:431–444
Buerge IJ, Buser HR, Kahle M, Muller MD, Poiger (2009) Ubiquitous occurrence of the artificial sweetener acesulfame in the aquatic environment: an ideal chemical marker of domestic wastewater in groundwater. Environ Sci Technol 43:4381–4385
Calamari D, Zuccato E, Castiglioni S, Bagnati R, Fanelli R (2003) Strategic survey of therapeutic drugs in the rivers Po and Lambro in northern Italy. Environ Sci Technol 37:1241–1248
Castiglioni S, Fanelli R, Calamari D, Bagnati R, Zuccato E (2004) Methodological approaches for studying pharmaceuticals in the environment by comparing predicted and measured concentrations in River Po, Italy. Regul Toxicol Pharm 39:25–32
Coiffard CAC, Coiffard LJM, de Roeck-Holtzhauer YMR (1999) Photodegradation kinetics of acesulfame-K solutions under UV light: effect of pH. Z Lebensm Unters Forsch A 208:6–9
Debiak M, Panas A, Steinritz D, Kehe K, Bürkle A (2011) Highthroughput analysis of DNA interstrand crosslinks in human peripheral blood mononuclear cells by automated reverse FADU assay. Toxicology 280:53–60
Fasani E, Tilocca F, Protti S, Merli D, Albini A (2008) An exploratory and mechanistic study of the defluorination of an (aminofluorophenyl)oxazolidone: SN1(Ar*) vs. SR+N1(Ar*) mechanism. Org Biomol Chem 6:4634–4642
Fatta-Kassinos D, Vasquez MI, Kümmerer K (2011) Transformation products of pharmaceuticals in surface waters and wastewater formed during photolysis and advanced oxidation processes - Degradation, elucidation of byproducts and assessment of their biological potency. Chemosphere 85:693–709
Garcia-Käufer M, Haddad T, Bergheim M, Gminski R, Gupta P, Mathur N, Kümmerer K, Mersch-Sundermann V (2012) Genotoxic effect of ciprofloxacin during photolytic decomposition monitored by the in vitro micronucleus test (MNvit) in HepG2 cells. Environ Sci Pollut Res 19:1719–1727
Ge L, Chen J, Wei X, Zhang S, Qiao X, Cai X, Xie Q (2010) Aquatic photochemistry of fluoroquinolone antibiotics: kinetics, pathways, and multivariate effects of main water constituents. Environ Sci Technol 44:2400–2405
Grice HC, Goldsmith LA (2000) Sucralose - an overview of the toxicity data. Food Chem Toxicol 38:S1–S6
Hjorth M, Hansen JH, Camus L (2010) Short-term effects of sucarlose on Calanus finmarchicus and Calanus glacialis in Disko Bay, Greenland. Chem Ecol 26:385–393
Huggett DB, Stoddard KI (2011) Effects of the artificial sweetener sucralose on Daphnia magna and Americamysis bahia survival, growth and reproduction. Food Chem Toxicol 49:2575–2579
Jia A, Wan Y, Xiao Y, Hu JY (2012) Occurrence and fate of quinolone and fluoroquinolone antibiotics in a municipal sewage treatment plant. Water Res 46:387–394
Kemper N (2008) Veterinary antibiotics in the aquatic and terrestrial Environment. Ecol Indic 8:1–13
Khetan SK, Collins TJ (2007) Human pharmaceuticals in the aquatic environment: a challenge to green chemistry. Chem Rev 107:2319–2364
Kim J-W, Jan H-S, Kim J-G, Ishibashi H, Hirano M, Nasu K, Ichikawa N, Takao Y, Shinohara R, Arizono K (2009) Occurence of pharmaceutical and personal care products (PPCPs) in surface water from Mankyung River, South Korea. J Health Sci 55:249–258
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36:1202–1211
Kümmerer K (2009) Antibiotics in the environment – a review – part II. Chemosphere 75:417–434
Li B, Zhang T (2010) Biodegradation and adsorption of antibiotics in the activated sludge process. Environ Sci Technol 44:3468–3473
Lin AYC, Yu TH, Lin CF (2008) Pharmaceutical contamination in residential, industrial, and agricultural waste streams: risk to aqueous environments in Taiwan. Chemosphere 74:131–141
Lubick N (2008) Artificial sweetener persists in the environment. Environ Sci Technol 42:3125
Marchese A, Schito GC (2001) The oxazolidinones as a new family of antimicrobial agent. Clin Microbiol Infect 7:66–74
Mawhinney DB, Young RB, Vanderford BJ, Borch T, Snyder SA (2011) Artificial sweetener sucralose in U.S. drinking water systems. Environ Sci Technol 45:8716–8722
Mead RN, Morgan JB, Avery GB, Kieber RJ, Kirk AM, Skrabal SA, Willey JD (2009) Occurrence of the artificial sweetener sucralose in coastal and marine waters of the United States. Mar Chem 116:13–17
Michael I, Rizzo L, McArdell CS, Manaia CM, Merlin C, Schwartz T, Dagot T, Fatta-Kassinos D (2013) Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: a review. Water Res 47:957–995
Moreno-Villanueva M, Pfeiffer R, Sindlinger T, Leake A, Müller M, Kirkwood T B, Bü̈rkle A (2009) A modified and automated version of the ‘fluorimetric detection of alkaline DNA unwinding’ method to quantify formation and repair of DNA strand breaks. BMC Biotechnol 9:1–9
Oppenheimer JA, Badruzzaman M, Jacangelo JG (2012) Differentiating sources of anthropogenic loading to impaired water bodies utilizing ratios of sucralose and other microconstituents. Water Res 46:5904–5916
Prabhakaran D, Sukul P, Lamshoft M, Maheswari MA, Zuhlke S, Spiteller M (2009) Photolysis of difloxacin and sarafloxacin in aqueous systems. Chemosphere 77:739–746
Püttmann W, Keil F, Oehlmann J, Schulte-Oehlmann U (2008) Wassertechnische Strategien zur Reduzierung der Trinkwasserbelastung durch Arzneimittelwirkstoffe. Umweltwissenschaften Schadstoff-Forschung 20:209–226
Rodero AB, Rodero LS, Azoubel R (2009) Toxicity of sucralose in humans: a review. Int J Morphol 27:239–244. URL:www.scielo.cl/pdf/ijmorphol/v27n1/art40.pdf
Scheurer M, Brauch HJ, Lange FT (2009) Analysis and occurrence of seven artificial sweeteners in German waste water and surface water and in soil aquifer treatment (SAT). Anal Bioanal Chem 394:1585–1594
Seigel A, Schroeck A, Hauser R, Spangenberg B (2011) Sensitive quantification of diclofenac and ibuprofen using thin layer chromatography coupled with A vibrio fisheri bioluminescence assay. J Liq Chrom Relat Tech 34:817–828
Soh, Connors KA, Brooks BW, Zimmerman J (2011) Fate of sucralose through environmental and water treatment processes and impact on plant indicator species. Environ Sci Technol 45:1363–1369
Sturini M, Speltini A, Maraschi F, Profumo A, Pretali L, Fasani E, Albini A (2012) Sunlight-induced degradation of soil-adsorbed veterinary antimicrobials Marbofloxacin and Enrofloxacin. Chemosphere 86:130–137
Sturini M, Speltin A, Pretali L, Fasani E, Profumo A (2009) Solid-phase ectraction and HPLC determination of fluoroquinolones in suface waters. J Sep Sci 32:2020–3028
Sukul P, Spiteller M (2007) Fluoroquinolone antibiotics in the environment. Rev Environ Contam Toxicol 191:131–162
Van Stempvoort DR, Roy JW, Brown SJ, Bickerton G (2011) Artificial sweeteners as potential tracers in groundwater in urban environments. J Hydrol 401:126–133
Watkinson A, Murby E, Costanzo S (2007) Removal of antibiotics in conventional and advanced wastewater treatment: implications for environmental discharge and wastewater recycling. Water Res 41:4164–4176
Weihrauch MR, Diehl V (2004) Artificial sweeteners - do they bear a carcinogenic risk? Ann Oncol 15:1460–1465
Wiklund AKE, Breitholtz M, Bengtsson BE, Dolfsson-Erici M (2012) Sucralose - an ecotoxicological challenger? Chemosphere 86:50–55
Yan S, Song W (2014) Photo-transformation of pharmaceutically active compounds in the aqueous environment: a review. Environ Sci Process Impacts 16:697–720
Acknowledgments
Marlies Bergheim wishes to thank the German Environment Foundation (DBU), Stiftung Viamedica (Director: Prof. Daschner), the Frankfurter Allgemeine Zeitung (FAZ), and the Vereinte Studienstiftung of the University of Freiburg for providing financial support through scholarships. We are grateful to two anonymous reviewers whose comments and suggestions helped improving this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Roland Kallenborn
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1S
Total ion chromatograms (TICs), extracted ion chromatograms (EICs) (m/z 338, m/z 267, m/z 289, m/z 294, m/z 316, m/z 372, m/z 350) and UV spectra (275 nm) of linezolid from test samples at 0 min, 4 min and 128 min irradiation with a Hg lamp (left) and a Xe lamp (right) (PPT 536 kb)
Rights and permissions
About this article
Cite this article
Bergheim, M., Gminski, R., Spangenberg, B. et al. Antibiotics and sweeteners in the aquatic environment: biodegradability, formation of phototransformation products, and in vitro toxicity. Environ Sci Pollut Res 22, 18017–18030 (2015). https://doi.org/10.1007/s11356-015-4831-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4831-x