Abstract
Foliar ionome, photosystem II activity, and leaf growth parameters of Ranunculus acris L., a potential biomonitor of trace element (TE) contamination and phytoavailability, were assessed using two riverbank soil series. R. acris was cultivated on two potted soil series obtained by mixing a TE (Cd, Cu, Pb, and Zn)-contaminated technosol with either an uncontaminated sandy riverbank soil (A) or a silty clay one slightly contaminated by TE (B). Trace elements concentrations in the soil-pore water and the leaves, leaf dry weight (DW) yield, total leaf area (TLA), specific leaf area (SLA), and photosystem II activity were measured for both soil series after a 50-day growth period. As soil contamination increased, changes in soluble TE concentrations depended on soil texture. Increase in total soil TE did not affect the leaf DW yield, the TLA, the SLA, and the photosystem II activity of R. acris over the 50-day exposure. The foliar ionome did not reflect the total and soluble TE concentrations in both soil series. Foliar ionome of R. acris was only effective to biomonitor total and soluble soil Na concentrations in both soil series and total and soluble soil Mo concentrations in the soil series B.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Worldwide, some 22 million hectares of land have been degraded by contamination, and case studies arise everywhere from increasing industrialization, urbanization, and lax environmental regulations or their lack of respect (GACGC 1995; Evangelou et al. 2012). In the European Union, on average, about 4.2 potentially contaminating sites are estimated to exist per 1,000 inhabitants and about 5.7 contaminated sites per 10,000 inhabitants (EEA 2014). A tentative extrapolation to cover 39 European countries results in an estimated 2.5 million potentially contaminated sites of which about 14 % (340 000 sites) is estimated to be contaminated and in need of remediation measures. Trace elements (TE) are of concern at 35 % of these sites (EEA 2014). Increases in TE (here, essential and non-essential metal(loid)s with common concentrations in plant shoots below 100 mg kg−1 dry weight, DW; Adriano 2001) in soils over time is mainly due to anthropogenic activities in both urban and agricultural soils (Burt et al. 2014; Capra et al. 2014; Washa et al. 2014). Cadmium and Zinc often deserve special emphasis due to their mobility in soils and their presence in many pollutant linkages (Defra 2012); indeed, there are many sources and pathways that lead to an increase of Cd and Zn in soils, which can result in risks for water resources or human health (Beesley et al. 2010; Bearup et al. 2014; Li et al. 2014; Rashti et al. 2014). Briefly, Cd inputs to agricultural soils are predominantly through aerial depositions, and applications of P-fertilizers, soil amendments, manure, and sewage sludges (McLaughlin and Singh 1999). Anthropogenic soil Zn contamination mainly results from short- and long-range industrial and urban emissions to the atmosphere (e.g., burning of coal and oil, waste incineration, industrial processes including non-ferrous metal smelting), and (long-term) applications of P- and Zn-fertilizers, sewage sludges, some livestock manures, agrochemicals, and industrial waste products (Alloway 2008; Belon et al. 2012). Consequently, many topsoils display higher TE (Cd, Zn, etc.) concentrations than background levels for their soil type, e.g., median values range from 9 to 131 mg Zn and from 0.06 to 0.77 mg Cd kg−1 soil DW in French agricultural soil series (Baize et al. 2007; GIS sol 2009). Floodplain and riverbank soils are among sites for dynamic biogeochemical research as they largely control pollutant storage and release, having interfaces with the atmosphere, soils, ground-, and surface waters (Barth et al. 2009). Riverbank soils often showed water and soil TE contaminations downstream efflux of water treatment plants (Vystavna et al. 2012; Marchand et al. 2014).
Physico-chemical analysis does not always provide evidence of the biological actions of soil TE contamination (Zhou et al. 2008). In addition, while providing relevant information on geochemistry and fractionation of TE in the soil profile, soil manipulation can disturb soil structure, enhances the surface area exposed to extractants, and may distance the data interpretation from field conditions (Beesley et al. 2010; Moreno-Jiménez et al. 2011). Biomonitoring and bioassays, notably with plants and stress enzyme activities (e.g., enzyme activities involved in plant responses to stress caused by exposure to TE in excess), complement the physico-chemical analysis of environmental matrices, accounting for the subtle biological changes in organisms affected by exogenous contaminants (Mench et al. 2000; Markert 2007; Marchand et al. 2011; Kolbas et al. 2013; Kumpiene et al. 2014). Plant morphological traits are generally considered poorly sensitive to fully assess potential phytotoxicity in moderately TE-contaminated soils (Meers et al. 2006), while one additional method would be to measure the photosynthetic performance (Cambrollé et al. 2012). Measuring the yield of photosystem II (PSII) chlorophyll fluorescence provides insights into the extent to which environmental stresses have damaged the photosynthetic apparatus of a plant (Maxwell and Johnson 2000).
The use of root-emerged macrophytes to biomonitor total and soluble TE concentrations in riverbank soils is under investigations at several sites such as the Jalle d’Eysines River, a Garonne tributary, SW of France (Bonanno 2013; Marchand et al. 2014; Delmail 2014). Total metal concentrations in the Jalle d’Eysines riverbank soil series were monitored by foliar metal concentrations: for Mo in Phragmites australis (Cav.) Trin. Ex Steud and Phalaris arundinacea L., for Cd in P. australis and Carex riparia Ehrh. (Marchand et al. 2014). Copper and Mo concentrations in the soil-pore water can be monitored by respectively P. arundinacea and P. australis.
Ranunculus acris L. (R. acris or meadow buttercup, Ranunculaceae) is a weed of old pasture and hay meadows, widely distributed throughout Europe and considered as a common plant species of the agricultural landscape (Schmitz et al. 2013; Bourdot et al. 2013). It is one of the most common species along the Jalle d’Eysines River course, where it was used to monitor total Ni concentration in soils, showing also a site-dependent foliar ionome (Marchand et al. 2014). It was not clear why R. acris was biomonitoring Ni and not Cd and Zn, which have a speciation in the soil controlled by similar factors, i.e., pH, content, and type of soluble organic matter, hydrous metal oxides and clay, presence of organic and inorganic ligands, and competition from other metal ions.
In addition to the Jalle d’Eysines river course, R. acris was present at the TE-contaminated Fresnes sur Escaut site, north of France. Therefore, this pot experiment aimed at appraising the photosystem II activity and phenotypic responses of the meadow buttercup to increasing soil TE contamination, with special attention to Zn and Cd, in two soil series differing by their soil texture. They were made using the fading technique with either a sandy loam (A) or silty clay (B) riverbank soil from the Jalle d’Eysines River, SW France, and a TE-contaminated technosol (F) developed over dredged sediments of the Escaut River channel, N France. Morphological traits, foliar ionome, and photosystem II activity of R. acris were compared on both soil series. In the general context of TE transfer in the soil-plant continuum, the relationships between total element concentration in the riverbank soil, the soluble element concentration, the foliar ionome of a rooted macrophyte, in this case R. acris, and its trait responses were monitored. Based on such relationships, the use of R. acris for biomonitoring TE concentrations in riverbank soils and their soil-pore water was questioned.
Material and methods
Soil preparation and analysis
The TE-contaminated technosol F (FAO World Reference Base for Soil Resources, thereafter referred to soil F) was sampled (0–25 cm soil layer) at the PHYTOSED Scale 1 platform (1 ha, Fresnes-sur-Escaut, France) in February 2012. It has developed over dredged sediments of the Escaut River channel deposited at this landfill site over the last century (Bert et al. 2012). Pseudo-total element concentrations in the soil F (aqua regia extraction) are reported in Table 1. For the soil F, emergence of Brassica rapa L. (ISO 11269–2:2005) was reduced to 80 % and its growth by 10 % after 18 days, compared to an uncontaminated VDLUFA (Association of German Agricultural Analytic and Research Institutes) soil. In the ISO 17512-1 (2008) test with Eisenia fetida, earthworms were, however, more attracted (18 %) by the soil F compared to the ISO soil. In the ISO 10872:2010 test with Caenorhabditis elegans Maupas, the nematode reproduction was inhibited by 71.5 % compared to the VDLUFA soil (Bert et al. 2012).
Riverbank soils used to make the soil series, displaying lower total soil TE concentrations (e.g., 16–274 mg Zn and 0.09–1.6 mg Cd kg−1 DW soil) were sampled (0–25 cm soil layer) in February 2012 at two sites, i.e., A and B corresponding to the sandy soil #2 and clay soil #4 in Marchand et al. (2014), along the Jalle d’Eysines River, a tributary of the Garonne River located in southwest France (44° 53′ 36″ N, 00° 40′ 40″ O), north of Bordeaux. From its source to its confluence with the Garonne River, the Jalle d’Eysines River is 32-km long. Water depth varies from 0.8 to 2.5 m annually and average flow volume is 3 m3 s−1. Sites A and B were, respectively, located at 20 and 30 km from the river’s source. Soil texture is sandy at site A with low clay proportion while the soil B contains more clay than sand (Table 2, Marchand et al. 2014).
Details on the geographic position of Escault and Jalle d’Eysines Rivers and the location of sampling points (A, B, and F) are presented in supplementary material (S1).
Soils A, B, and F were air-dried and sieved at 5 mm prior to their use with the fading technique. It consists in mixing contaminated and uncontaminated soils, preferably of the same type, in various proportions to obtain a realistic contaminated soil series. Here, the soil series displayed a wide range of metal (Cd, Cu, Pb, and Zn) exposures, well distributed, allowing to gain dose-effect relationships, upper critical TE concentrations for plant parameters, and effective TE concentrations in the soil-pore water. This technique allows to better model the field soil conditions than hydroponics (Japenga et al. 2007). The A soil series was made by carefully mixing (run-over-run) the soil F with the soil A in a ratio from 0:100 to 100:0 % with a 20 % gradual step. Soil samples (1 kg DW) were placed in plastic pots (1.3 L) to prepare six treatments (four pots/treatment) labeled from A (0 %) to F (100 %), the percentage between brackets being the soil F proportion. Similarly, the B soil series was made by mixing soils F and B. Soils were watered twice a week between February and April 2012 with deionized water and maintained at 70 % of field capacity (water holding capacity, 10 % of air-dried soil mass). In week 10, one Rhizon MOM moisture sampler (Eijkelkamp, The Netherlands) was inserted with a 45° angle into each potted soil. For all soils, soil-pore waters were collected (three times 10 mL during weeks 16 and 17 to make a 30-mL sample) and kept at 4 °C prior to TE analysis by ICP-AES (Varian liberty 200, Germany) and ICP-MS (Thermo X series 200, USA) at the INRA USRAVE laboratory (Villenave d’Ornon, France). Trace element concentrations in soil-pore water along the Jalle d’Eysines River and at the PHYTOSED Scale 1 platform were detailed by Marchand et al. (2014) and Kidd et al. (2014), respectively. The soil-soil-pore water partition coefficients [KdTE (L kg−1) = total soil TE concentration (mg kg−1)/TE concentration in the soil-pore water (mg L−1)] were calculated for three (Zn, Cd, and Cu) out of the four TE which exceeded both the French guideline values for dredged-sediment management and the inquiry threshold values for French soils on the soil series A and B. The Kd values were not calculated for Pb since its concentrations in the soil-pore water remained below the detection limit (<0.8 μg L−1).
Plant material
In June 2011, 50 individuals of meadow buttercup (R. acris L.) were sampled along the Jalle d’Eysines River. Plant samples were kept separate in buckets, standardized (similar belowground and aboveground volumes), and then placed in water in a greenhouse at the Centre INRA-Bordeaux Aquitaine (Villenave d’Ornon, France). Individuals were thereafter grown in separate polyethylene containers (volume, 60 × 40 × 15 cm3) containing perlite soaked with a quarter-strength Hoagland nutrient solution (HNS, Hoagland and Arnon, 1950): KNO3 (1.62 mM), Ca(NO3)2 (0.69 mM), NH4H2PO4 (0.25 mM), MgSO4 (0.5 mM), H3BO3 (11.53 μM), MnCl2 (2.29 μM), CuSO4·5H2O (0.08 μM), (NH4)6Mo7O24 (0.13 μM), ZnSO4·7H2O (0.19 μM) and FeSO4 (48.6 μM). Water volume was maintained constant by adding tap water. Water was renewed and nutrients were added every month during the growing season and every 2 months during winter to avoid anoxia and nutrient depletion in the growth medium. In March 2012 (week 10), one standardized plant (3–5 leaves) was transplanted into each potted soil. All pots were randomly placed on a bench in the same greenhouse during 7 weeks [day (9–21 h) 1911 ± 1232 μM photons m−2 s−1, 28 ± 5 °C, night (21–9 h) 19 ± 3 °C].
Chlorophyll fluorescence
Chlorophyll fluorescence was measured once a week for all plants in a fully developed and randomly selected leaf (n = 4 replicates for all soils) using a portable modulated fluorometer (Pam-2500 Waltz, Germany) during the 7-week growth period.
In the morning, in a dark chamber, the minimal fluorescence level in the dark-adapted state (F 0) was measured using a modulated pulse (<0.05 μmol m−2 s−1 for 1.8 μs) which was too small to induce significant physiological changes in the plant. Maximal fluorescence in this state (F m ) was measured after applying a saturating actinic light pulse of 15,000 μmol m−2 s−1 for 0.7 s. The value of F m was recorded as the highest average of two consecutive points. Values of variable fluorescence (F v = F m − F0) and maximum quantum efficiency of PSII photochemistry (F v /F m ) were calculated from F 0 and F m . This ratio of variable to maximal fluorescence correlates with the number of functional PSII reaction centers, and dark-adapted values of F v /F m can be used to quantify photoinhibition (Cambrollé et al. 2012).
Growth parameters
In week 17, after measuring the survival rate (S2), plants were harvested. The total leaf area (TLA, cm2plant−1) of each individual was immediately measured by digital image analysis (Epson Expression 10000XL, WINFOLIA software, Regent Instrument, Canada). Leaf samples were then carefully washed with tap water, rinsed with deionized water, blotted with filter paper, placed in paper bags, and oven-dried for 48 h at 55 °C to a constant weight. In week 18, the leaf dry weight (DW) yield (g plant−1) was measured [n = 4 replicates for all soils except for B (0 %): n = 1 survivor plant and B (80 %): n = 2 survivor plants, due to powdery mildew]. The specific leaf area (SLA, cm2 g−1) was calculated as the ratio of the total leaf area (TLA) over the leaf DW yield.
Mineral analysis
Dried leaf samples were ground (<1.0-mm particle size, Retsch MM200) and stored in plastic containers (100 mL) at room temperature, in dark conditions. For all soil treatments, four leaf aliquots (0.5 g DW) from individual replicates were wet digested under microwaves (180 °C, CEM Marsexpress, USA) with 5 mL supra-pure 14 M HNO3, 2 mL 30 % (v/v) H2O2 not stabilized by phosphates, and 1 mL Milli-Q water. Certified reference material (BIPEA maize V463) and blank reagents were included in all series. Macroelements and TE concentrations in solutions were then determined by ICP-AES (Varian liberty 200) and ICP-MS (Thermo X series 200) at the INRA USRAVE laboratory [n = 3 out of four replicates, randomly selected, except n = 2 for B (80 %)]. All elements were recovered (>95 %) according to the standard values and the standard deviation for replicates (n = 3) was <5 %. All element concentrations in plant and soil samples are presented on DW basis.
Statistical analysis
The influence of sampling sites A and B on the main soil characteristics and the total TE concentrations in riverbank soils were tested using a Student’s T test. The dilution rate effect on the foliar ionome of R. acris was assessed with a one-way ANOVA for both the soil series A and B. A principal component analysis (PCA) was conducted on soil-pore waters and a second one on the foliar ionome of R. acris after a 50-day exposure to the soil series A and B. Pearson correlation coefficients between soil TE concentrations, soil-pore water TE concentrations, and foliar ionome of R. acris grown on the soil series A and B were calculated. The x,y data sets were curve-fitted by the Excel software (Microsoft, USA) for the Kd values of Zn, Cd, and Cu vs. the dilution rate for the soil series A and B. The maximum efficiency of PSII (F v /F m ) in leaves of R. acris depending on the soil series A and B, dilution rate (%), and exposure time (weeks) were represented using a surface response analysis. Finally, the crossed effects of the soil series and the dilution rates after a 50-day exposure were tested on the leaf DW yield, the TLA, and the SLA using a two-way ANOVA. Normality and homoscedasticity of residuals were met for all tests. Differences were considered statistically significant at p < 0.05. All statistical analyses were performed using the R software (version 3.0.1, R Foundation for Statistical Computing, Vienna, Austria).
Results
Physico-chemical analyses of soils and soil-pore waters
Total soil Cu, Cd, Fe, Mo, Pb, and Zn varied in the decreasing order: F > B > A, while total soil Cr, Co, Ni, and Mn ranked as F ≈ B > A (Table 1). All TE concentrations in the soil A were lower than both the inquiry threshold value for French soils (Baize et al. 2007; Table 1) and the French guideline values for dredged-sediment management (Arrêté du 9 août 2006, Table 1). Total soil Zn was within its common range (20–200 mg Zn kg −1 DW, Blum et al. 2012) for both soils A and B. Total soil Cd and Pb were within their common ranges (i.e., 0.05–1 mg Cd and 10–30 mg Pb kg−1 DW) in the soil A, but were slightly higher in the soil B. Total Cu, Zn, Pb, and Cd concentrations in the soil B exceeded the inquiry threshold values for French soils, i.e., 2-fold for Zn and Cd, but were below French guideline values for dredged-sediment management (Table 1). Total soil concentrations of the other TE were within the common ranges and below both the French guideline values for dredged sediment management and the inquiry threshold values for French soils. Higher total soil Al, K, Mg and Na, and extractable P in the soil B likely resulted from its higher clay and OM contents (Tables 1 and 2). For the soil F, total soil Cd, Cu, Pb, and Zn largely surpassed both the inquiry threshold values for French soils and French guideline values for dredged-sediment management, i.e., 20-, 10-, and 5-fold for Zn, Pb, and Cd, respectively (Table 1), and total soil As as well for these guidelines.
Soil physico-chemical parameters at site A differed from those at site B (Table 2). Soil texture was sandy at site A with a low clay content while the soil B contained more clay than sand. Similarly the soil A had lower C, N, and OM contents and lower CEC, EC, and WHC values than the soil B. Mean soil pH (7.5–7.9) did not significantly differ between soils A and B and was similar to pH value for the soil F (7.2). The soil F texture was silty loam with an intermediate OM content between the soil A and B values. The CEC values of soils F and B were similar and higher than for the soil A.
Total element concentrations in the soil-pore water did not follow the gradient of total element concentrations in soils (Table 3, Fig. 1). For convenience, we referred to element concentration in the soil-pore water as soluble element concentration throughout the paper.
Soil series A
Mean values of soluble Ni, Fe, B, P, K, and As concentrations peaked for the soil A (0 %), soluble Cu and Mo ones for the soil A (20 %) but their values were similar compared to A (0 %), and soluble Cr, Cd, and Zn concentrations for the soil A (60 %) (Table 3). As the percentage of soil F increased, soluble concentrations decreased for Na, K, P, Ni, Mo, Mg, Fe, and As; remained steady for Al, B, Cu, Mn, and Pb; and rapidly raised at A (20 %) to reach a plateau for Zn (39 μg L−1) and Cd (0.3 μg L−1).
Soil series B
The soil B displayed the highest soluble Na and Mg concentrations, and they decreased thereafter as the soil F percentage raised (Table 3). Lowest soluble concentrations were for As, Cd, Mo, K, and Zn in the soil B (0 %) and for Cu and Cr, respectively, in the soil B (40 %) and the soil B (60 %). Soluble P and Fe concentrations were low at all dilution rates. Soluble Zn and Cd concentrations were the lowest in soil B (0 and 20 %) and the highest in soil F (Table 3, Fig. 1). Soluble Zn and Cd concentrations displayed maximum values around 22 μg Zn and 0.2 μg Cd L−1 in soil F (Table 3). Soluble Ni concentration was the lowest for the soil F, and soluble Al, Mn, and Pb concentrations remained below detection limits for all soils.
PCA on the composition of the soil-pore water
The first axis (PC1, 33.4 %) corresponded to the dilution rate of the soil series A, while the second axis (PC2, 21.3 %) matched with the dilution rate of the soil series B. Accordingly, the soil-pore water can be divided in three groups. Group 1 (A 0% and A 20%) included soil-pore water with the highest B, Fe, Mn, P, K, As, Mo, and Cu concentrations. Group 2 (B 0 to 40 %) gathered soil-pore water with the highest Ca, Mg, and Na concentrations, but with the lowest Cd and Zn ones. Group 3 (A 40 to 80 %, B 60, and 80 %, F) had the highest soluble Cr, Zn, and Cd concentrations (Fig. 1).
Partition coefficients
The Log KdZn values ranged from 2.8 (soil A) and 4.6 (soil B) to 5.6 L kg−1 (soil F). The Log KdCd values varied from 2.9 (soil A) and 4.2 (soil B) to 4.7 L kg−1 (soil F). Values of the Log KdCu increased from 2.5 (soil A) and 3.5 (soil B) to 4.4 (soil F). For Cd, Cu, and Zn, the distribution of Log Kd values vs. the dilution rate was best curve-fitted with a quartic polynomial equation for both the soil series A and B (Fig. 2).
Foliar ionome of R. acris
No symptoms of element toxicity such as spotted necrosis and chlorosis were visible on any R. acris leaves but four plants on the soil B (0 %) and two plants on the soil B (80 %) suffered from a powdery mildew attack leading to the death of three plants for the soil B (0 %) and two for the soil B (80 %).
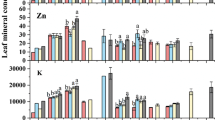
The foliar element concentrations did not follow the patterns of total element concentrations in the soils and the soil-pore waters (Fig. 5; Table 4). The ranges of foliar concentrations (in mg kg−1 DW) were: Cd 0.1–0.3, Cu 6.5–12.7, Cr 0.4–0.8, Ni 0.4–0.7, and Pb 1.9–3.5 (Table 4). Foliar Zn concentrations peaked for plants cultivated on the soils A (80 %), B (80 %), and F, i.e., 124, 115, and 112 mg kg−1, respectively. In both soil series, foliar Al, Ca, Cu, Mg, P, K, and Na concentrations did not significantly depend on the dilution rate. Foliar Mo, Pb, and Zn did not vary with the dilution rate in the soil series A, as well as foliar Cd concentration in the soil series B. Conversely, foliar As, B, Cd, Cr, Fe, Mn, and Zn concentrations, respectively, peaked at 0–20, 20, 80, 80, 20–80, 0–20–80, and 80 % in the soil series A and at 80 % for all elements in the soil series B except for As (40 %) and Cd (no variation with the dilution rate).
The PCA based on the foliar ionome discriminated two groups. The first one (B 60 %, A 40 %, and A 60 %) displayed lower foliar Ni, Mn, Mg, Fe, Cu, Cr, and B concentrations compared to the second group (A 0%, A 20%, A 80 %, B 0 %, B 20 %, B 40 %, B 80 %, and F) (Fig. 5). Several foliar element concentrations were correlated: Cr/As/Na/Fe/Zn, P/K/B/Cd/Cu, and Mn/Mg/Ca/Mo.
Relationships between soil TE concentrations, soil-pore water TE concentrations, and the foliar ionome of R. acris
The Pearson coefficient (r) between the total concentration in the soil and the soluble concentration was significant (p < 0.05) for As, Cu, Fe, Mo, Ni, K, and Na for the soil series A and for As, Cd, Mg, Mo, K, Na, and Zn for the soil series B. This Pearson coefficient calculated between the total concentration in the soil and the foliar ionome of R. acris was only significant for Na in the soil series A and for Mg, Mn, Mo, and Na in the soil series B. Finally, the Pearson coefficient between the soluble element concentrations and the foliar ionome of R. acris was significant for Mo and Na in both the soil series A and B (Table 5).
Chlorophyll fluorescence and growth parameters
Leaves of R. acris displayed steady maximum quantum efficiency of PSII (F v /F m ) at dawn over a 50-day exposure period for both soil series A and B (F v /F m ranged between 0.65 and 0.8) (Fig. 3). The leaf DW yield ranged between 0.24–0.78 and 0.01–0.4 g plant−1 for the soil series A and B, respectively (Fig. 4). However, neither the influence of the soils A and B, nor the dilution rate, nor their interaction was significant on this leaf DW yield (p > 0.05, Table 5). Substantial variations in leaf DW yield between replicates at each dilution rate and for both soil series were noted. Similarly, the mean TLA values ranged between 40–110 and 10–80 cm−2plant−1 for both soil series A and B, with relatively large differences between replicates and no significant differences for the TLA according to the soil series, the dilution rate and their interaction (Table 6, Fig. 4, p > 0.05). Conversely, the SLA was significantly lower for the soil series A (80–120 cm−2 g−1 plant−1) compared to the soil series B (110–220 cm−2 g−1 plant−1) (Table 6, Fig. 4, p < 0.05).
Discussion
Relationships between total and soluble TE concentrations in the soil series
The fading technique, previously used by Japenga et al. (2007) and Kolbas et al. (2013), allowed to obtain the soil series A and B, with a realistic gradual soil TE contamination but differing by their soil texture. Trace element concentrations of soil-pore water in the soil series A and B slightly exceeded the As and Cu values, but not the Zn one, for an uncontaminated soil with similar pH but slightly lower OM content (in μg L−1: As [1–3]; Cu [2–10]; and Zn [<1–360], Beesley and Dickinson 2010). Soluble Cr concentrations were however similar to previous findings in uncontaminated conditions (Burbridge et al., 2012; Marchand et al. 2014). Our soluble TE concentrations are generally lower than values reported in the soil-pore water of contaminated soils (in μg L−1): Cu [370–1780] (Bes et al. 2010); Cu [1050] and Pb [80] (Karami et al. 2011); Cu [168–270], Zn [57–610], and Pb [1.25–14.9] (Kabala et al. 2014) and Moreno-Jiménez et al. (2011). Unlike the gradient of total concentrations created by fading the soil F with either the soil A or soil B, the soil-pore water in both soil series generally did not display gradual increases in soluble element concentrations in line with the percentage of TE-contaminated soil F. Generally, soil TE availability depends on the characteristics of particle surfaces, e.g., organic matter (OM), oxyhydroxides of Fe, Al, and Mn (FeMnOx), phyllosilicate minerals, carbonates, and sulphides, their sorption properties and/or reactions with the solution in contact. Such reactions are driven by soil physico-chemical parameters such as pH, CEC, and the redox potential and dissolution-precipitation reactions (Violante et al. 2008; Moreno-Jiménez et al. 2011; Manzano et al. 2014). The clay content, total OM content, and the CEC in our soils were in the increasing order B > F > A, while the coarse sand content was in the decreasing order B < F < A. The Fe/Mn (hydr)oxide concentrations were the highest in the soil F and the lowest in the soil. Consequently, the CEC, total OM, clay, and Fe/Mn (hydr)oxide contents increased in the soil series A, while they decreased, except for Fe/Mn (hydr)oxides, in the soil series B.
The slight increase in soluble Zn/Cd in both soil series was mainly related to the increment of total soil Zn/Cd, but only correlated in the soil series B. The soil texture of the soil series indeed influenced markedly the soluble Zn/Cd concentrations. Their values were higher in the soil series A than in the B one, even though the slight decrease of pH in the soil series B from 7.9 to 7.2 may in theory favor metal desorption from Fe/Mn (hydr)oxides. This was reflected by differences in the KdZn and KdCd modeling soluble vs. total soil Zn/Cd. Both KdZn and KdCd, and also KdCu were higher in the soil series B, notably at dilution rates between 0 and 20 %, suggesting that the OM and clay contents and the hydrous Fe/Mn oxides would more buffer soluble Zn, Cd, and Cu concentrations in this soil series despite a significant supply of total Zn, Cd, and Cu. Such results confirmed that the kinetically labile solid-phase pool of metal would be key factor for plant Zn and Cd uptake along with the labile metal in soil solution (Nolan et al. 2005). The soil and SPW pH remained roughly steady (7.2–7.9) in both soil series, likely explaining low soluble Al, Mn, and Pb concentrations, as these elements likely (co)precipitate as (hydr)oxides and with phosphates and carbonates and react with clay and OM. Soluble Ca and B concentrations remained steady in both soil series despite contrasting soil textures and increase in total soil Ca in line with the soil F percentage.
In the soil series A, soluble As, Fe, Ni, and Mg concentrations progressively decreased despite the gradual increase of their total soil concentrations. This occurred also for soluble Cu and Mo concentrations but at high soil F addition rates. Such decreases may be driven in theory by increases in soil CEC, hydrous Fe/Mn oxides, clay, and OM contents in line with the progressive addition of soil F. Decrease in soluble P concentration may reflect sorption of phosphates on hydrous Fe oxides and clay as for arsenates and molybdates. Iron and Mn (hydr)oxides can react by sorption and co-precipitation with cations, e.g., Zn, Cd, Cu, and Ni, and anions, e.g., arsenates (As(V)) (Violante et al. 2010), molybdates (Caporale et al. 2011), and phosphates (Yan et al. 2010). Such pH-dependent reactions may simultaneously occur with oxyanions and cations as soil pH was near 7 in the soil series A. Total soil Na and K were similar in the soils F and A, and decreases in soluble Na and K concentrations may mirror the increase in soil CEC. Metal mobilization by dissolved organic compounds is reported for Zn, Cr, and Cu (Alvim Ferraz and Lourenço 2000), Ni (Tipping et al. 1998; Wells et al. 1998), and As (Kalbitz and Wennrich 1998). Due to gradual increase in soil OM derived from the soil F, dissolved OM might raise in the soil-pore water, but its potential effect was not evidenced here on soluble As, Ni, and Cu concentrations, whereas soluble Cr concentration transiently increased in the soils A (60) and A (80). Conversely, in the riverbank soil series of the Jalle d’Eysines River, displaying gradual increases in OM and clay contents but steady soil pH, total, and soluble soil Cr concentrations were negatively correlated (Marchand et al. 2014).
In the soil series B, total and soluble soil As, Cd, Mg, Mo, Zn, Na, and K concentrations were positively correlated. For Mo, this confirmed previous findings with the Jalle d’Eysines riverbank soils (Marchand et al. 2014). Increased total soil Mo as well as slightly lower OM and clay contents may explain higher soluble Mo in the soils B (80) and F. Higher hydrous Fe oxide and clay contents in the B soil series likely explain low and steady soluble As, Ni, Fe, and P concentrations compared to the soil series A. Slight soluble As increase in soils B (80) and F may be related to lower clay and OM contents. Organic matter is able to react with arsenite and arsenate, limiting As mobility through sorption (Goldberg 2002) and formation of insoluble complexes (Wang and Mulligan 2006). Conversely, dissolved organic matter (DOM) can promote As desorption from Fe/Mn (ox)hydroxides (Bauer and Blodau 2006). Increase in soluble K concentration was mainly due to higher total soil K in the soil B and, may be, to changes in soil texture from silty clay loam to silty loam. Total soil Na and Mg were faded in the soil series B and consequently soluble Na and Mg concentrations decreased. As soil OM and clay contents were high in both the soils B and F, soluble Cu concentration remained steady and lower than in the soil series A. Further investigations on the TE speciation in the solid phases and the soil-pore water, e.g., extended x-ray absorption fine structure (EXAFS), μXANES imaging, and DGT, are needed to better characterize the influence of soil texture and TE-bearing phases on TE exposure in the studied soil series (Davison and Zhang 2012; Grafe et al. 2014).
Relationships between the foliar ionome, morphological traits, and indicators of soil TE exposures
The soil fading did not significantly affect the leaf DW yield, the TLA, and the SLA of R. acris on the 50-day exposure period for both soil series A and B. Thus, a dilution effect on foliar TE concentrations due to changes in leaf biomass across each soil series was unlikely. Similarly, PSII maximum efficiency of R. acris was not impacted by the gradual soil TE contamination. Indeed, photosystem II activity and foliar traits of R. acris gave coinciding responses for each soil series studied.
In plant photosynthesis, Zn is involved in the catalytic function of the chloroplastic b-carbonic anhydrase (b-CA) enzyme, which rapidly inter-converts CO2 and H2O into HCO3 ions. Additionally, Zn-finger proteins regulating transcription through site-specific interactions play a role in photosynthesis (Yruela 2013). Under high Zn exposure, a decline in the photosynthetic function may occur (Cambrollé et al. 2012). Foliar Zn concentrations of R. acris were in compliance with common concentrations in aboveground plant parts, i.e., 20–100 mg kg−1 DW (Blum et al. 2012) and slightly higher than those of this species along the Jalle d’Eysines river (37–55 m kg−1, Marchand et al. 2014) due to a higher Zn exposure (Fig. 5). Phytotoxicity symptoms usually become visible at foliar Zn concentration over 300 mg kg−1 DW (Marschner 2011). Stress-enzyme activities however indicate early phytotoxic effect in leaf dwarf bean when 100 mg Zn kg−1 DW is exceeded (Mench et al. 2000). This upper critical threshold value was surpassed in R. acris from the soils A (80), B (80), and F. Indeed, 2-week-old beans grown on the soil F showed a slight decrease of the root biomass and increased activities of guaiacol peroxidase, isocitrate dehydrogenase, and malic enzyme in primary leaves, ranking this soil as slightly phytotoxic (JO Janssen, Hasselt University, personal communication). Here, photosystem II activity was not affected, as well as leaf growth parameters, and the foliar ionome did not evidence antagonism with Zn. For both soil series A and B, the foliar Zn concentrations of R. acris did not reflect the total Zn concentrations in the soil and the soil-pore water. This confirmed previous findings suggesting R. acris as a Zn excluder storing more Zn in its belowground parts (Marchand et al. 2014).
Cadmium is a non-essential element for plants, easily taken up by roots, likely following the Zn and Fe uptake pathways (Hassan and Aarts 2011). It is also transported through membranes by the natural resistance associated macrophage protein (NRAMP) family (Pottier et al. 2014). Phytotoxic effects of excess Cd are dose-dependent (Semane et al. 2010; Lin and Aarts 2012; D’Alessandro et al. 2013). Foliar Cd concentrations of R. acris, i.e., 0.1–0.3 mg Cd kg−1, were very low compared to the phytotoxic range (5–700 mg kg−1, Chaney 1989), similar to those for R. acris sampled along the Jalle d’Eysines River (0.1–0.3 mg Cd kg−1, Marchand et al. 2014), and in line with common Cd concentrations in aerial plant parts (0.05–0.5 mg kg−1, Blum et al. 2012). Bonanno (2013) reported Cd concentrations in roots, stems and leaves of Typha domingensis Pers., P. australis (Cav.) Trin. ex Steud., and Arundo donax L. below 0.1 mg kg−1 DW, suggesting macrophytes act as Cd excluders. Conversely, Marchand et al. (2014) found higher foliar Cd concentrations, e.g., up to 1.13 mg kg−1 in leaves of Iris pseudacorus L. The inter-specific diversity, but also the development stage of macrophytes, shoot biomass, and sampling time, in line with soil physico-chemical and microbial conditions are key factors for foliar Cd concentrations and may explain such variations (Wu et al. 2013). Our results confirmed the lack of relationship between foliar Cd concentration of R. acris and soluble Cd concentration in the soils. This plant species was likely a Cd excluder in both soil series A and B and not a relevant biomonitor for both total and soluble soil Cd.
Soluble Ni concentrations in the soil series A, i.e., 2.9–15.9 μg L−1 slightly surpassed the values for the Jalle d’Eysines riverbank soils, i.e., 3.1–5.3 μg L−1 (Marchand et al. 2014), but foliar Ni concentrations in this pot experiment were at least 3-fold lower compared to those for R. acris leaves collected along the Jalle d’Eysines riverbanks, i.e., 1.2–3.3 mg kg−1 (Marchand et al. 2014). No correlation was found between Ni concentrations in R. acris leaves, the soil and the soil-pore water, which disagreed with Marchand et al. (2014). Similarly, soluble Cu concentrations in the soil series A, i.e., 11.1–31.2 μg L−1 were slightly higher than the values for the Jalle d’Eysines riverbank soils, i.e., 11.1–19.6 μg L−1, but foliar Cu concentrations of R. acris grown on the soil series A were 2-fold lower compared to those sampled on the Jalle d’Eysines riverbanks, i.e., 15.8–16.9 mg kg−1 (Marchand et al. 2014). The lack of correlation between foliar Cu concentration of R. acris and total and soluble soil Cu in both soil series A and B confirmed previous findings (Marchand et al. 2014).
Foliar Na concentrations of R. acris were positively correlated with both total and soluble soil Na for the soil series A and B. Similarly, foliar Mo concentrations were positively correlated with both total and soluble soil Mo for the soil series B, but negatively for the more sandy soil series A. A bias resulting from the high variability of foliar concentrations on this soil series prevented to clearly rule on the relationship between the Mo concentration in the soil and the pore water and the leaf Mo content. In line with Marchand et al. (2014) reporting no significant correlation between total and soluble soil Mo and the foliar Mo concentration of R. acris, this plant species would not be relevant for biomonitoring Mo without accounting for the soil type.
Even for the soil F with high TE contamination, photosystem II activity and leaf morphological parameters did not indicate deleterious effects on R. acris growth. This agreed with previous findings showing no major adverse effects of this soil on the abundance and diversity of the springtail communities, no deleterious effect on the soil bacterial communities and a vegetation cover close to 100 % in plots at the PHYTOSED Scale 1-platform (Bert et al. 2012). Plant assays accounting for stress-enzyme activities may however be relevant to detect early phytotoxic effects if any in the soil series A and B (Kumpiene et al. 2014).
Conclusions
Relationships between total TE concentrations in the soil and soil-pore water, foliar ionome of R. acris L., its photosystem II activity and foliar morphological traits were assessed for a sandy (A) and a silty clay loam (B) soil series obtained by fading a TE-contaminated technosol developed on dredged sediments (soil F) by two riverbank soils. The TE concentrations in soil-pore water of both soil series did not reflect the gradual increase in soil TE contamination. For Zn, Cu, and Cd, the partition coefficient Kd between the soil and the soil-pore water was influenced by the soil series texture. Increases in total soil TE did not affect leaf DW yield, total and specific leaf areas, and the photosystem II activity of R. acris over a 50-day exposure period. The foliar ionome of R. acris was not effective for biomonitoring total and soluble element concentrations in both soil series A and B, except for Na. Such results highlight that total TE concentration in the soil but also (1) the soil texture, (2) its physico-chemical parameters, (3) TE chemical speciation, and (4) the plant species strategy implemented for facing a TE exposure are key factors when selecting a macrophyte for biomonitoring TE exposure in a riverbank soil. Additional parameters such as (5) the development stage of the plant, (6) the intraspecific variability as a driver of TE tolerance and TE transfer to aerial parts in macrophytes, (7) the bacterial and fungal communities in the soil, and the soil-pore water and the plants (e.g., endophytic bacteria) should be considered in further investigations. Such set of drivers may limit the use of rooted macrophytes as relevant biomonitors of TE exposure.
Abbreviations
- b-CA:
-
b-Carbonic anhydrase
- CEC:
-
Cationic exchange capacity
- DGT:
-
Diffusive gradients in thin-film
- DOC:
-
Dissolved organic carbon
- DW:
-
Dry weight
- EC:
-
Electric conductivity
- EEA:
-
European Environmental Agency
- EXAFS:
-
Extended x-ray absorption fine structure
- F 0 :
-
Minimal fluorescence level in the dark adapted state
- F m :
-
Maximum fluorescence
- F v :
-
F m − F 0
- GACGC:
-
German Advisory Council on Global Change
- ISO:
-
International Organization of Standardization
- Kd:
-
Partition coefficient
- NRAMP:
-
Natural resistance-associated macrophage protein
- OM:
-
Organic matter
- PCA:
-
Principal component analysis
- PSII:
-
Photosystem II
- SLA:
-
Specific leaf area
- TE:
-
Trace element
- TLA:
-
Total leaf area
- VDLUFA:
-
Association of German Agricultural Analytic and Research Institutes
- WHC:
-
Water holding capacity
- XANES:
-
X-ray absorption near edge structure
References
Adriano DC (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals, 2nd edn. Springer, Verlag
Alloway BJ (2008) Zinc in soils and crop nutrition. Second edition, Eds. IZA and IFA, Brussels, Belgium and Paris, France. 135 p. ISBN 978-90-8133-310-8. Available at http://www.zinc.org/general/Zinc_in_Soils_and_Crop_Nutrition_ALLOWAY.pdf
Alvim Ferraz MCM, Lourenço JCN (2000) The influence of organic matter content of contaminated soils on the leaching rate of heavy metals. Environ Prog 19:53–58. doi:10.1002/ep.670190118
Arrêté du 9 août 2006, relatif aux niveaux à prendre en compte lors d’une analyse de rejets dans les eaux de surface ou de sédiments marins, estuariens ou extraits de cours d’eau ou canaux relevant respectivement des rubriques 2.2.3.0, 4.1.3.0 et 3.2.1.0 de la nomenclature annexée au décret n° 93–743 du 29 mars 1993. Fr Off J 222 :14082–14085
Baize D, Deslais W, Saby N (2007) Teneurs en huit éléments traces (Cd, Cr, Cu, Hg, Ni, Pb, Se, Zn) dans les sols agricoles en France - Résultats d’un collecte de données à l’échelon national. ADEME - Gis Sol - INRA. 86 p
Barth JAC, Grathwohl P, Fowler HJ, Bellin A, Gerzabek MH, Lair GJ, Barcelo D, Petrovic M, Navarro A, Negrel P, Petelet-Giraud E, Darmendrail D, Rijnaarts H, Langenhoff A, de Weert J, Slob A, van der Zaan BM, Gerritse J, Frank E, Gutierrez A, Kretzschmar R, Gocht T, Steidle D, Garrido F, Jones KC, Meijer S, Moeckel C, Marsman A, Klaver G, Vogel T, Burger C, Kolditz O, Broers HP, Baran N, Joziasse J, Von Tumpling W, Van Gaans P, Merly C, Chapman A, Brouyere S, Aguilar JB, Orban P, Tas N, Smidt H (2009) Mobility, turnover and storage of pollutants in soils, sediments and waters: achievements and results of the EU project AquaTerra. A review. Agron Sustain Dev 29:161–173. doi:10.1051/agro:2007060
Bauer M, Blodau C (2006) Mobilization of arsenic by dissolved organic matter from iron oxides, soils and sediments. Sci Total Environ 354:179–190. doi:10.1016/j.scitotenv.2005.01.027
Bearup L, Mikkelson KM, Wiley JF, Navarre-Sitchler AK, Maxwell RM, Sharp JO, McCray JE (2014) Metal fate and partitioning in soils under bark beetle-killed trees. Sci Total Environ 496:348–367. doi:10.1016/j.scitotenv.2014.07.052
Beesley L, Moreno-Jiménez E, Clemente R, Lepp N, Dickinson N (2010) Mobility of arsenic, cadmium and zinc in a multi-element contaminated soil profile assessed by in-situ soil-pore water sampling, column leaching and sequential extraction. Environ Pollut 158:155–160. doi:10.1016/j.envpol.2009.07.021
Beesley L, Dickinson NM (2010) Carbon and trace element mobility in an urban soil amended with green waste compost. J Soils Sediments 10:215–222. doi:10.1007/s11368-009-0112-y
Belon E, Boisson M, Deportes IZ, Eglin TK, Feix I, Bispo AO, Galsomies L, Leblond S, Guellier CR (2012) An inventory of trace element inputs to French agricultural soils. Sci Total Environ 439:87–95. doi:10.1016/j.scitotenv.2012.09.011
Bert V, Lors C, Ponge JF, Caron L, Biaz A, Dazy M, Masfaraud JF (2012) Metal immobilization and soil amendment efficiency at a contaminated sediment landfill site: a field study focusing on plants, springtails, and bacteria. Environ Pollut 169:1–11. doi:10.1016/j.envpol.2012.04.021
Bes C, Mench M, Aulen M, Gasté H, Taberly J (2010) Spatial variation of plant communities and shoot Cu concentrations of plant species at a timber treatment site. Plant Soil 330:267–280. doi:10.1007/s11104-009-0198-4
Blum WEH, Horak O, Mentler A, Puschenreiter M (2012) UNESCO – EOLSS Environmental and Ecological chemistry – Vol.II – Trace elements. http://www.eolss.net/Sample-Chapters/C06/E6-13-03-04.pdf (verified on October, 1, 2014)
Bonanno G (2013) Comparative performance of trace element bioaccumulation and biomonitoring in the plant species Typha domingensis, Phragmites australis and Arundo donax. Ecotoxicol Environ Saf 97:124–130. doi:10.1016/j.ecoenv.2013.07.017
Bourdot GW, Lamoureaux SL, Watt MS, Kriticos DJ (2013) The potential global distribution of tall buttercup (Ranunculus acris ssp. acris): opposing effects of irrigation and climate change. Weed Sci 61:230–238. doi:10.1614/WS-D-12-00106.1
Burbridge DJ, Koch I, Zhang J, Reimer KJ (2012) Chromium speciation in river sediment pore water contaminated by tannery effluent. Chemosphere 89:838–843. doi:10.1016/j.chemosphere.2012.05.005
Burt R, Hernandez L, Shaw R, Tunstead R, Ferguson R, Peaslee S (2014) Trace element concentration and speciation in selected urban soils in New York City. Environ Monit Assess 186:195–215. doi:10.1007/s10661-013-3366-1
Cambrollé J, Mancilla-Leytón JM, Muñoz-Vallés S, Luque T, Figueroa ME (2012) Zinc tolerance and accumulation in the salt-marsh shrub Halimione portulacoides. Chemosphere 86:867–874. doi:10.1016/j.chemosphere.2011.10.039
Caporale AG, Pigna M, Dynes JJ, Cozzolino V, Zhu J, Violante A (2011) Effect of inorganic and organic ligands on the sorption/desorption of arsenate on/from Al-Mg and Fe-Mg layered double hydroxides. J Hazard Mater 198:291–298. doi:10.1016/j.jhazmat.2011.10.044
Capra GF, Coppola E, Odierna P, Grilli E, Vacca S, Buondonno A (2014) Occurrence and distribution of key potentially toxic elements (PTEs) in agricultural soils: a paradigmatic case study in an area affected by illegal landfills. J Geochem Explor 145:169–180. doi:10.1016/j.gexplo.2014.06.007
Chaney RL (1989) Toxic element accumulation in soils and crops: protecting soil fertility and agricultural food chains. In: Bar-Yosef B, Barrow NJ, Goldshmid J (eds) Inorganic contaminants in the Vadose Zone. Springer, Berlin, pp 140–158
D’Alessandro A, Taamalli M, Gevi F, Timperio AM, Zolla L, Ghnaya T (2013) Cadmium stress responses in Brassica juncea: hints from proteomics and metabolomics. J Proteome Res 12:4979–4997. doi:10.1021/pr400793e
Davison W, Zhang H (2012) Progress in understanding the use of diffusive gradients in thin films (DGT)—back to basics. Environ Chem 9:1–13. doi:10.1071/EN11084
Defra (2012) Environmental Protection Act 1990: part 2A, contaminated land statutory guidance April 2012. PB13735 Defra, London, UK. https://www.gov.uk/government/publications/contaminated-land-statutory-guidance (accessed March 2015)
Delmail D (2014) Risk management of European inland waters using macrophyte biomonitoring. Front Environ Sci. doi:10.3389/fenvs.2014.00031
European Environmental Agency (2014) Progress in management of contaminated sites (LSI 003)—assessment published May 2014. Copenhagen, Denmark: European Environmental Agency. Available at http://www.eea.europa.eu/data-and-maps/indicators/progress-in-management-of-contaminated-sites-3/assessment#toc-2 on October 7, 2104.
Evangelou MWH, Robinson BH, Conesa HM, Schulin R (2012) Biomass production on trace element (TE) contaminated land—a review. Environ Eng Sci 29:823–839. doi:10.1089/ees.2011.0428
GACGC (1995) World in transition: the threat to soils. Annual report—1994. German Advisory Council on Global Change, Bonn
GIS sol (2009) Nouvelle collecte nationale d’analyses d’éléments traces métalliques - Statistiques par région agricole. Available at http://www.gissol.fr/programme/bdetm/Collecte_2/index.php. Access on October 7, 2014
Goldberg S (2002) Competitive adsorption of arsenate and arsenite on oxides and clay minerals. Soil Sci Soc Am J 66:413–421. doi:10.2136/sssaj2002.4130
Grafe M, Donner E, Collins RN, Lombi E (2014) Speciation of metal(loid)s in environmental samples by X-ray absorption spectroscopy: a critical review. Anal Chim Acta 822:1–22. doi:10.1016/j.aca.2014.02.044
Hassan Z, Aarts GM (2011) Opportunities and feasibilities for biotechnological improvement of Zn, Cd or Ni tolerance and accumulation in plants. Environ Exp Bot 72:53–63. doi:10.1016/j.envexpbot.2010.04.003
Hoagland DR, Arnon DL (1950) The water culture method for growing plants without soil. California agricultural experiment station. Circular 347
ISO 11269-2 (2005) Soil quality. Determination of the effects of pollutants on soil flora. Effects of chemicals on the emergence and growth of higher plants. ISO/TC 190/SC 4
ISO 17512-1 (2008) Soil quality—avoidance test for determining the quality of soils and effects of chemicals on behaviour—part 1: test with earthworms (Eisenia fetida and Eisenia andrei). ISO/TC 190/SC 4
ISO 10872 (2010) Water quality—determination of the toxic effect of sediment and soil samples on growth, fertility and reproduction of Caenorhabditis elegans (Nematoda). ISO/TC 147/SC 5
Japenga J, Koopmans GF, Song J, Romkens PFAM (2007) A feasibility test to estimate the duration of phytoextraction of heavy metals from polluted soils. Int J Phytorem 9:115–132. doi:10.1080/15226510701232773
Kabala C, Karczewska A, Medynska-Juraszek A (2014) Variability and relationships between Pb, Cu, and Zn concentrations in soil solutions and forest floor leachates at heavily polluted sites. J Plant Nutr Soil Sci 177:573–584. doi:10.1002/jpln.201400018
Kalbitz K, Wennrich R (1998) Mobilization of heavy metals and arsenic in polluted wetland soils and its dependence on dissolved organic matter. Sci Total Environ 209:27–39. doi:10.1016/S0048-9697(97)00302-1
Karami N, Clemente R, Moreno-Jiménez E, Lepp NW, Beesley L (2011) Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J Hazard Mater 191:41–48. doi:10.1016/j.jhazmat.2011.04.025
Kidd P, Mench M, Álvarez-López V, Bert V, Dimitriou I, Friesl-Hanl W, Herzig R, Janssen JO, Kolbas A, Müller I, Neu S, Renella G, Ruttens A, Vangronsveld J, Puschenreiter M (2014) Agronomic practices for improving gentle remediation of trace-element-contaminated soils. Int. J. Phytoremediation (accepted, BIJP-2014-0174).
Kolbas A, Mench M, Marchand L, Herzig R, Nehnevajova E (2013) Phenotypic seedling responses of a metal-tolerant mutant line of sunflower growing on a Cu-contaminated soil series: potential uses for biomonitoring of Cu exposure and phytoremediation. Plant Soil 376:377–397. doi:10.1007/s11104-013-1974-8
Kumpiene J, Bert V, Dimitriou I, Eriksson J, Friesl-Hanl W, Galazka F, Herzig R, Janssen JO, Kidd P, Mench M, Muller I, Neu S, Oustriere N, Puschenreiter M, Renella G, Roumier PH, Siebielec G, Vangronsveld J, Manier N (2014) Selecting chemical and ecotoxicological test batteries for risk assessment of trace element-contaminated soils (phyto)managed by gentle remediation options (GRO). Sci Total Environ 496:510–522. doi:10.1016/j.scitotenv.2014.06.130
Li L, Holm PE, Marcussen H, Bruun Hansen HC (2014) Release of cadmium, copper and lead from urban soils of Copenhagen. Environ Pollut 187:90–97. doi:10.1016/j.envpol.2013.12.016
Lin YF, Aarts MGM (2012) The molecular mechanism of zinc and cadmium stress response in plants. Cell Mol Life Sci 69:3187–3206. doi:10.1007/s00018-012-1089-z
Manzano R, Penalosa JM, Esteban E (2014) Amendment application in a multi-contaminated mine soil: effects on trace element mobility. Water Air Soil Pollut 225:1874–1884. doi:10.1007/s11270-014-1874-4
Marchand L, Mench M, Marchand C, Lecoustumer P, Kolbas A, Maalouf JP (2011) Phytotoxicity testing of lysimeter leachates from aided phytostabilized Cu-contaminated soils using duckweed (Lemna minor L.). Sci Total Environ 411:146–153
Marchand L, Nsanganwimana F, Vystavna Y, Huneau F, Lecoustumer P, Lamy JB, Cook B, Mench M (2014) Trace element transfer from soil to leaves of macrophytes along the Jalle d’Eysines River, France and their potential use as contamination biomonitors. Ecol Indic 46:425–437. doi:10.1016/j.ecolind.2014.07.011
Markert B (2007) Definitions and principles for bioindication and biomonitoring of trace metals in the environment. J Trace Elem Med Biol 21:77–82
Marschner P (2011) Mineral nutrition of higher plants, 3rd edn. Academic, London
Maxwell K, Johnson GN (2000) Chorophyll fluorescence—a practical guide. J Exp Bot 51:659–668. doi:10.1093/jexbot/51.345.659
McLaughlin MJ, Singh BR (1999) Cadmium in soil and plants. Kluwer Academic Publishing, Dordrecht
Mench MJ, Manceau A, Vangronsveld J, Clijster H, Mocquot B (2000) Capacity of soil amendments in lowering the phytoavailability of sludge-borne zinc. Agronomie 20:383–397. doi:10.1051/agro:2000135
Meers E, Ruttens A, Geebelen W, Vangronsveld J, Samson R, Vanbroekhoven K, Vandegehuchte M, Diels L, Tack FMG (2006) Potential use of the plant antioxidant network for environmental exposure assessment of heavy metals in soils. Environ Monit Assess 120:243–267. doi:10.1007/s10661-005-9059-7
Moreno-Jiménez E, Beesley L, Lepp NW, Dickinson NM, Hartley W, Clemente R (2011) Field sampling of soil-pore water to evaluate trace element mobility and associated environmental risk. Environ Pollut 159:3078–3085. doi:10.1016/j.envpol.2011.04.004
Nolan AL, Zhang H, McLaughlin MJ (2005) Prediction of zinc, cadmium, lead, and copper availability to wheat in contaminated soils using chemical speciation, diffusive gradients in thin films, extraction, and isotopic dilution techniques. J Environ Qual 34:496–507. doi:10.2134/jeq2005.0496
Pottier M, Masclaux-Daubresse C, Yoshimoto K, Thomine S (2014) Autophagy as a possible mechanism for micronutrient remobilization from leaves to seeds. Front Plant Sci 5(11):1–8. doi:10.3389/fpls.2014.00011
Rashti MR, Esfandbod M, Adhami E, Srivastava P (2014) Cadmium desorption behaviour in selected sub-tropical soils: effects of soil properties. J Geochem Explor 144(B):230–236. doi:10.1016/j.gexplo.2014.01.023
Schmitz J, Schafer K, Bruhl CA (2013) Agrochemicals in field margins—assessing the impacts of herbicides, insecticides, and fertilizer on the common buttercup (Ranunculus acris). Environ Toxicol Chem 32:1124–1131. doi:10.1002/etc.2138
Semane B, Dupae J, Cuypers A, Noben JP, Tuomainen M, Tervahauta A, Kärenlampi S, Van Belleghem F, Smeets K, Vangronsveld J (2010) Leaf proteome responses of Arabidopsis thaliana exposed to mild cadmium stress. J Plant Physiol 167:247–254. doi:10.1016/j.jplph.2009.09.015
Tipping E, Lofts S, Lawlor AJ (1998) Modelling the chemical speciation of trace metals in the surface waters of the Humber system. Sci Total Environ 210–211:63–77. doi:10.1016/S0048-9697(98)00045-X
Violante A, Huang PM, Gadd GM (2008) Biophysico-chemical processes of heavy metals and metalloids in soil environments. Wiley, Hoboken, 678 pp
Violante A, Cozzolino V, Perelomov L, Caporale AG, Pigna M (2010) Mobility and bioavailability of heavy metals and metalloids in soil environments. J Soil Sci Plant Nutr 10:268–292. doi:10.4067/S0718-95162010000100005
Vystavna Y, Huneau F, Grynenko V, Vergeles Y, Celle-Jeanton H, Tapie N, Budzinski H, Le Coustumer P (2012) Pharmaceuticals in rivers of two regions with contrasted socio-economic conditions: occurrence, accumulation, and comparison for Ukraine and France. Water Air Soil Pollut 223:2111–2124. doi:10.1007/s11270-011-1008-1
Wang S, Mulligan CN (2006) Effect of natural organic matter on arsenic release from soils and sediments into groundwater. Environ Geochem Health 28:197–214. doi:10.1007/s10653-005-9032-y
Washa M, Bini C, Zilioli D, Spiandorello M, Gallo M (2014) Potentially harmful elements in terraced agroecosystems of NE Italy: geogenic vs anthropogenic enrichment. J Geochem Explor 144:355–362. doi:10.1016/j.gexplo.2014.01.012
Wells ML, Kozelka PB, Bruland KW (1998) The complexation of “dissolved” Cu, Zn, Cd and Pb by soluble and colloidal organic matter in Narragansett Bay. RI Mar Chem 62:203–217. doi:10.1016/S0304-4203(98)00041-3
Wu J, Wang L, Ma F, Yang J, Li S, Li Z (2013) Effects of vegetative-periodic-induced rhizosphere variation on the uptake and translocation of metals in Phragmites australis (Cav.) Trin ex, Steudel growing in the Sun Island wetland. Ecotoxicol 22:608–618. doi:10.1007/s10646-013-1052-2
Yan LG, Xu YY, Yu HQ, Xin XD, Wei Q, Du B (2010) Adsorption of phosphate from aqueous solution by hydroxy-aluminum, hydroxy-iron and hydroxy-iron-aluminum pillared bentonites. J Hazard Mater 179:244–250. doi:10.1016/j.jhazmat.2010.02.086
Yruela I (2013) Transition metals in plant photosynthesis. Metallomics 5:1090–1109. doi:10.1039/c3mt00086a
Zhou QF, Zhang JB, Fu JJ, Shi JB, Jiang GB (2008) Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal Chim Acta 606:135–153. doi:10.1016/j.aca.2007.11.018
Acknowledgments
This work was financially supported by (1) AXA foundation (Ph.D. grant of L. Marchand); (2) the Aquitaine Region Council (Phytorem project n°20091205013), Bordeaux, France; (3) ADEME, Department of Urban Landfills and Polluted Sites, Angers, France; (4) Euskampus Fundazioa (contract of C. Quintela-Sabarís); and (5) the European Commission under the Seventh Framework Programme for Research (FP7-KBBE-266124, GREENLAND). This study was carried out in the framework of the Cluster of Excellence COTE. The authors greatly thank Dr. M. Urli, UMR BIOGECO INRA 1202, for her advices in measuring photosynthetic parameters.
Compliance with ethical standards
The manuscript has not been submitted to more than one journal for simultaneous consideration.
The manuscript has not been published previously (partly or in full), unless the new work concerns an expansion of previous work (please provide transparency on the re-use of material to avoid the hint of text-recycling (“self-plagiarism”)).
A single study is not split up into several parts to increase the quantity of submissions and submitted to various journals or to one journal over time (e.g., “salami-publishing”).
No data have been fabricated or manipulated (including images) to support your conclusions
No data, text, or theories by others are presented as if they were the author’s own (“plagiarism”). Proper acknowledgements to other works must be given (this includes material that is closely copied (near verbatim), summarized and/or paraphrased), quotation marks are used for verbatim copying of material, and permissions are secured for material that is copyrighted.
Consent to submit has been received explicitly from all co-authors, as well as from the responsible authorities—tacitly or explicitly—at the institute/organization where the work has been carried out before the work is submitted.
Authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Highlight
Gradual increase in total trace element (TE) concentrations in a sandy and a silty clay soil series was generally not mirrored by changes in TE concentrations of the soil-pore water and leaves of Ranunculus acris.
Rights and permissions
About this article
Cite this article
Marchand, L., Lamy, P., Bert, V. et al. Potential of Ranunculus acris L. for biomonitoring trace element contamination of riverbank soils: photosystem II activity and phenotypic responses for two soil series. Environ Sci Pollut Res 23, 3104–3119 (2016). https://doi.org/10.1007/s11356-015-4646-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4646-9