Abstract
Aims
This study aimed to screen and identify low-cadmium (Cd) hot pepper (Capsicum annuum L.) cultivars and to clarify the mechanisms of low Cd accumulation in fruits.
Methods
A pot experiment was conducted to investigate the variations of fruit Cd concentration among 30 hot pepper cultivars and to determine the differences in uptake and translocation of Cd between low- and high-Cd cultivars in the control and two Cd treatments.
Results
There are significant differences among the cultivars in their ability to accumulate Cd in fruits. Fruit Cd concentrations are positively and significantly correlated with the translocation of Cd from roots to aboveground parts and the Cd concentrations of leaves and stems. However, no correlation was observed between the fruit’s Cd concentration and the root’s Cd uptake ability.
Conclusions
Two hot pepper cultivars, Yeshengchaotianjiao (No. 16) and Heilameixiaojianjiao (No. 23), were identified as low-Cd cultivars, and two, Jinfuzaohuangjiao (No. 13) and Shuduhong (No. 18), were treated as high-Cd cultivars. The difference in fruit Cd concentrations between low- and high-Cd cultivars is attributable to the difference in Cd translocation from roots to aboveground parts and from leaves and stems to fruits, rather than to the root’s Cd uptake ability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a non-essential and highly toxic element to plants and can pose a human health risk through the food chain (Grant et al. 2008). Moreover, Cd levels in agricultural soils have been elevated continuously due to anthropogenic activities, such as mining and smelting, electroplating, and the use of sewage sludge and phosphate fertilizers (Wong et al. 2002; Wong 2003). Therefore, effective technologies to reduce Cd accumulation in crops are urgent for food safety.
In recent years, many soil cleanup techniques, including the excavation and removal of the top contaminated soil layer, phytoremediation, and the washing of contaminated soils with strong acids and heavy metal chelators, have been used to remove Cd from Cd-contaminated agricultural soils. However, most of these techniques are costly, time-consuming, cause soil disturbances, and are not readily accepted by the general public (Zhou and Song 2004; Bhargava et al. 2012). Recently, researchers found that the uptake and accumulation of Cd varied greatly not only among plant species but also among cultivars (Greger and Löfstedt 2004; Liu et al. 2007; Grant et al. 2008; Liu et al. 2009). Genotypic variations and genotype dependence in the Cd accumulation of many crop species, such as potato (Solanum tuberosum L.) (Dunbar et al. 2003), wheat (Triticum aestivum L.) (Greger and Löfstedt 2004), rice (Oryza sativa L.) (Yu et al. 2006; Liu et al. 2007), Chinese cabbage (Brassica pekinensis L.) (Liu et al. 2009), and water spinach (Ipomoea aquatica Forsk.) (Huang et al. 2009; Xin et al. 2010), have been well reported. On this basis, the concepts of pollution-safe cultivars (Yu et al. 2006), Cd-excluder genotypes (Liu et al. 2009), Cd-excluding cultivars (Li et al. 2012), and pollution free cultivars (Liu et al. 2012), have been proposed. These are essentially low-Cd cultivars (Clarke et al. 2002), referring to cultivars whose edible parts accumulate Cd at a low enough level for safe consumption when grown in Cd-contaminated soils. Therefore, the selection of low-Cd crop cultivars is an effective approach to reduce the risk of soil Cd entering the human diet without fallowing agricultural soils.
Hot pepper (Capsicum annuum L.) is one of the most important crops in the world and is the second largest vegetable crop in China. At present, the cultivated area per year in China is about 1.3 million hm2, producing 27 million tons of pepper (Xu et al. 2008). As about 70 % of the Cd intake by humans originates from vegetable foodstuffs (Günther et al. 2000), it is extremely important to minimize the Cd concentrations in hot pepper fruits by using low-Cd cultivars. However, relevant information on screening for low-Cd hot pepper cultivars is still quite limited. Additionally, it is necessary to have a good understanding of the Cd accumulation mechanisms in hot pepper fruits to identify low-Cd cultivars.
As reported by many researchers, the transport processes mediating Cd accumulation in the aerial plant parts includes the uptake of Cd by roots, root-to-shoot Cd translocation, and xylem-to-phloem Cd transfer (Herren and Feller 1997; Uraguchi et al. 2009; Xin et al. 2012; Su et al. 2013). After Cd reaches root cell membranes via the apoplasmic pathway, it enters the plant root system by some other nutrient metabolic pathway, such as iron, calcium, or zinc (Cosio et al. 2004). A portion of the Cd that enters the root system is translocated to the aboveground parts via the xylem in the form of Cd-complexes with organic acids, phytochelatins, and metallothioneins (Salt et al. 1995). In hot pepper plants, cadmium is probably either translocated directly via the xylem to the fruits or is translocated from leaves to the fruits via the phloem. Consequently, the accumulation of Cd in hot pepper fruits may be affected by root Cd uptake and Cd translocation via the xylem or phloem. Furthermore, the mechanisms responsible for Cd accumulation in fruits are also unknown.
The objectives of this study were to screen for low-Cd cultivars from the tested hot pepper cultivars and to determine differences in the uptake and translocation of Cd in hot pepper cultivars to elucidate the mechanisms of low Cd accumulation in fruits. We hypothesized that there would be significant differences in the fruit Cd concentration among the 30 hot pepper cultivars. We also hypothesized that the Cd accumulation in hot pepper fruits would be related to root Cd uptake and the Cd translocation from roots to aboveground parts.
Materials and methods
Experimental site and soil
A pot experiment was carried out in an experimental garden of Hunan Institute of Technology (112°41′E, 26°52′N), Hunan Province, China. The experimental soil was collected from a vegetable farm at the institute, was air-dried, and ground to pass through a 5 mm sieve. The soil was a sandy loam whose main physical and chemical properties were measured according to the analytical methods described by Lu (2000). The soil pH, organic matter content, cation exchange capacity, total nitrogen, available P, available K, total Cd and DTPA-extractable (bio-available) Cd were 6.35, 1.84 %, 8.9 cmol kg−1, 1.7 g kg−1, 90.3 mg kg−1, 115.4 mg kg−1, 0.28 mg kg−1 and 0.09 mg kg−1, respectively. According to the Farmland Environmental Quality Evaluation Standards for Edible Agricultural Products (HJ 332-2006), the maximum level (ML) for Cd is 0.3 mg kg−1; therefore, the tested soil is clean and severed as the control in this experiment. Two Cd treatments, low-Cd and high-Cd with target concentrations of 1.0 and 2.5 mg kg−1, respectively, were created by mixing the clean soil with appropriate amounts of Cd in the form of Cd(NO3)2·4H2O. Each of the two soils was placed in a large basin, watered, and left to equilibrate outdoors under a waterproof tarpaulin for about 4 months. This long period allowed for the balancing of the various sorption mechanisms in the soils (Alexander et al. 2006). For low-Cd and high-Cd treatments, the final soil total Cd concentrations were 1.16 and 2.69 mg kg−1, respectively, and the DTPA-extractable Cd concentrations were 0.58 and 1.62 mg kg−1, respectively.
Plant materials and experimental design
There were 30 hot pepper cultivars (Table 1) used in this study. Seeds of the cultivars were collected from a seed market in Changsha, China. Plastic pots, with diameters of 18 cm (top) and 13 cm (bottom) and a height of 15 cm, were each filled with 3.0 kg of the prepared soil. The seeds were sown into the soil in the pots on March 25, 2012 and watered daily with tap water. The experiment was laid out in a completely randomized design with three replicate pots per treatment. Fifteen days after germination, the seedlings were thinned to one per pot. A solid compound fertilizer (N:P:K = 15:15:15) was applied into the soil at 3.0 g per pot every 2 weeks thereafter.
Sampling and chemical analysis
The whole plants were harvested after the 120-day growth period. All of the fruits from each plant were harvested to reduce the influence of fruit position on the fruit Cd concentrations. Fruits, leaves, stems and roots were separately rinsed with tap water, and roots were desorbed for 15 min in ice-cold 5 mM CaCl2 solution (5 mM Mes-Tris, pH 6.0). All samples were thoroughly washed with deionized water, dried at 105 °C for 20 min and then at 70 °C to a constant weight, and the fresh and dry weights were recorded. The dried plant samples were crushed to pass through a 0.149 mm sieve for chemical analysis. Concentrations of Cd in the dry samples were determined with a flame atomic absorption spectrophotometer (Hitachi Z-2300, Japan) after digestion with HNO3-H2O2 (10:3, v/v) in a microwave oven (Microwave digester XT-9900A, Shanghai Xintuo Analytical Instruments Co., Ltd., China). A Certified Reference Material (CRM) of plant GBW07605 (provided by the National Research Center for CRM, China) was used for quality assurance and quality control (QA/QC) of the Cd analysis.
Safety standard and statistical methods
The General Standard for Contaminants and Toxins in Food and Feed (CODEX STAN 193-1995, Revision 4, 2009, http://www.codexalimentarius.org/standards/list-of-standards/en/?no_cache=1) was used to evaluate the safety of consuming fruits of the tested hot pepper cultivars. The Codex ML for Cd in fruiting vegetables is 0.05 mg kg−1 (fresh weight, fw). The cadmium uptake ability of plants can be estimated by the net uptake of Cd via roots, which was calculated as the total amount of Cd in the whole plant divided by the root dry weight. The Cd translocation to the aboveground parts (including fruits, leaves and stems) was calculated as the amount of Cd in the aboveground parts in relation to the total amount of Cd in the whole plant (Greger and Löfstedt 2004). Data were statistically analyzed using the least significant difference (LSD) test based on a two-way ANOVA using Excel 2003 and SPSS 13.0. Correlations were assessed using the Pearson correlation coefficient. Results were expressed on a dry weight (dw) basis.
Results
Fruit biomass response to Cd exposure
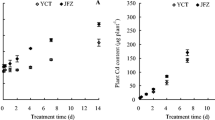
Fruit biomass (dw) of hot pepper was significantly affected by cultivars, Cd treatments, and interaction between the two factors (p < 0.01) (Table 2), indicating that fruit biomass of hot pepper was determined not only by genetic factors, but also by Cd levels in soils. All of the cultivars had some tolerance to Cd toxicity. The fruit biomasses of 30 cultivars in the two Cd treatments did not decrease significantly (p > 0.05) compared with those in the control (Fig. 1). Furthermore, the fruit biomasses of No. 3, 8, 12, 23, 25, 26, and 30 in the high-Cd treatment were significantly higher (p < 0.05) than those in the control. This indicated that the seven cultivars had a greater tolerance to Cd toxicity than did the other cultivars.
Variations in Cd accumulation
Significant differences (p < 0.05) in fruit Cd concentration were found among the 30 cultivars in each treatment (Table 2), ranging from 0.07 to 0.23, from 0.39 to 1.23, and from 1.32 to 3.28, with the averages of 0.15, 0.78 and 2.39 mg kg−1 for control, low- and high-Cd treatments, respectively. In the same three treatments, the differences between the highest and lowest fruit Cd concentrations were 3.3-, 3.2- and 2.5-fold, respectively. In addition, the fruit Cd concentrations in the high-Cd treatment were, on average, 16.8 (10.8–27.7) and 3.1 (2.1–5.0) times higher than those in the control and low-Cd treatment, respectively. The differences in fruit Cd concentrations were much greater than the differences in soil total Cd concentrations between high-Cd and control (9.6 times) and between high-Cd and low-Cd (2.3 times) but close to the differences of DTPA-extractable Cd (18.0 and 2.8 times, respectively). The fruit Cd concentrations of all cultivars (fw) in the control treatment were lower than 0.5 mg kg−1; thus they were below the Codex ML for Cd (0.05 mg kg−1) because the water content of hot pepper fruits was 90 % (the mean value in this study). However, only two cultivars, Nos.16 and 23, met the standard in the low-Cd treatment, and the fruit Cd concentrations (fw) of all the cultivars exceeded 0.05 mg kg−1 in the high-Cd treatment. These results indicate that most of the hot pepper cultivars easily take up Cd, even from slightly Cd-contaminated soils. Furthermore, Nos.16 and 23 always accumulated less Cd, whereas Nos. 13 and 18 had consistently higher fruit Cd concentrations than most of the other cultivars in the three treatments. Cd concentrations in fruits of hot pepper were also significantly influenced by cultivars, Cd treatments and the interaction between the two factors (p < 0.01) (Table 2), indicating that fruit Cd concentration was determined by genetic factors besides Cd exposure levels. Thus, the trait of Cd accumulation in hot pepper fruits can be used to select low-Cd cultivars in certain Cd-contaminated soils.
Cd uptake by roots
The highest root Cd concentration was always observed in No. 16 (low-Cd cultivar) and was significantly higher (p < 0.05) than that of No. 23 (low-Cd cultivar). However, no significant differences were found among No. 23 and the two high-Cd cultivars, Nos. 13 and 18, except that root Cd concentration of No. 18 was higher (p < 0.05) than that of No. 13 in the high-Cd treatment (Table 4). Interestingly, there were significant differences (p < 0.05) in the root Cd uptake among the four cultivars (Table 4). Under the three treatments, No. 16 always had a higher (p < 0.05) Cd uptake ability through the roots than No. 23, and the two high-Cd cultivars had similar Cd uptake abilities. Moreover, the Cd uptake ability of the high-Cd cultivars was not obviously superior to that of the low-Cd cultivars in each treatment. These results indicate that the difference of Cd accumulation in fruits between the low- and high-Cd cultivars is not affected by the root’s uptake of Cd.
Ratios of Cd concentrations in different organs
In general, there was a tendency for the low-Cd cultivars to have lower leaf Cd concentrations than the high-Cd cultivars, but this was only consistently significant in the low-Cd treatment (Fig. 2). The Cd concentrations in the leaves of the four cultivars were 2.72–6.83, 2.69–5.55 and 2.71–4.70 times higher than those in the fruits of the control, low-Cd and high-Cd treatments, respectively (Table 5). The highest leaf: fruit ratio of Cd concentration was always observed in No. 16 in each treatment, but the leaf: fruit ratio in the other low-Cd cultivar (No. 23) was higher than those in the two high-Cd cultivars in the low-Cd treatment (Table 5). These results demonstrate that Cd translocation from leaves to fruits is more difficult in No. 16 than in other cultivars.
Concentrations of Cd in the leaves and stems of low- and high-Cd hot pepper (Capsicum annuum L.) cultivars under different treatments. Different lower-case and upper-case letters on bars indicate a significant difference of Cd concentrations in leaves and in stems at p < 0.05 among cultivars, respectively. Error bars represent the standard deviation (n = 3)
The stem Cd concentrations in the low-Cd cultivars were significantly lower (p < 0.05) than those in the high-Cd cultivars in each treatment except the Cd concentration of No. 16 was similar with that of No. 18 in the low-Cd treatment (Fig. 2). The stem: fruit ratios of Cd concentrations for the four cultivars were 2.69–8.71, 3.86–6.99 and 3.08–5.26 in the control, low-Cd and high-Cd treatments, respectively (Table 5). The stem: fruit ratios in the low-Cd cultivars were significantly higher (p < 0.05) than those in the high-Cd cultivars in each treatment except that no difference was observed between Nos. 13 and 23 in the high-Cd treatment (Table 5). This indicates that Cd translocation from stems to fruits is more difficult in low-Cd cultivars than in high-Cd cultivars.
The highest root: fruit ratio of Cd concentration was always observed in No. 16 and was significantly higher than that in No. 23 in each treatment, but no significant difference was observed between the two high-Cd cultivars (Table 5). The root: fruit ratio of Cd concentration in the two low-Cd cultivars were always significantly higher (p < 0.05) than those in the two high-Cd cultivars except for Nos. 18 and 23 in the high-Cd treatment (Table 5). Additionally, the two low-Cd cultivars always had lower translocation levels of Cd from roots to aboveground parts than the two high-Cd cultivars (Fig. 3). These results indicate that low-Cd cultivars may have a greater ability to retain Cd in roots, which is probably associated with the mechanism involved in Cd translocation from the roots to the aboveground plant parts.
Percentages of Cd translocation to aboveground parts in low- and high-Cd hot pepper (Capsicum annuum L.) cultivars under different treatments. Different lower-case letters on bars indicate a significant difference at p < 0.05 among cultivars under the same treatment. Error bars represent the standard deviation (n = 3)
Correlation tests
To estimate Cd translocation to the fruits of hot pepper cultivars, the relationships between Cd concentrations in fruits and Cd concentrations in other organs, including leaves, stems and roots were analyzed (Table 6). A significant positive correlation (p < 0.01) was observed between Cd concentrations in fruits and leaves in each treatment. There was also a significant positive correlation (p < 0.01) between Cd concentration in fruits and stems in the two Cd treatments but not in the control. However, no significant correlation (p > 0.05) was found between Cd concentrations in fruits and roots in the three treatments. Furthermore, the Cd concentration in fruits was significantly positively correlated (p < 0.01) with the Cd translocation from roots to aboveground parts, but not with root Cd uptake (Table 6). These correlations suggest that a low level of Cd in the leaves or stems, or a low translocation of Cd to the aboveground parts, leads to a low level of Cd in the fruits.
Discussion
Fruit biomass response to soil Cd toxicity
In this study, the fruit biomass of all tested hot pepper cultivars did not significantly decrease in the two Cd treatments (1.16 and 2.69 mg kg−1) compared with the control, and the biomass of the seven cultivars even significantly increased in the high-Cd treatment (Fig. 1). These findings suggest that hot pepper cultivars have a high tolerance to the levels of Cd contamination commonly found in agricultural soils (Liu et al. 2009, 2012) compared with water spinach (Xin et al. 2010), Chinese cabbage (Liu et al. 2009) and pakchoi (Brassica rapa L. ssp. chinensis) (Chen et al. 2012). It has also been reported that the growth of many crop species was stimulated under exposure to lightly to moderately Cd-contaminated soils (Yu et al. 2006; Liu et al. 2009). The stimulatory effect of Cd on plant growth may be a case of hormesis (Stebbing 1982), but the mechanism is not well known. One possible reason is that the Cd ion serves as an activator of enzyme(s) in the metabolism of cytokinin, thus accelerating the growth of plants (Kaminek 1992; Nyitrai et al. 2004). However, the detailed mechanisms responsible for the activators still need to be further investigated. In addition, all hot pepper cultivars grew well in Cd-contaminated soils without visible symptoms of Cd toxicity in this study. Thus, farmers may not get sufficient warning regarding Cd contamination in hot pepper fruits only based on yield reduction or phytotoxicity symptoms, which greatly increases the human health risks from Cd-contaminated products. Therefore, the selection and breeding of low-Cd cultivars to reduce Cd accumulation in edible plant parts is an effective method for ensuring food safety.
Selection of low-Cd hot pepper cultivars
There was more than a 2.5-fold difference in the fruit Cd concentrations among the 30 hot pepper cultivars in all treatments (Table 3). The variation was wide enough when compared with variations of Cd concentrations among other crop cultivars to allow for the selection of low-Cd cultivars (Greger and Löfstedt 2004; Xin et al. 2010). Thus, the first aim of this study was achievable using our experimental results. We intended to identify low-Cd cultivars according to their fruit Cd concentrations and tolerance to Cd toxicity. The Codex ML for Cd was used as a standard for selecting low-Cd hot pepper cultivars, and only the two cultivars Nos.16 and 23 met the criterion to be considered as low-Cd cultivars in the low-Cd treatment. The fruit Cd concentrations of Nos. 16 and 23 were always lower than the soil total Cd concentration, which is less likely to pose a human health risk. According to the results of the fruit biomasses, all hot pepper cultivars had a high tolerance to Cd, especially No. 23, so Nos. 16 and 23 were finally identified as low-Cd cultivars.
However, no hot pepper cultivars produced fruits safe for consumption when Cd concentrations in the soils reached 2.69 mg kg−1. Similarly, Liu et al. (2009) reported that no low-Cd Chinese cabbage cultivars were found under moderate Cd exposure (2.5 mg Cd kg−1 soil). Xin et al. (2010) found that shoot Cd concentrations in low-Cd water spinach cultivars exceeded the Codex ML for Cd in leafy vegetables when grown in the soil containing a Cd level of 0.61 mg kg−1. Therefore, the safety of low-Cd cultivars is conditioned by the level of soil Cd. In addition, Nos. 13 and 18 always had high fruit Cd concentrations and tolerance to Cd toxicity, which can pose a high risk to human health through the food chain, even when grown in sites only slightly Cd-contaminated. Therefore, the two cultivars were identified as high-Cd cultivars.
Root Cd uptake ability
In the present study, the Cd uptake in low- and high-Cd cultivars through the roots was characterized to elucidate the mechanisms responsible for their different levels of Cd accumulation in fruits. The amassing of Cd in aboveground plant parts of has been proposed to be the results of several transport processes (Herren and Feller 1997; Uraguchi et al. 2009; Xin et al. 2012; Su et al. 2013). Because plants take up Cd via the roots, many researchers investigate the uptake ability in an attempt to clarify the differences in Cd accumulation between cultivars (Liu et al. 2007; Arao et al. 2003). The root’s net Cd uptake in No. 23 (low-Cd cultivar) was always the lowest among the four cultivars and was significantly lower than that in the other low-Cd cultivar (No. 16) (Table 4). Thus, the reason for the lower fruit Cd concentration in No. 23 may be its decreased ability to uptake Cd through its roots. However, the Cd uptake ability in the two high-Cd cultivars was not always significantly higher than that in the two low-Cd cultivars, and a similar phenomenon was also observed in the root’s Cd concentrations (Table 4). Additionally, there was no correlation between the fruit Cd concentration and the root’s Cd uptake ability (Table 6). Uraguchi et al. (2009) reported that the low-Cd rice cultivar ‘Sasanishiki’ had a higher Cd uptake through the roots than the high-Cd cultivar ‘Habataki’, but no significant difference in root Cd concentrations was found between the two cultivars when grown in Cd-contaminated soils. The results of this study on hot pepper illustrated that the root’s Cd uptake is not a major process determining the distinct difference in fruit Cd concentrations between low- and high-Cd cultivars. Thus, it is unreasonable to hypothesize that the Cd accumulation in hot pepper fruits is related to root Cd uptake.
Cd translocation
Although the root’s Cd uptake ability was not superb in the high-Cd cultivars in comparison with that in the low-Cd cultivars, a greater Cd translocation from roots to aboveground parts was observed in high-Cd cultivars (Fig. 3). Additionally, a positive relationship was found between fruit Cd concentration and Cd translocation to aboveground parts (Table 6). Relationships were also observed between the Cd concentration in fruits and the Cd concentrations in leaves and stems (Table 6). Movement of Cd into fruits occurs via xylem, which is largely controlled by transpiration stream, and/or phloem, which is a process of retranslocation from leaves and stems. Therefore, Cd translocation to aboveground parts may be crucial for the accumulation of Cd in fruits. However, if the translocation from roots to aboveground parts was the only parameter that decided the accumulation of Cd in fruits, the cultivar’s Cd distribution patterns in leaves and stems (Fig. 2) would be the same as in their fruits (Table 3). That is not the case. It was found that the order of Cd concentrations of leaves and stems (Fig. 2) among the four cultivars was not consistent with that of fruits (Table 3). Similarly, the order of Cd translocation (Fig. 3) was also different from that of the fruit Cd concentration.
These results indicate a redistribution of Cd within aboveground parts that is important for the Cd accumulation in fruits. Liu et al. (2007) investigated the uptake and translocation of Cd in low- and high-Cd rice cultivars. They found that the redistribution of Cd within rice shoots was highly correlated with the Cd concentration in grains. This redistribution is in accordance with the theory that the flag leaf is the major provider of photosynthates to the grain (Sultana et al. 1999). Greger and Löfstedt (2004) also found the difference in grain Cd concentrations among durum wheat cultivars is related to the redistribution of Cd via the flag leaves. In this study, a positive correlation was found between the Cd concentrations in fruits and in leaves. If redistribution via leaves were the only influence on the fruit Cd concentration, the leaf: fruit ratio of Cd concentrations should be constant for all the cultivars, but significant differences in the ratios among the cultivars existed (Table 5). Similar results were also observed for the stem: fruit ratios (Table 5). Therefore, it is likely that both leaves and stems prevent Cd accumulation in fruits, and the preventative ability varies among different hot pepper cultivars. Furthermore, the xylem-to-phloem transfer of Cd in rice occurs in the nodes and plays a critical role in Cd translocation to the grains (Fujimaki et al. 2010). However, it is still unclear where Cd transfer from xylem to phloem takes place in hot peppers and what role it plays in fruit Cd accumulation.
In conclusion, the results of this study support the hypothesis that there are significant differences among the hot pepper cultivars in their ability to accumulate Cd in fruits. The scientific screening method used in this study identified two low-Cd hot pepper cultivars, Yeshengchaotianjiao (No. 16) and Heilameixiaojianjiao (No. 23), which can be grown to increase food safety. The differences in fruit Cd concentrations between low- and high-cultivars were owing to variations of Cd translocation from roots to aboveground parts and within aboveground parts, rather than to the root’s Cd uptake ability. In addition, it is worth studying whether the accumulation of Cd in hot pepper fruits mainly originates from xylem transport or phloem transport, and the result could be incorporated into breeding programs for low-Cd cultivars.
References
Alexander PD, Alloway BJ, Dourado AM (2006) Genotypic variations in the accumulation of Cd, Cu, Pb and Zn exhibited by six commonly grown vegetables. Environ Pollut 144:736–745
Arao T, Ae N, Sugiyama M, Takahashi M (2003) Genotypic differences in cadmium uptake and distribution in soybeans. Plant Soil 251:247–253
Bhargava A, Carmona FF, Bhargava M, Srivastava S (2012) Approaches for enhanced phytoextraction of heavy metals. J Environ Manag 105:103–120
Chen Y, Li T, Han X, Ding Z, Yang X, Jin Y (2012) Cadmium accumulation in different pakchoi cultivars and screening for pollution-safe cultivars. J Zhejiang Univ Sci B 13(6):494–502
Clarke JM, Norvell WA, Clarke FR, Buckley WT (2002) Concentration of cadmium and other elements in the grain of near-isogenic durum lines. Can J Plant Sci 82:27–33
Cosio C, Martinoia E, Keller C (2004) Hyperaccumulation of cadmium and zinc in Thlaspi caerulescens and Arabidopsis halleri at the leaf cellular level. Plant Physiol 134:716–725
Dunbar KR, McLaughlin MJ, Reid RJ (2003) The uptake and partitioning of cadmium in two cultivars of potato (Solanum tuberosum L.). 54(381):349–354
Fujimaki S, Suzui N, Ishioka NS, Kawachi N, Ito S, Chino M, Nakamura S (2010) Tracing cadmium from culture to spikelet: noninvasive imaging and quantitative characterization of absorption, transport, and accumulation of cadmium in an intact rice plant. Plant Physiol 152:1796–1806
Grant CA, Clarke JM, Duguid S, Chaney RL (2008) Selection and breeding of plant cultivars to minimize cadmium accumulation. Sci Total Environ 390:301–310
Greger M, Löfstedt M (2004) Comparison of uptake and distribution of cadmium in different cultivars of bread and durum wheat. Crop Sci 44:501–507
Günther K, Ji G, Kastenholz B (2000) Characterization of high molecular weight cadmium species in contaminated vegetable food. Fresenius J Anal Chem 368:281–287
Herren T, Feller U (1997) Transport of cadmium via xylem and phloem in maturing wheat shoots: comparison with the translocation of zinc, strontium and rubidium. Ann Bot 80:623–628
Huang B, Xin J, Yang Z, Zhou Y, Yuan J, Gong Y (2009) Suppression subtractive hybridization (SSH)-based method for estimating Cd-induced differences in gene expression at cultivar level and identification of genes induced by Cd in two water spinach cultivars. J Agric Food Chem 57:8950–8962
Kaminek M (1992) Progress in cytokinin research. Trends Biotechnol 10:159–164
Li X, Zhou Q, Wei S, Ren W (2012) Identification of cadmium-excluding welsh onion (Allium fistulosum L.) cultivars and their mechanisms of low cadmium accumulation. Environ Sci Pollut Res 19:1773–1780
Liu J, Qian M, Cai G, Yang J, Zhu Q (2007) Uptake and translocation of Cd in different rice cultivars and the relation with Cd accumulation in rice grain. J Hazard Mater 143:443–447
Liu W, Zhou Q, Sun Y, Liu R (2009) Identification of Chinese cabbage genotypes with low cadmium accumulation for food safety. Environ Pollut 157:1961–1967
Liu L, Hu L, Tang J, Li Y, Zhang Q, Chen X (2012) Food assessment of planting patterns of four vegetable-type crops grown in soil contaminated by electronic waste activities. J Environ Manage 93:22–30
Lu R (2000) Soil and agro-chemical analysis methods. Agricultural Science and Technology Press, Beijing
Nyitrai P, Bóka K, Gáspár L, Sárvári É, Keresztes Á (2004) Rejuvenation of ageing bean leaves under the effect of low-dose stressors. Plant Biol 6(6):708–714
Salt DE, Blaylock M, Kumar NPBA, Dushenkov V, Ensley BD, Chet I, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology 13:468–474
Stebbing ARD (1982) Hormesis—the stimulation of growth by low levels of inhibitors. Sci Total Environ 22:213–234
Su Y, Wang X, Liu C, Shi G (2013) Variation in cadmium accumulation and translocation among peanut cultivars as affected by iron deficiency. Plant Soil 363:201–213
Sultana N, Ikeda T, Itoh R (1999) Effect of NaCl salinity on photosynthesis and dry matter accumulation in developing rice grains. Environ Exp Bot 42(3):211–220
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60:2677–2688
Wong MH (2003) Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 50:775–780
Wong SC, Li XD, Zhang G, Qi SH, Min YS (2002) Heavy metals in agricultural soils of the Peral River Delta, South China. Environ Pollut 119:33–44
Xin J, Huang B, Yang Z, Yuan J, Dai H, Qiu Q (2010) Responses of different water spinach cultivars and their hybrid to Cd Pb and Cd-Pb exposures. J Hazard Mater 175:468–476
Xin J, Huang B, Yang J, Yang Z, Yuan J, Mu Y (2012) Role of roots in cadmium accumulation of two water spinach cultivars: reciprocal grafting and histochemical experiments. Plant Soil. doi:10.1007/s11104-012-1439-5
Xu X, Li Y, Wang H (2008) Present situation, development trend and countermeasures of pepper industry in China. Chin Agric Sci Bull 24(11):332–338
Yu H, Wang J, Fang W, Yuan J, Yang Z (2006) Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice. Sci Total Environ 370:302–309
Zhou Q, Song Y (2004) Principles, methods of contaminated soil remediation. Science Press, Beijing
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No. 41101303 and No. 41201320) and Hunan Provincial Natural Science Foundation of China (Grant No. 11JJ6013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juan Barcelo.
Rights and permissions
About this article
Cite this article
Xin, J., Huang, B., Liu, A. et al. Identification of hot pepper cultivars containing low Cd levels after growing on contaminated soil: uptake and redistribution to the edible plant parts. Plant Soil 373, 415–425 (2013). https://doi.org/10.1007/s11104-013-1805-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1805-y