Abstract
Cadmium (Cd) contamination in agricultural products presents a threat to humans when consumed. Sweet potato is the world’s seventh most important food crop. The aims of this study were to screen for low-Cd sweet potato cultivars and clarify the mechanisms of low-Cd accumulation in edible roots. A pot experiment was conducted to investigate the variation of Cd uptake and translocation among 30 sweet potato cultivars grown in contaminated soils with three different Cd concentrations. Cadmium concentrations in edible roots were significantly different among cultivars and were significantly affected by Cd treatment, and the interaction between cultivar and Cd treatment. High-Cd cultivars have higher ratios of edible root/shoot Cd concentration and edible root/feeder root Cd concentration than low-Cd cultivars; however, the ratio of shoot/feeder root Cd concentration seems unrelated to the ability of Cd accumulation in edible roots. Four sweet potato cultivars, Nan88 (No. 10), Xiang20 (No. 12), Ji78-066 (No. 15), and Ji73-427 (No. 16), were identified as low-Cd cultivars. Cadmium translocation from feeder roots to edible roots via the xylem, and from shoots to edible roots via the phloem, controls Cd accumulation in edible roots of sweet potato cultivars.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd), a non-essential and highly toxic element, can be easily taken up by agricultural crops and subsequently pose a risk to human health (Grant et al. 2008). Cadmium contamination caused by anthropogenic activities, such as mineral exploitation and the use of phosphate fertilizer and sewage sludge, has been a worldwide environmental problem (Xin et al. 2013). For example, Cd contamination of agricultural soils was widely reported in the Jinzu River Basin of Japan (Ishihara et al. 2001), Mae Tao watershed of Thailand (Kosolsaksakul et al. 2014), midstream and downstream of the Xiangjiang River in Hunan Province (Wang et al. 2008), and Pearl River Delta in Guangdong Province (Wong et al. 2002) of China. Therefore, efficient technologies must be developed to deal with the problem of Cd-contaminated soils.
The uptake and accumulation of Cd varied greatly not only among plant species but also among cultivars (Grant et al. 2008). Thus, several scientists proposed that selection of low-Cd crop cultivars is an effective method to reduce Cd entering food chain. Many low-Cd cultivars were selected from different crops such as rice (Liu et al. 2005; Yu et al. 2006), durum wheat (Berkelaar and Hale 2000), Chinese cabbage (Liu et al. 2010), peanut (Bell et al. 1997), hot pepper (Xin et al. 2013), and so on. Low-Cd cultivars, similar as the concepts of Cd-safe cultivars (Liu et al. 2010), pollution-safe cultivars (Yu et al. 2006), pollution free cultivars (Liu et al. 2012), Cd-excluding cultivars (Li et al. 2012), and Cd-excluder genotypes (Liu et al. 2009), refer to cultivars whose edible parts can accumulate Cd at a low enough level for safe consumption even when grown in Cd-contaminated soils. Therefore, it may be a feasible way to provide sufficient and safe food for the people located in the areas where the soils are lightly contaminated by Cd.
Sweet potato (Ipomoea batatas (L.) Lam.) is the world’s seventh most important food crop after wheat, rice, maize, potato, barley, and cassava (Ray and Ravi 2005). Its nutritional advantages, including high levels of β-carotene, anthocyanins, and minerals (calcium, iron, and potassium), make it a popular food in the world (Woolfe 1992). China produces about 90 % of the world’s production of ~117 million tons of sweet potatoes (Jung et al. 2011). However, it was reported that more than 13,000 ha of farmland are contaminated by Cd in the 11 provinces of China (Biao and Nan 2000), and the soil environmental quality is becoming worse and worse (Zhao et al. 2015). As a result, the selection of sweet potato cultivars with low Cd accumulation can help produce safe sweet potatoes in China.
The edible roots of sweet potato are true roots, so sweet potato may have a particular pathway of Cd uptake and distribution when compared with non-root crops. It is well known that calcium (Ca) is not phloem mobile and it is transported only in the xylem, but Cd can move both in the xylem and phloem. For instance, in potato, whose tubers have a quite low concentration of Ca because of the low transpiration rates, Cd was mainly transported from shoots to tubers via the phloem (Reid et al. 2003). However, in contrast with potato, sweet potato has a high Ca content in its edible roots. Therefore, the determination of which pathway, xylem or phloem, is more important in Cd accumulation in the edible roots of sweet potato and is vital to control the concentration of Cd in sweet potato.
The objectives of this study were to screen for low-Cd cultivars from the tested cultivars of sweet potato and to determine differences in the uptake and translocation of Cd in cultivars of sweet potato. Low-Cd cultivars were also compared with high-Cd cultivars to provide some insight into the likely pathways for movement of Cd into edible roots of sweet potato.
Materials and methods
Experiment site and soil
A pot experiment was conducted in an experimental garden of the Hunan Institute of Technology (112° 41′ E, 26° 52′ E), Hunan Province, China. The tested soil was collected from a Cd-contaminated farm near the Hunan Institute of Technology. The soil was air-dried and ground to pass through a 5-mm sieve. The general physicochemical properties of the soil are listed in Table 1. The total Cd concentration in the tested soil (0.95 mg kg−1) exceeded the maximum level (ML) (0.3 mg kg−1) regulated by the Farmland Environmental Quality Evaluation Standards for Edible Agricultural Products (State Environmental Protection Administration of China 2006). Therefore, the tested soil is considered as Cd-contaminated and served as a treatment (T1) without adding Cd2+. Two other Cd treatments (T2 and T3) were obtained by mixing T1 soil with appropriate amounts of Cd in the form of Cd(NO3)2·4H2O in this experiment. Each of the T2 and T3 soils were placed in a large basin and watered. The moist soils equilibrated for 4 months in order to balance the various absorption mechanisms in the soils (Alexander et al. 2006). For T2 and T3 treatments, the final soil total Cd concentrations were 1.67 and 2.91 mg kg−1, respectively; the DTPA-extractable Cd concentrations were 1.03 and 1.46 mg kg−1, respectively.
Plant materials
Thirty sweet potato cultivars (Table 2) provided by Hunan Crop Research Institute were used in this study. Five kilograms of prepared soil was put into each plastic pot with diameters 24 cm (top) and 20 cm (bottom) and a height of 30 cm. Shoots with uniform size were selected and used for cutting propagation. One shoot was put into each pot in order to get one seedling on May 1, 2013. All plants were watered daily with tap water (no Cd detected). A solid compound fertilizer (N:P:K = 15:15:15) was applied to the soil at the rate of 5.0 g per pot every 2 weeks.
Sampling and chemical analysis
The plants were harvested on October 1, 2013 and separated into shoots, edible roots, and feeder roots. Edible roots and feeder roots were rinsed with tap water to remove soil and then desorbed for 15 min in ice-cold 5 mM CaCl2 solution (5 mM Mes-Tris, pH 6.0). Shoots were rinsed with tap water, and then all samples were washed twice with deionized water for approximately 3 min. Edible roots were cut into pieces about 3-mm thick, and then all samples were dried first at 105 °C for 20 min and then at 70 °C in an oven until completely dry. Fresh and dry weights were recorded. The plant samples were ground into powders, passed through a 0.149-mm sieve and then digested with HNO3-H2O2 (10:3, v/v) in a microwave oven (Microwave digester XT-9900A, Shanghai Xintuo Analytical Instruments Co., Ltd., China). Concentrations of Cd in digested solutions were determined with a flame atomic absorption spectrophotometer (Hitachi Z-2300, Japan). Quality control of the analytical measurements was performed using blank samples and certified reference materials of plant GBW07605 (provided by the National Research Center for CRM, China).
Safety standard and statistical methods
To evaluate the safety of edible roots of the tested sweet potato cultivars, the General standard for Contaminants and Toxins in Food and Feed (CODEX STAN 193-1995, Revision 4, 2009, http://www.codexalimentarius.org/standards/list-of-standards/en/?no_cache=1) was used in this study. The Codex ML for Cd is 0.1 mg kg−1 (fw) in root and tuber vegetables. All the data was statistically processed on a computer using the Excel 2003 and SPSS 13.0. The data was analyzed using a least significant difference (LSD) test based on a two-way ANOVA. Correlations were assessed using the Pearson correlation coefficient.
Results
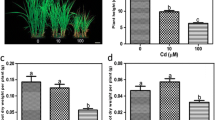
Edible root biomass response to Cd exposure
The variation of biomass is considered as a good indicator of plant response to Cd exposure (Shi and Cai 2009). Edible root biomass of sweet potato was significantly influenced by Cd treatment, cultivar, and interaction between the two factors (p < 0.01) (Table 3), which indicated that edible root biomass of sweet potato was determined not only by the levels of Cd in soils but also by genetic factors. The edible root biomasses of 16 cultivars in both T2 and T3 treatments did not decrease significantly compared with those in T1 treatment (Fig. 1). Furthermore, the edible root biomasses of four out of the 16 cultivars (Nos. 3, 8, 11, and 17) in T3 treatment were even significantly higher (p < 0.05) than those in T1 treatment (Fig. 1). These results indicated that the four cultivars had a greater tolerance to Cd toxicity than those cultivars whose edible root biomasses significantly decreased with increasing Cd in soil.
Accumulation of Cd in edible roots
Significant differences (p < 0.05) in edible root Cd concentration were found among the 30 cultivars in each treatment (Table 4), ranging from 0.00 to 0.41, from 0.01 to 0.87, and from 0.33 to 2.65, with averages of 0.14, 0.26, and 0.83 mg kg−1 for T1, T2, and T3 treatments, respectively. The differences between the highest and lowest edible root Cd concentration exceeded eightfold in the three treatments. The edible root Cd concentrations in T3 treatment were, on average, 5.9 and 3.2 times higher than those in T1 and T2 treatments, respectively. The Codex ML for Cd is 0.1 mg kg−1 (fw) in root and tuber vegetables, and the average water content in sweet potato edible roots is 72 %. As a result, the edible roots whose Cd concentration was lower than 0.36 mg kg−1 (dw) are safe for consumption. The edible root Cd concentrations of only two cultivars, Nos. 7 and 11, exceeded 0.36 mg kg−1 in T1 treatment. Most cultivars still met the standard in T2 treatment; only the Cd edible root concentrations in the five cultivars, Nos. 3, 7, 11, 19, and 29, exceeded the Codex ML for Cd. However, in T3 treatment, only two cultivars, Nos. 15 and 26, met the standard. It was also found that Cd concentrations in edible roots of sweet potato were significantly affected by Cd treatment, cultivar, and the interaction between the two factors (p < 0.01) (Table 3). This result indicates that the Cd concentrations in edible roots of sweet potato depended on genetic factors besides soil Cd levels. Consequently, the characteristic of Cd accumulation in sweet potato cultivars can be used to select low- and high-Cd cultivars. The stability of Cd accumulation in edible roots was considered when we identified low-Cd sweet potato cultivars. The seven cultivars, Nos. 10, 12, 14, 15, 16, 26, and 28, were always among the top ten cultivars with the lowest edible root Cd concentration in each Cd treatment (Table 4). Furthermore, there was no significant difference in edible root Cd concentration among Nos. 10, 12, 15, and 16 in each treatment. Therefore, the four cultivars were identified as low-Cd cultivars. On the other hand, Nos. 3, 7, 11, and 19 were always among the top four cultivars with the highest edible root Cd concentration in the T1 and T2 treatments, and they were the second, seventh, sixth, and eighth cultivars with the highest edible root Cd concentration in the T3 treatment, respectively (Table 4). Thus, these four cultivars were selected as high-Cd cultivars.
Cd concentration in feeder roots and shoots
Cd concentration in feeder roots differs in different cultivars (Fig. 2), but there is no tendency that low-Cd cultivars have higher or lower feeder root Cd concentration than high-Cd cultivars. For example, in T2 treatment, Cd concentrations in feeder roots of high-Cd cultivars, Nos. 3, 7, and 11, are not significantly different than those in low-Cd cultivars Nos. 10, 15, and 16. There is also no correlation that low-Cd cultivars have higher or lower shoot Cd concentration than high-Cd cultivars. For example, in T1 treatment, Cd concentrations in the shoots of high-Cd cultivars, Nos. 3, 7, 11, and 19, have no significant difference with those in low-Cd cultivars, Nos. 10, 12, and 16. This result indicates that low Cd concentration in shoot or feeder root does not mean low Cd levels in edible roots.
Ratios of Cd concentration in different organs
The highest shoot/feeder root ratio of Cd concentration was observed in No. 19, a high-Cd cultivar (Table 5). However, a high shoot/feeder root ratio of Cd concentration was also found in the low-Cd cultivar (No. 15). There was no correlation of high-Cd cultivars to a higher shoot/feeder root ratio (0.11–0.32) than low-Cd cultivars (0.07–0.38). These results indicate that Cd translocation from feeder root to shoot is not a limiting factor that influences Cd concentration in edible roots.
There was a tendency of high-Cd cultivars to have a higher edible root/shoot ratio of Cd concentration (0.25–0.60) than low-Cd cultivars (0.00–0.18) (Table 5). This indicates that Cd translocation from shoots to edible roots is more difficult in low-Cd cultivars than in high-Cd cultivars. Furthermore, the edible root/feeder root ratio in the low-Cd cultivars (0.00–0.04) was significantly lower than that (0.05–0.11) in the high-Cd cultivars in each treatment. This result demonstrates that Cd translocation from feeder roots to edible roots is more difficult in low-Cd cultivars than in high-Cd cultivars. Low Cd accumulation in low-Cd cultivars may have a greater ability to retain Cd in feeder roots and shoots, which is probably associated with the mechanism involved in Cd translocation from feeder roots and shoots to edible roots, but not Cd translocation from feeder root to shoots, which is considered the most important step in Cd concentration in leafy vegetables and grain or fruits.
Cd partitioning in low- and high-Cd cultivars
The overall distribution of Cd in all tissues of low- and high-Cd cultivars of sweet potato after a 5-month growth is shown in Fig. 3. In all treatments, the proportions of total Cd that were retained in edible roots of low-Cd cultivars, Nos. 10, 12, 15, and 16, were lower than those in high-Cd cultivars, Nos. 3, 7, 11, and 19, except in the case of No. 7 in T3 treatment, which had a similar Cd proportion retained in edible roots as low-Cd cultivars; this exception is possibly due to the low edible root biomass of No. 7 in T3 treatment. On the other hand, there is no trend that a higher proportion of Cd was retained in the feeder roots of low-Cd cultivars when compared with high-Cd cultivars. The low cultivars did not also have a higher proportion of Cd retained in shoots. However, the total proportions of Cd retained in shoots and feeder roots were higher in low-Cd cultivars than those in high-Cd cultivars. These results indicate that the genotypic differences in edible root Cd concentration are due to differences in partitioning of total Cd among different organs.
Discussion
Edible root biomass response to Cd toxicity
In this study, different sweet potato cultivars showed different responses to Cd toxicity. Similarly, different responses to Cd among cultivars were also found in rice (Yu et al. 2006), Chinese cabbage (Liu et al. 2010), hot peppers (Xin et al. 2013), mustard (Gill et al. 2011), etc. Cadmium accumulation in plants may affect nutrient uptake, alter the chloroplast ultrastructure, inactivate enzymes of CO2 fixation, inhibit photosynthesis and induce lipid peroxidation and antioxidant machinery, and ultimately affect plant growth and yield (Gill et al. 2011). The different abilities of cultivars to detoxify Cd result in different tolerance to Cd toxicity among cultivars. It was reported that the enhanced activities of antioxidative enzymes, ascorbate peroxidase, catalase, and glutathione reductase, and lowered activity of superoxide dismutase in a higher tolerance cultivar Varuna alleviated Cd stress and protected the photosynthetic activity (Mobin and Khan 2007). Different parts of the same plant may have different responses to Cd toxicity. For example, a delayed response was observed in Arabidopsis leaves with respect to roots due to limited Cd translocation (Jozefczak et al. 2014). Thus, the leaves were protected up to a certain level, allowing them time to activate their defense mechanisms. The mechanisms of Cd detoxification in the edible roots of sweet potato are still not well known and need further investigation.
Selection of low Cd cultivars
The first aim of this study is to select sweet potato cultivars with low-Cd accumulation in their edible roots. A more than eightfold difference in the edible Cd concentrations was observed among the 30 tested sweet potato cultivars in all the Cd treatments. The variation was wide enough to select low-Cd cultivars (Xin et al. 2013). We intended to identify low-Cd sweet potato cultivars according to their edible root Cd concentrations and the stability of Cd accumulation. The edible root Cd concentrations in Nos. 10, 12, 15, and 16 always showed no significant differences in all the treatments and were lower than the Codex ML for Cd in the T1 and T2 treatments. The four cultivars were identified as low-Cd cultivars. It was worth noting that the biomasses of Nos. 12 and 15 were not affected by soil Cd levels. However, with increasing Cd in soil, the biomass of No. 10 significantly decreased and that of No. 16 increased firstly and then decreased. These results indicate that the low-Cd cultivars have different sensitivity to Cd toxicity.
The Cd concentration in edible roots of No. 15 was very close to the Codex ML for Cd in the T3 treatment. Thus, when Cd concentration in soil exceeds 2.91 mg kg−1, no sweet potato cultivars produced edible roots for safe consumption. Wright et al. (2012) also found that sweet potato grown in a location with 2.65 ± 0.04 mg kg−1 Cd in soil accumulated 0.61 ± 0.02 mg kg−1 (dw) Cd in its edible roots. Xin et al. (2013) reported that fruit Cd concentrations in low-Cd hot pepper cultivars exceeded the safety standard for human consumption when Cd levels in the soils reached 2.69 mg kg−1. Therefore, the safety of low-Cd crop cultivars is conditioned by the soil Cd level. In addition, Nos. 3, 7, 11, and 19 always had significantly higher edible root Cd concentrations than the low-Cd cultivars, which can pose a human health risk through the food chain, when grown in moderately Cd-contaminated soils. Therefore, the four cultivars were identified as high-Cd cultivars.
Cd translocation in sweet potato
In this study, it was found that the genotypic differences in edible root Cd are due to differences in partitioning. Some studies also found that Cd partitioning plays an important role in different Cd accumulations between cultivars. For example, it was found that in a high-Cd accumulator potato cultivar Kennebec, 75 % of the total plant Cd was found in the tubers, whereas in a low Cd accumulator potato cultivar Wilwash, tuber Cd only accounted for 43 % (Dunbar et al. 2003). It was also found that accumulation of Cd in the shoots was 13 % (low-Cd NIL) or 37 % (high-Cd NIL) of whole-plant Cd accumulation at grain maturity (Harris and Taylor 2013). However, it was reported that the significant differences in the Cd-partitioning ratio among rice genotypes had no correlation with Cd concentration of brown rice (He et al. 2006), meaning that the role of Cd partitioning in Cd accumulation between cultivars may differ among species, especially among the species with different edible parts.
The edible parts of sweet potato are its tuberous roots. Different from leafy vegetables, tuberous roots have low rates of transpiration, like grains and fruits. While Cd translocation to the shoot of some plants is mainly driven by mass flow due to transpiration (Liu et al. 2010; Salt et al. 1995), metal transport to the shoot primarily takes place through the xylem (Salt et al. 1998). For example, Ca is believed to be transported with water in the xylem (Busse and Palta 2006). Cd shares many physical similarities, such as charge and ionic radius, with Ca (Reid et al. 2003). Cd may follow a similar route to Ca for movement from soil into leaves. However, in the tubers of potatoes, where Ca content is very low, xylem was not considered an important way to translocate Cd to tubers due to the low transpiration rate in tuberous stem (Reid et al. 2003). Sweet potato, whose edible parts were its tuberous roots, also have low rates of transpiration. However, it was found that low-Cd cultivars had a significant difference in the edible root/feeder root ratio of Cd concentration when compared with high-Cd cultivars. This result indicates that Cd translocation through the xylem from feeder roots to edible roots maybe an important way to redistribute Cd in sweet potato. There are two kinds of feeder roots in sweet potato. One kind consists of the feeder roots which directly connect with the edible root and can provide water and iron for the edible roots. Cd absorbed by the feeder roots of the edible roots might translocate Cd to the edible root via the xylem (Fig. 4). This is consistent with the relative higher Ca concentration in sweet potato edible roots, which is different from Ca deficiency in potato (Busse and Palta 2006; Treche 1996). So, an important way of accumulating Cd is Cd translocation from feeder roots, probably partly from the feeder roots where edible roots develop from, to edible roots through the xylem. The other type is the common feeder roots, which can directly transport water and iron to shoots without passing through edible roots. These feeder roots may transport Cd to the shoot via the xylem and then to edible root via the phloem, just like Cd transport in potatoes. More experiments need to be conducted to investigate if the two kinds of feeder roots play different roles in Cd accumulation in edible roots.
Possible transport pathways of Cd from soil to edible roots of sweet potato. Cd absorbed by the feeder roots where edible roots develop might be loaded into the xylem and transported to edible roots. Cd absorbed by other feeder roots might be transported to the leaves via the xylem and then remobilized to edible roots via the phloem
In this study, we also found that the ratio of edible root/shoot Cd concentration also were significantly different between high and low Cd accumulation cultivars, which indicates that Cd translocation between the shoot and edible root is also an important factor that influences Cd concentration in roots. The phloem pathway may also play an important role in Cd accumulation in sweet potato (Fig. 4). It was reported that the phloem also plays an important role in Cd translocation to low-transpiration organs such as grains (Yoneyama et al. 2010), fruits (Xin et al. 2013), and tubers (Reid et al. 2003). Most Cd in rice grains accumulates through the phloem transport. Low-affinity cation transporter 1 (OsLCT1) in rice facilitates Cd loading to the sieve tube by supplying Cd from the parenchyma cells (Uraguchi and Fujiwara 2013). It was also suggested that in addition to directional xylem Cd transport, the phloem is a major vascular system for long-distance source to sink transport of Cd as PC-Cd and glutathione-Cd complexes in Brassica napus (Mendoza-Cózatl et al. 2008). However, Cd has limited phloem mobility in wheat; only a minor quantity of Cd is transported to the grain via the phloem in control shoots while a high percentage of Cd is retained in the peduncle (Herren and Feller 1997). In sweet potato, shoots usually have higher Cd than edible roots. The efficiency of Cd translocation from the shoot to root, which is probably through the phloem, might be an important factor that can affect Cd content in the edible root. The determination of whether the shoot or feeder root provides a bigger contribution in Cd accumulation in the edible root of sweet potato needs more experiments to test.
In conclusion, sweet potato cultivars Nos. 10, 12, 15, and 16 were selected as low-Cd cultivars in this study. The difference in edible roots’ Cd concentrations between low- and high-Cd cultivars resulted from the variations of Cd translocation from feeder roots to edible roots, and those from shoots to edible roots, but not those from feeder roots to shoots. Both xylem and phloem pathways are important for Cd accumulation in edible roots. In addition, more experiments should be conducted to investigate the contribution of feeder root (either directly connected with edible root or indirectly connected) and shoot to Cd accumulation in sweet potato.
References
Alexander P, Alloway B, Dourado A (2006) Genotypic variations in the accumulation of Cd, Cu, Pb and Zn exhibited by six commonly grown vegetables. Environ Pollut 144:736–745
Bell M, McLaughlin MJ, Wright G, Cruickshank A (1997) Inter-and intra-specific variation in accumulation of cadmium by peanut, soybean, and navybean. Aust J Agric Res 48:1151–1160
Berkelaar E, Hale B (2000) The relationship between root morphology and cadmium accumulation in seedlings of two durum wheat cultivars. Can J Bot 78:381–387
Biao ZJ, Nan HW (2000) Advances on physiological and ecological effects of cadmium on plants. Acta Ecol Sin 20:514–523
Busse JS, Palta JP (2006) Investigating the in vivo calcium transport path to developing potato tuber using 45Ca: a new concept in potato tuber calcium nutrition. Physiol Plant 128:313–323
Dunbar KR, McLaughlin MJ, Reid RJ (2003) The uptake and partitioning of cadmium in two cultivars of potato (Solanum tuberosum L.). J Exp Bot 54:349–354
Gill SS, Khan NA, Tuteja N (2011) Differential cadmium stress tolerance in five Indian mustard (Brassica juncea L.) cultivars: an evaluation of the role of antioxidant machinery. Plant Signal Behav 6:293–300
Grant C, Clarke J, Duguid S, Chaney R (2008) Selection and breeding of plant cultivars to minimize cadmium accumulation. Sci Total Environ 390:301–310
Harris NS, Taylor GJ (2013) Cadmium uptake and partitioning in durum wheat during grain filling. BMC Plant Biol 13:103
He J, Zhu C, Ren Y, Yan Y, Jiang D (2006) Genotypic variation in grain cadmium concentration of lowland rice. J Plant Nutr Soil Sci 169:711–716
Herren T, Feller U (1997) Transport of cadmium via xylem and phloem in maturing wheat shoots: comparison with the translocation of zinc, strontium and rubidium. Ann Bot 80:623–628
Ishihara T, Kobayashi E, Okubo Y, Suwazono Y, Kido T, Nishijyo M, Nakagawa H, Nogawa K (2001) Association between cadmium concentration in rice and mortality in the Jinzu River basin, Japan. Toxicology 163:23–28
Jozefczak M, Keunen E, Schat H, Bliek M, Hernández LE, Carleer R, Remans T, Bohler S, Vangronsveld J, Cuypers A (2014) Differential response of Arabidopsis leaves and roots to cadmium: glutathione-related chelating capacity vs antioxidant capacity. Plant Physiol Biochem 83:1–9
Jung J-K, Lee S-U, Kozukue N, Levin CE, Friedman M (2011) Distribution of phenolic compounds and antioxidative activities in parts of sweet potato (Ipomoea batata L.) plants and in home processed roots. J Food Compos Anal 24:29–37
Kosolsaksakul P, Farmer JG, Oliver IW, Graham MC (2014) Geochemical associations and availability of cadmium (Cd) in a paddy field system, northwestern Thailand. Environ Pollut 187:153–161
Li X, Zhou Q, Wei S, Ren W (2012) Identification of cadmium-excluding welsh onion (Allium fistulosum L.) cultivars and their mechanisms of low cadmium accumulation. Environ Sci Pollut Res 19:1773–1780
Liu J, Zhu Q, Zhang Z, Xu J, Yang J, Wong MH (2005) Variations in cadmium accumulation among rice cultivars and types and the selection of cultivars for reducing cadmium in the diet. J Sci Food Agric 85:147–153
Liu W, Zhou Q, Sun Y, Liu R (2009) Identification of Chinese cabbage genotypes with low cadmium accumulation for food safety. Environ Pollut 157:1961–1967
Liu W, Zhou Q, An J, Sun Y, Liu R (2010) Variations in cadmium accumulation among Chinese cabbage cultivars and screening for Cd-safe cultivars. J Hazard Mater 173:737–743
Liu L, Hu L, Tang J, Li Y, Zhang Q, Chen X (2012) Food safety assessment of planting patterns of four vegetable-type crops grown in soil contaminated by electronic waste activities. J Environ Manag 93:22–30
Mendoza-Cózatl DG, Butko E, Springer F, Torpey JW, Komives EA, Kehr J, Schroeder JI (2008) Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant J 54:249–259
Mobin M, Khan NA (2007) Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164:601–610
Ray R, Ravi V (2005) Post harvest spoilage of sweetpotato in tropics and control measures. Crit Rev Food Sci Nutr 45:623–644
Reid RJ, Dunbar KR, McLaughlin MJ (2003) Cadmium loading into potato tubers: the roles of the periderm, xylem and phloem. Plant Cell Environ 26:201–206
Salt DE, Prince RC, Pickering IJ, Raskin I (1995) Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol 109:1427–1433
Salt DE, Smith R, Raskin I (1998) Phytoremediation. Annu Rev Plant Biol 49:643–668
Shi G, Cai Q (2009) Cadmium tolerance and accumulation in eight potential energy crops. Biotechnol Adv 27:555–561
State Environmental Protection Administration of China (2006) Farmland environmental quality evaluation standards for edible agricultural products (HJ 332–2006). China Environment Press, Beijing
Trèche S (1996) Tropical root and tuber crops as human staple food. In: Congresso Latino Americano de Raizes Tropicais 1, Sao Pedro, ORSTOM, Montpellier, pp 10
Uraguchi S, Fujiwara T (2013) Rice breaks ground for cadmium-free cereals. Curr Opin Plant Biol 16:328–334
Wang L, Guo Z, Xiao X, Chen T, Liao X, Song J, Wu B (2008) Heavy metal pollution of soils and vegetables in the midstream and downstream of the Xiangjiang River, Hunan Province. J Geogr Sci 18:353–362
Wong S, Li X, Zhang G, Qi S, Min Y (2002) Heavy metals in agricultural soils of the Pearl River Delta, South China. Environ Pollut 119:33–44
Woolfe JA (1992) Sweet potato: An untapped food resource. Cambridge University Press, Cambridge
Wright V, Jones S, Omoruyi FO (2012) Effect of bauxite mineralized soil on residual metal levels in some post harvest food crops in Jamaica. Bull Environ Contam Toxicol 89:824–830
Xin J, Huang B, Liu A, Zhou W, Liao K (2013) Identification of hot pepper cultivars containing low Cd levels after growing on contaminated soil: uptake and redistribution to the edible plant parts. Plant Soil 373:415–425
Yoneyama T, Gosho T, Kato M, Goto S, Hayashi H (2010) Xylem and phloem transport of Cd, Zn and Fe into the grains of rice plants (Oryza sativa L.) grown in continuously flooded Cd-contaminated soil. Soil Sci Plant Nutri 56:445–453
Yu H, Wang J, Fang W, Yuan J, Yang Z (2006) Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice. Sci Total Environ 370:302–309
Zhao F, Ma Y, Zhu Y, Tang Z, McGrath SP (2015) Soil contamination in China: current status and mitigation strategies. Environ Sci Technol 45(2):750–759
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 41201320 and No. 41101303), Hunan Provincial Natural Science Foundation of China (No. 2015JJ6026 and No. 14JJ7082), and China Scholarship Council (CSC). We also thank Hunan Crop Research Institute to provide the sweet potato cultivars.
Compliance with Ethical Standards
The authors declare no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Huang, B., Xin, J., Dai, H. et al. Identification of low-Cd cultivars of sweet potato (Ipomoea batatas (L.) Lam.) after growing on Cd-contaminated soil: uptake and partitioning to the edible roots. Environ Sci Pollut Res 22, 11813–11821 (2015). https://doi.org/10.1007/s11356-015-4449-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4449-z