Abstract
The methylmercury (MeHg) cycling at water–sediment interface in an acid mine drainage (AMD)-polluted reservoir (Aha Reservoir) and a reference site (Hongfeng Reservoir) were investigated and compared. Both reservoirs are seasonal anoxic and alkaline. The concentrations of sulfate, sulfide, iron, and manganese in Aha Reservoir were enriched compared to the reference levels in Hongfeng reservoir due to the AMD input. It was found that the MeHg accumulation layer in Aha Reservoir transitioned from the top sediment layer in winter to the water–sediment interface in spring and then to the overlying water above sediment in summer. It supported the assumption that spring methylation activity may start in sediments and migrate into the water column with seasonal variation. The weaker methylation in sediment during spring and summer was caused by the excessive sulfide (∼15–20 μM) that reduced the bioavailability of mercury, while sulfate reduction potential was in the optimal range for the methylation in the overlying water. This led to a transport flux of MeHg from water to sediment in spring and summer. In contrast, such inversion of MeHg accumulation layer did not occur in Hongfeng Reservoir. The sulfate reduction potential was in the optimal range for the methylation in top sediment, and dissolved MeHg was positively related to sulfide in pore water of Hongfeng Reservoir (r = 0.67, p < 0.001). This result suggested that accumulation of MeHg in lake water and cycling of MeHg at sediment–water interface associate with some sensitive environmental factors, such as sulfur.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Methylmercury (MeHg) levels in fish and invertebrates in some lakes or marine systems were found to exceed the state, federal, or international health guidelines (Chase et al. 2001). As a result, mercury contamination of the aquatic system is a worldwide health concern (Clarkson 1990; Fitzgerald and Clarkson 1991). The sediment and/or bottom water has long been recognized as the key locations of microbial-mediated Hg methylation (Hammerschmidt and Fitzgerald 2004; Eckley et al. 2005). Therefore, in order to understand geochemical cycling of mercury at sediment–water interface, it is essential to know the modes of transfer of mercury to the overlying water and biota.
Numerous studies are linked with cycling of MeHg at the sediment–water interface. Several mechanisms have been suggested to explain the seasonal accumulation of MeHg in the anoxic hypolimnia of lakes including the following:
-
1.
Diffusion and release from the sediments to the water column under anoxic conditions (Gill et al. 1999; Regnell et al. 2001; Ullrich et al. 2001). In freshwater aquatic ecosystems, warm, shallow, and organic-rich lake sediments are often important zones of net methylation (Munthe et al. 2007).
-
2.
Sedimentation of catchment-derived particulate MeHg (Verta and Matilainen 1995) or dense layers of seston that have accumulated Hg in the upper water layers (Hurley et al. 1991). The study of Watras et al. (2006), however, showed that MeHg in the anoxic hypolimnia of Little Rock Lake did not come from the settling plankton from above.
-
3.
In situ methylation in the anoxic water column (Watras et al. 1995; Mauro et al. 2002; Eckley et al. 2005). The study of Watras et al. (2006) showed MeHg production within a discrete layer of microbes at various depths in the anoxic water column in Little Rock Lake. A peak in methylation potential was typically observed just below the oxycline, and it decreased with increasing depth in the water columns of five lakes across Canada (Eckley and Hintelmann 2006). Thus, it was assumed that spring methylation activity may start in the sediments in spring and migrate into the water column in summer as anaerobic microbes move upward and toward the source of limiting nutrients, like sulfate and dissolved organic carbon (DOC) (Watras et al. 2006; Eckley and Hintelmann 2006; Watras et al. 1995). However, the data from sediment and pore water are limited to support the assumption that methylation activity may start in sediments and migrate into the water column with seasonal variation, as the data from the sediments and pore waters have not been reported in those articles. In this article, the research group investigated the cycling of MeHg at water–sediment interface of Aha Reservoir, which is polluted by acid mine drainage (AMD) and Hongfeng Reservoir which is not affected by AMD, as a reference site. The assumption that methylation activity may migrate between sediment and water column with seasonal variation will be investigated and discussed based on data from lake water and pore water. The effect of high sulfate and iron input from AMD on cycling of MeHg at water–sediment interface will also be discussed.
Under anoxic conditions, sulfur (S) and iron cycling may have a significant influence on Hg complexation and methylation. Both sulfate-reducing bacterium (SRB) and iron-reducing bacterium (IRB) can methylate Hg in freshwater ecosystems (Benoit et al. 1999; Fleming et al. 2006; Gilmour et al. 2013). Numerous studies observed that the sulfate-reducing activity would result in an increase in the production of MeHg (e.g., Shao et al. 2012; Eckley and Hintelmann 2006), while high sulfide inhibits its methylation, presumably by affecting the speciation of the dissolved Hg–S complexes (Benoit et al. 1999; Hammerschmidt et al. 2008). Thus, the impact of sulfur is a complex process regarding the cycling of mercury in the environment. Cycling of iron in water and sediment also can affect bioavailability of mercury directly or indirectly (Regnell et al. 2001; Mehrotra and Sedlak 2005). Aha Reservoir is polluted by AMD from coal mines, which results in unusually high SO4 2−, Fe, and Mn concentrations in the water and sediment compared to Hongfeng Reservoir. These specific characteristics of the Aha Reservoir provide us with an opportunity to investigate the effects of sulfur and iron cycles on mercury distribution in aquatic systems.
Methodology
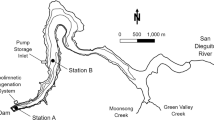
Description of the sampling site
The Aha Reservoir was initially impounded in 1960 and then enlarged in 1982 to a final surface area of 4.5 km2 and a volume of 4.45 × 107 m3 (Table 1). The reservoir has been contaminated by coal mining activities for many years. Approximately 220 small coal mines were in operation around the catchments of the reservoir during the 1980s, which constantly discharged acidic pollutants such as SO4 2−, Fe, and Mn into the water (Wang 2003). Since 1995, two water treatment plants have been built to purify the inlet water of Aha Reservoir by using lime neutralization and aeration oxidation, but the SO4 2− concentrations in water of the reservoir still reached 303 mg/L (Luo 2003), which were significantly elevated compared to the adjacent reservoirs. At the same time, the Aha Reservoir has been polluted by the industrial and domestic waste water in the catchment. Most of the coal mines were completely closed by 2011.
Hongfeng Reservoir was constructed in 1960 with a surface area of 57.2 km2 and a volume of 6.01 × 108 m3 (Table 1). Its main bedrocks at the watershed are limestone and dolomite (Zhang 1999). There are nearly two dozen factories in the drainage area of the reservoir, and a large volume of improperly treated waste water has been discharged into the reservoir. Accordingly, the reservoir has become a polluted and eutrophic body of water. As a result, the reservoir exhibits alkaline conditions with seasonally anoxic and eutrophic features. Eutrophication, however, was just developed in some sites, such as Houwu, but not at the Daba station (He et al. 2008), which served as a reference site in this study.
Lake water, sediment, and pore water sampling
The lake water, pore water, and sediment samples were collected from Aha and Hongfeng reservoirs in February, June, and September of 2012 using clean metal protocols (Method 1669 1996; Method 1630 2001). The sampling sites in the reservoirs were chosen as shown in Fig. 1. Among two sites in Aha Reservoir, the Daba sampling site of Aha Reservoir (AHDB) is close to the dam of the reservoir while Changtang sampling site of Aha Reservoir (AHCT) is located at the center of the reservoir. For the reference site, Daba of Hongfeng (HFDB) was chosen, which is close to the dam of Hongfeng Reservoir. As Hongfeng Reservoir was mainly constructed for the purpose of hydroelectric power generation, flood control, drinking water supply, etc. The HFDB site is near the dam of the reservoir, the water levels at the Daba site exhibited significant fluctuations. The water depth was 24 m in June but increased to 32 m in September. Water samples were obtained from different depth levels (0, 4, 8, 12, 16, 20, 24, 28, 32, and 36 m) using a 10-L Niskin sampler. For the filtered water samples, they were prepared by filtering water samples using a 0.45-μm polyvinylidene fluoride (PVDF) filter (Millipore) on site. All water samples were filled in borosilicate glass bottles, acidified with 0.5 % HCl, double-bagged and transported to the laboratory within 24 h, and finally stored at 3–4 °C in the dark until subsequent analysis. Prior to sample collection, the borosilicate glass bottles were cleaned by acid leaching, rinsing with ultrapure deionized water (18 MΩ cm), and heating at 500 °C for several hours in a muffle furnace (He et al. 2008; Feng et al. 2011). As a precaution, collected water samples and standard stock samples were stored separately. Similarly, the pre-cleaned and packed apparatus that would contact samples was opened or exposed only in a clean bench and for a short time. When not in use, the apparatus was packed in separate clean ziplock bags and stored in a clean plastic box. Clean, talc-free gloves were worn during all operations involving handling of the apparatus, samples, and blanks, and they were changed frequently during the whole procedures to minimize any possible contamination.
Sediment cores of approximately 25 cm in length were collected and sectioned at 1- and 2-cm intervals. Each core section was sliced and placed into 45-mL centrifuge tubes under a nitrogen atmosphere. The pore water was extracted from the wet sediment within 48 h by centrifugation at 3000 rpm for 30 min at in situ bottom water temperature (5–15 °C), followed by filtration through syringe filter units (single use, 0.45-μm PVDF membrane, Millipore). The resulting pore water was collected in borosilicate glass bottles and acidified to a 0.5 % HCl solution. All bottles were capped and then sealed with parafilm. The whole process was performed in a nitrogen bag with gloves. All the resulting pore water samples were stored in a refrigerator at 3–4 °C until further analysis. Solid-phase samples were freeze-dried and homogenized using a mortar and pestle.
Mercury analysis
The total Hg (THg), dissolved total Hg (FTHg), total methylmercury (TMeHg), and dissolved MeHg (FMeHg) concentrations were determined for each sample. The analytical methods used for Hg speciation in water have been described in detail elsewhere (Bloom and Fitzgerald 1988; Horvat et al. 1993; Method 1630 2001; Method 1631 2002; He et al. 2008; Feng et al. 2011). All methods rely on cold vapor atomic fluorescence spectroscopy.
For THg and FTHg, the water samples were oxidized with 0.5 % BrCl at least for 24 h. After oxidation, 0.2 % NH2OH·HCl was added to destroy any free halogens before adding stannous chloride (SnCl2) to convert Hg(II) to volatile Hg(0). The resulting sample was then purged with Hg-free N2, and Hg(0) was adsorbed onto a gold trap (Bloom and Fitzgerald 1988).
TMeHg and FMeHg concentrations in water were determined using the standard distillation–ethylation GC separation–cold vapor atomic fluorescence spectrometry (CVAFS) technique (USEPA 2001). A 45-mL aliquot of an acidified sample was placed in a fluoropolymer distillation vessel and distilled at 125 °C under Hg-free N2 flow until approximately 35∼36 mL of water was collected in the receiving vessel. The distillation process was stopped to avoid more artifactual methylation of inorganic Hg when the volume of distillate was up to 80 % of distilland. The pH of the collected sample was adjusted to 4.9 using an acetate buffer. The Hg in the sample was ethylated in a closed 200-mL bubbler by the addition of sodium tetraethyl borate. The ethyl analog of CH3Hg, CH3CH2HgCH3, was separated from the solution by purging with N2 onto a Tenax trap. The trapped CH3CH2HgCH3 was then thermally desorbed, separated from other mercury species using an isothermal GC column, and then decomposed to Hg(0) in a pyrolytic decomposition column (700 °C). The resulting analyte was examined by CVAFS (Brooks Rand II). All PTFE vials for distillation were cleaned by heating for 48 h in concentrated nitric acid (Horvat et al. 1993).
The analysis of MeHg in sediment was performed following the procedure developed by Liang et al. (2004). Approximately 0.3 g of sediment was placed into a 50-mL centrifuge tube, into which 1.5 mL of 1 M CuSO4, 7.5 mL of 3 M HNO3, and 10 mL of CH2Cl2 were added. The tube was closed and shaken for 30 min. Five milliliters of the CH2Cl2 layer was pipetted into another 50-mL centrifuge tube after the tube was centrifuged at 3000 rpm for 30 min. Any of inorganic part, such as sediment, water, and acid, could not be transferred to another tube with CH2Cl2. It would result in artificial methylation of inorganic Hg in the sample if inorganic mercury in the sample was transferred with CH2Cl2. Approximately 40 mL of double-deionized water was added to the tube. The tube was heated at 45 °C in a water bath until no visible solvent was left in the tube. The remaining liquid was then purged with nitrogen for 8 min in a water bath at 80 °C to remove the solvent residue. The sample was brought to 50 mL with double-deionized water before an appropriate volume (generally 15 mL) of the sample was transferred to a borosilicate bubbler for MeHg analysis following the procedure described previously.
Physical and chemical characteristics
Ancillary water analyses, including the pH, dissolved oxygen, temperature, total dissolved solids (TDS), dissolved concentrations of sulfide, sulfate, iron, and manganese, were performed. The sulfide levels in the lake water and pore water were measured by colorimetry using Cline’s method (Cline 1969). The sulfate content of the water samples was determined by ion chromatography. Iron and manganese were analyzed by inductively coupled plasma atomic emission spectrometry. Water quality parameters such as T, dissolved oxygen (DO), pH, and TDS were measured using a portable analyzer (Radiometer Analytical, plONneer 65) in winter and the analyzer (YSI 6600V2) in September on site.
Quality assurance
Quality assurance and quality control of the analytical process were carried out using duplicates, method blanks, field blanks, matrix spikes, and certified reference materials (ERM 580, marine sediment). Field blanks and duplicates were taken regularly (>10 % of samples) throughout each sampling campaign. The estimated detection limit is 0.10 mg/L for SO4 2−, 0.002 mg/L for iron, and 0.001 mg/L for Mn. The relative average deviations on precision tests for the duplicate samples vary from 1.3 to 4.0 % for SO4 2−, from 3.5 to 9.2 % for sulfide, from 1.4 to 7.1 % for iron, and from 0.4 to 6.4 % for Mn in filtered water. Detection limit of MeHg for 45-mL water sample was estimated as three times the standard deviation of the blank measurement (0.027 ± 0.003 ng/L, n = 12, consecutive determination) and is 0.009 ng/L. The relative average deviations on precision tests for the duplicate samples vary from 0.7 to 8.1 %, and recoveries for matrix spikes are in the range of 80–101 % for MeHg analysis in lake water. The mean MeHg concentration of 74.7 ± 5 ng g−1 (n = 10) was obtained from ERM 580 with a certified value of 75.5 ± 4 ng g−1.
Results
Physical and chemical characteristics of the water and sediment
Tables 2 and 3 list the physical and chemical characteristics of the water and the sediment. The two reservoirs are seasonally stratified and anoxic. Li et al. (2008) analyzed monthly variation in DO distribution in Hongfeng Reservoir and showed that anaerobic hypolimnion (DO <1 mg/L) began to develop in deeper water in May and this zone in deeper water expanded below the depth of 8–10 m in September.
As both Aha Reservoir and Hongfeng Reservoir are located in the Karst region of Guiyang city, many physical and chemical characteristics are the same, such as pH, DO, and temperature (Table 2). The main difference between the two reservoirs is sources of pollution. The sources of pollution in Hongfeng Reservoir are mostly due to industrial and domestic waste water (He et al. 2008). For Aha Reservoir, it was mainly polluted by AMD, in addition to industrial and domestic waste. As both reservoirs are polluted by industrial and domestic waste water, the organic matter concentrations are generally higher than the values observed in other reservoirs (e.g., Dongfeng Reservoir, 1.98∼3.09 % in sediment) located in Guizhou (Jiang 2005). Dissolved organic matter (DOC) in lake water and organic matter in sediment in Hongfeng Reservoir were slightly higher than those in Aha Reservoir (Tables 2 and 3). Although total mercury of the surface sediment in Hongfeng Reservoir is two to three times higher than that in Aha Reservoir, dissolved total mercury in lake water and pore water of the two reservoir had no statistical difference (one-way ANOVA, p = 0.29). The main discernible differences in the physical and chemical characteristics of Aha Reservoir and Hongfeng Reservoir are the sulfate, sulfide, iron, and manganese concentrations, which may result in obviously different MeHg cycling at water–sediment interface.
The sulfate and sulfide in the lake water and pore water
Figure 2 shows the spatial and temporal distributions of sulfate in Aha and Hongfeng reservoirs. In both water columns and sediment pore water of Aha Reservoir, the sulfate concentrations in February were higher than those in June and September (Mann–Whitney test, p < 0.001). In particular, in the top 2 cm of pore water in February, the sulfate concentrations were 2–11 times higher than those of June and September. Moreover, in the summer months, there was a sharp significant decrease in the sulfate levels at the depth of 1 cm in pore water. These seasonal variations suggest that the top 2 cm of the sediment was in presence of oxygen in winter, so sulfate-reducing activity was weak in top 2 cm of the sediment. In Hongfeng Reservoir, the sulfate concentrations ranged from 53 to 92 mg/L in lake water and 0.8 to 21 mg/L in pore water, which were distinctly lower than those of Aha Reservoir (Mann–Whitney test, p < 0.001). These results indicated that Aha Reservoir was still affected by acid mining discharge, which gives rise to high sulfate concentrations. Since Guizhou has geologically higher sulfur content, the sulfate concentrations in Hongfeng reservoir without additional sulfate pollution were much higher than those in some lakes in other regions (e.g., Eckley and Hintelmann 2006).
Figure 3 shows the spatial and temporal distributions of dissolved sulfide in Aha and Hongfeng reservoirs. Sulfide mainly accumulated in the anoxic water and the upper several centimeters in pore water and then declined with increasing depth in pore water. This distribution pattern was consistent with those observed in some anoxic lakes (e.g., Feyte et al. 2012; Couture et al. 2010). In the typical profiles observed in a coastal marine deposit, however, dissolved sulfide usually increased with increasing depth in pore water (Hammerschmidt et al. 2008; Hollweg et al. 2009). The difference of sulfide distribution could be a result of much lower sulfate but higher iron in lake and reservoir, which likely resulted in the precipitation of iron sulfide in the pore water.
Sulfide is the reduction product of sulfate, so to some extent, sulfide values can show sulfate reduction potential. At AHDB sampling site, the sulfide contents increased sharply at the depth of 16–24 m in bottom water in September and the highest value was as high as 13 μM while it generally stayed lower than 10 μM for most of the samples. This indicated that the sulfate reduction potential in lake water was relatively strong in September. In the top of the pore water, the sulfide levels were generally higher than 10 μM.
In lake water of HFDB, the sulfide contents were very low in June and September, which showed weaker sulfate reduction potential than that in lake water of Aha Reservoir. Sulfate reduction rate is mainly controlled by sulfate, redox potential, availability of organic substrates, and temperature (Kosolapov et al. 2003). Relatively low DOC concentrations (Table 2) limited the sulfate reduction activities in lake water of Hongfeng and Aha reservoirs. Although there were higher sulfide concentrations in lake water of Aha Reservoir affected by sulfate pollution, it was still much lower than those in some lakes with high DOC concentrations (Eckley et al. 2005).
In the pore water of HFDB, the sulfide contents were relatively higher than those in lake water in June. Nevertheless, with less than 10 μM, it was still significantly lower than those in the pore water of Aha Reservoir, indicating weaker sulfate reduction activity than that in Aha Reservoir. In contrast, the sulfide in the pore water obtained in September increased and was at the same level as that of Aha Reservoir, which corresponds to the relatively low sulfate content of the Hongfeng Reservoir in September. These results revealed that the sulfate reduction potential became high in September in Hongfeng Reservoir.
Iron and manganese in pore water
The spatial and temporal distributions of total iron (Fe) and manganese (Mn) in the pore water in Hongfeng and Aha reservoirs are shown in Fig. 4. Wang (2003) indicated that Aha Reservoir had been polluted by Fe and Mn from AMD. The total Fe and total Mn in the solid phase of the sediment were approximately 60–100 and 2–40 mg/g at the AHDB, respectively, whereas the values were approximately 40 and <2 mg/g for Fe and Mn in the Hongfeng Reservoir, respectively (Wang 2003). However, the total Fe concentrations in the pore water of three sampling sites revealed no seasonal and spatial differences except for the slightly higher values in September. The difference of iron distribution between solid phase and pore water might be attributed to the stronger precipitation of Fe sulfide in Aha Reservoir with higher sulfate and sulfide in pore water.
THg and TMeHg in the water columns and sediment
The vertical distributions of THg in water column at the three sampling sites in September are presented in Fig. 5. THg concentrations in the lake water of the reservoirs were relatively low compared with those reported before by He et al. (2008) and Feng et al. (2011) because of the control in the sources of mercury pollution from the catchment. Nevertheless, the mercury concentrations in the lake water of the two reservoirs were distinctly higher than those of other natural waters reported in Europe and North America (e.g., Bloom et al. 2004; Sullivan and Mason 1998).
The seasonal distributions of TMeHg in the water column are shown in Fig. 5. In Aha Reservoir, TMeHg concentrations changed from 0.04 to 3.09 ng/L with an average of 0.67 ng/L, and the values were distinctly elevated in the bottom water in June and September. The increased TMeHg concentrations in the hypolimnion were probably related with increased methylation rates, the accumulation of settled particulate matter, and/or the release of MeHg from the sediment (e.g., Regnell and Ewald 1997; Verta and Matilainen 1995). At the sampling site of AHDB, the highest value in September was located at the depth of 16 m, not at water–sediment interface, which indicated in situ methylation and/or that the accumulation of settled particulate matter contributed to most of MeHg in hypolimnion. At the HFDB sampling site, TMeHg ranged from 0.04 to 0.73 ng/L with an average of 0.22 ng/L, which was much lower than that at Aha Reservoir. The highest values were observed at the sediment–water interface, and MeHg distribution in the water column showed a strong increasing gradient toward the sediment. This distribution showed that TMeHg in the bottom water was probably from the release of MeHg in the sediment in Hongfeng Reservoir.
Conversely, MeHg in the sediment of Hongfeng Reservoir was much higher than that of Aha Reservoir (Fig. 6). There were no discernible seasonal differences in the distribution of MeHg in the sediment, with the exception of the slightly lower MeHg levels in the top several centimeters of the Aha Reservoir in September.
There were good correlations between FMeHg and TMeHg in lake water and sediment (Fig. 7). So, the spatial and temporal variations of TMeHg between Aha and Hongfeng reservoirs need to be discussed in detail together with FMeHg in lake and pore water which is provided in later section.
FMeHg in the lake water and pore water
There were significant temporal and spatial differences in the distribution of FMeHg in the lake and sediment pore water samples of both reservoirs (Fig. 8). In Aha Reservoir, the FMeHg in the hypolimnion water generally increased in June and September. In the pore water of the sediment, however, it seemed surprising that the highest FMeHg levels were observed in February instead of June and September. The entire profile of the FMeHg distributions showed that the MeHg accumulation layer migrated between lake water and pore water with the seasons. At the AHDB sampling site, the highest FMeHg levels (0.53, 0.92, and 1.93 ng/L) were observed at a depth of 2 cm in pore water in February, at a depth of 1 cm in pore water in June, and at a depth of 16 m in lake water in September, respectively. At the AHCT sampling site, the highest FMeHg levels (1.60, 0.51, and 0.89 ng/L) were at a depth of 1 cm in the pore water in February, at the sediment–water interface in June, and at a depth of 12 m in the lake water in September, respectively. The distributions suggested that MeHg accumulation layer shifted from the top of pore water to the bottom of lake water with the migration of the oxic/anoxic boundary layer over the course of seasonal variations. At the reference site HFDB in Hongfeng Reservoir, however, the MeHg accumulation layer was always in the top of pore water. Although the FMeHg contents increased in the hypolimnion of the lake water, the values were much lower than those of Aha Reservoir. In contrast, the FMeHg levels in the top 10 cm of the pore water were much higher than those in Aha Reservoir with a maximum of 4.11 ng/L in summer, which corresponded to the higher total MeHg concentrations in the sediment of Hongfeng Reservoir.
The sediment–water fluxes were calculated from the concentration gradient between surface pore water (2 cm) and bottom water using Fick’s law, with a value of 1.3 × 10−5 cm2 s−1 for D w (the diffusion coefficient of the solute in water in the absence of the sediment matrix). In Hongfeng Reservoir, the largest diffusive flux of MeHg from sediment to water column was in June, up to 17.7 ng/m2 day, while negative fluxes were obtained in Aha Reservoir in summer.
Discussion
Seasonal migration of methylation activity between water and sediment
A number of studies found that %MeHg was a reasonable approximation of the relative rates of Hg methylation in uncontaminated sediments (e.g., Benoit et al. 2003; Sunderland et al. 2004). In Aha Reservoir, as described above, the MeHg accumulation layer migrated from the top of the sediment to the bottom of lake water with the migration of the oxic/anoxic boundary layer. The highest FMeHg concentrations were observed at the surface or the subsurface of the sediment cores in February, at the surface of sediment or at the sediment–water interface in June, and in lake water in September, respectively. In the aquatic systems, the higher MeHg is usually observed in pore water compared to lake water, and MeHg in summer is usually higher than those in winter in sediment and pore water (Hines et al. 2004; Gill GA et al. 1999; Guédron et al. 2012). Some lines of research, however, found unusual MeHg distributions at the water–sediment interface, for example, Regnell et al. (2001) observed MeHg in surface sediment and showed a steady decline while MeHg increased in the overlying water during summer. The several studies showed that MeHg production within a discrete layer of microbes in the anoxic water column and highest methylation potential were typically observed just below the oxycline, which decreased with increasing depth in the anoxic water columns (Eckley and Hintelmann 2006; Watras et al. 1995; Regnell and Ewald 1997), and a peak in MeHg also was present near the lower boundary of the thermocline or right below it.(Regnell and Ewald 1997; Watras et al. 2006). Thus, it was assumed that spring methylation activity may start in sediments and migrate into the water column in summer as anaerobic microbes move upward and toward the source of limiting nutrients, like sulfate and DOC (Watras et al. 2006; Watras et al. 1995). In those articles, however, the data from sediment and pore water were not provided, so it is hard to support this assumption. In Aha Reservoir, the trends of seasonal distributions of MeHg in the surface pore water were FMeHg in February (0.87 ng/L) > FMeHg in June (0.64 ng/L) > FMeHg in September (0.15 ng/L), while those of average FMeHg in hypolimnion are FMeHg in February (0.12 ng/L) < FMeHg in June (0.42 ng/L) < FMeHg in September (0.59 ng/L). TMeHg in the sediment had same seasonal distributions with those in pore water. It is obvious that MeHg in hypolimnion was not from the diffusion of sediment and the MeHg accumulation layer migrated from the top of sediment to the bottom of lake water with the migration of the oxic/anoxic boundary layer. The distributions in water and sediment profile in Aha Reservoir supported the hypothesis that spring methylation activity may start in sediments and migrate into the water column in summer. In Hongfeng Reservoir, however, the highest MeHg values were always presented in surface pore water, and MeHg distributions in the water columns showed a strong increasing gradient toward the sediment. This MeHg distribution suggested that MeHg in the bottom water was probably from the release of MeHg in the sediment of Hongfeng Reservoir which is also a stratified lake and the deeper lake water was under anaerobic conditions in spring and summer. This result showed that accumulation of MeHg in lake water and cycling of MeHg at the sediment–water interface were associated with some sensitive environmental factors.
The MeHg contents in water and sediment are influenced by a wide variety of environmental factors, such as the total and reactive mercury content, temperature, redox potential, and pH, as well as the inorganic mercury, sulfur, iron, and organic matter (Ullrich et al. 2001). However, these factors cannot be viewed independently as they often interact, forming a complex system of synergistic and antagonistic effects.
Many physical and chemical characteristics such as pH, DO, and temperature of both Aha Reservoir and Hongfeng Reservoir are similar (Table 2), as both reservoirs are located in the Karst region of Guiyang city. Both DOCs in lake water and organic matter in the sediments of the Hongfeng Reservoir were slightly higher than those of Aha Reservoir (Tables 2 and 3). Even though total mercury of the surface sediment in Hongfeng Reservoir is two to three times higher than that in Aha Reservoir, not only the THg pool is important but also other factors such as microbial activity and the concentration of bioavailable Hg are important, as they affect the efficiency of microbial Hg methylation (Ullrich et al. 2001). Furthermore, dissolved total mercury in lake water and pore water of two reservoir had no statistical difference (one-way ANOVA, p = 0.29). The main discernible differences in the physical and chemical characteristics of Aha Reservoir and Hongfeng Reservoir are the sulfate, sulfide, iron, and manganese concentrations, which may have resulted in obvious difference in MeHg cycling at the water–sediment interface.
Impact of sulfur on MeHg cycling at sediment–pore water interface
In Aha Reservoir, as described above, the MeHg accumulation layer migrated from the top of the sediment to the bottom of lake water with the migration of the oxic/anoxic boundary layer. The highest MeHg contents, however, were always at the top of the pore water even in June and September in Hongfeng Reservoir. The difference in the FMeHg distributions was likely caused by a difference in the sulfate reduction potential at the two reservoirs. Under anoxic conditions, sulfur (S) cycling may have a significant influence on Hg complexation and methylation. Numerous studies observed that sulfate-reducing activity would result in an increase in the production of MeHg (e.g., Shao et al. 2012; Eckley and Hintelmann 2006), while high sulfide inhibits its methylation, presumably by affecting the speciation of the dissolved Hg–S complexes (Benoit et al. 1999; Hammerschmidt et al. 2008). In Aha Reservoir, the higher sulfate content in February indicated that the sulfate reduction potential was lower in the water and sediment profile. The highest sulfate reduction potential occurred at the subsurface of the sediment, which corresponds to the highest MeHg concentration at the subsurface of the sediment in February. In June and September, lower sulfate content and higher sulfide concentrations confirmed that the sulfate reduction potential was stronger throughout the deeper water column and sediment and the sulfide levels on the top of the pore water were as high as approximately 20 μM. Berman and Bartha (1986) observed that MeHg levels in the sediments were initially directly related to the sulfide concentrations but decreased sharply beyond the content of some sulfides. Although the presence of sulfide can enhance the solubility of inorganic Hg in the sediment of pore fluids, it also reduces the fraction of Hg as HgS(0) (Benoit et al. 1999), which was hypothesized to be available to the methylating bacteria (Benoit et al. 2001). Hammerschmidt et al. (2008) found that sulfide enhanced the solubility of Hg(II) but inhibited its methylation when the sulfide content is greater than approximately 10 μM. The research of Schaefer and Morel (2009) also indicated that the formation of specific mercury complexes in anoxic waters modulates the efficiency of the microbial methylation of mercury, and they found that the addition of 10 μM cysteine resulted in 50 times the rate of methylation observed in control bottles, but methylation of mercury was seriously reduced when 10 μM sulfide was added with cysteine at the same time. The high sulfide contents (approximately 15–20 μM) in the pore water of the Aha Reservoir might enhance the solubility of Hg(II) in the top of the sediment cores, whereas the excessive sulfide inhibits the methylation of mercury in the sediment in June and September. Thus, there were no relationships between FMeHg and sulfide (or THg), but a positive correlation between THg and sulfide was observed in pore water of Aha Reservoir (Figs. 9, 10, and 11). Although the sulfate reduction potential was also high in the deeper lake water of the Aha Reservoir in June and September, most of the sulfide values were lower than 10 μM. Therefore, the optimal sulfate reduction potential promoted the accumulation of MeHg in the deeper lake water of the Aha Reservoir in June and September.
At the HFDB site, the sulfide levels in the deeper lake water were very low, which exhibited that sulfate reduction potential was weak, likely because of low organic substrates (Table 2). Therefore, relatively low MeHg in this site is probably because sulfate reduction potential was too low to promote the methylation of mercury. In the pore water in June, the sulfide content was relatively higher than that in lake water. Still, they were less than 10 μM, which was in the optimal range for the methylation of mercury. Therefore, high MeHg was observed in the top centimeters of sediment and pore water. Compared to Aha Reservoir, FMeHg was positively correlated to sulfide in pore water of Hongfeng Reservoir (Fig. 9). Simultaneously, a positive correlation between FMeHg and FTHg was observed (Fig. 10), as sulfate reduction product (sulfide) enhanced the solubility of Hg(II) (Fig. 11) when sulfate reduction activity promoted methylation of mercury in the sediment of Hongfeng Reservoir. However, higher MeHg levels were still observed in the pore water in September when sulfide already accumulated to approximately 20 μM. This result was observed most likely because MeHg, which had been produced before the appearance of the high sulfide concentration, accumulated in sediment, or for some other reasons.
Impact of iron on MeHg cycling at sediment–pore water interface
The role of iron-reducing activity in methylation of mercury still remains unclear. Warner et al. (2003) found that potential rates of methylation were suppressed under iron-reducing conditions in the sediment. However, some studies (Fleming et al. 2006) have found that IRBs could methylate mercury at environmentally significant rates and solid-phase ferric iron can also be an electron acceptor, especially in the sediments polluted by AMD. Avramescu et al. (2011) have demonstrated that iron reduction might influence the net methylation of the sediment by decreasing the demethylation rather than favoring methylation. The lines of research of Mehrotra and Sedlak (2005) have shown that addition of ferrous iron reduced the net rate of mercury methylation by decreasing the concentration of dissolved sulfide. In the sediment of Aha Reservoir, total iron was much higher than that in Hongfeng Reservoir, so it could not be precluded that low MeHg in the top of pore water and sediment of Aha Reservoir was related with high iron.
There were similar distribution trends for the FMeHg and total dissolved Fe in the middle and bottom of the sediment cores. Usually, total dissolved Fe presents as Fe2+ in anoxic environment (Wang 2004). The distributions of dissolved iron indicated that the reduction or dissolution of iron mainly occurred in the middle and bottom of sediment cores (Fig. 4). Accordingly, there were some small FMeHg and MeHg peaks in the middle of sediment cores of two reservoirs, especially in Aha Reservoir (Fig. 8). Therefore, we proposed that the reduction of iron most likely promoted the methylation of mercury in the middle of the sediment cores, as some lines of research indicated that IRBs can methylate mercury (Fleming et al. 2006).
Summary
We investigated MeHg distribution at the two sampling sites of Aha Reservoir and at a reference site of Hongfeng Reservoir. The main differences between the two reservoirs are mainly chemical characteristics such as sulfate, iron, and manganese concentrations while the physical parameters were similar. The MeHg accumulation layer migrated from the top of pore water into the bottom of lake water with the migration of the oxic/anoxic boundary layer in Aha Reservoir. In February, the highest FMeHg contents occurred at the surface or the subsurface of sediment cores while the highest FMeHg values appeared in the water columns in September. In contrast, the maximum MeHg contents were always in the top of pore water in Hongfeng Reservoir rather than in the water column even in summer. In Hongfeng Reservoir, the largest diffusive flux of MeHg from sediment to water column was in June, up to 17.7 ng/m2 day, while negative fluxes were obtained in Aha Reservoir in summer. The differences in the FMeHg distributions were likely caused by the differences in the sulfate reduction potential of the two reservoirs. In the sediment of Aha Reservoir, the methylation of mercury was inhibited by the high sulfide concentration because the sulfide ions reduced the bioavailability of mercury. In the lake water of Aha Reservoir and the pore water of Hongfeng Reservoir, sulfate reduction potential was in the optimal range for methylation of mercury. Our result supported the assumption that spring methylation activity may start in sediments and migrate into the water column with seasonal variation in some seasonal anoxic reservoirs or lakes, but this transition may occur only in some lake with special environmental factors, such as unusual sulfate reduction potential.
References
Avramescu ML, Yumihoze E, Hintelmann H, Ridal J, Fortin D, Lean D (2011) Biogeochemical factors influencing net mercury methylation in contaminated freshwater sediments from the St. Lawrence River in Cornwall, Ontario, Canada. In: Proceedings of 10th International Conference on Mercury as a Global Pollutant, Nova Scotia, Canada
Benoit JM, Gilmour CC, Mason RP (1999) Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters. Environ Sci Technol 33:951–957
Benoit JM, Gilmour CC, Mason RP (2001) The influence of sulfide on solid-phase mercury bioavailability for methylation by pure cultures of Desulfobulbus propionicus (1pr3). Environ Sci Technol 35:127–132
Benoit JM, Gilmour CC, Heyes A, Mason RP, Miller CL (2003) Geochemical and biological controls over methylmercury production and degradation in aquatic systems. In: Cai, Y., Braids, O.C. (eds.), Biogeochemistry of environmentally important trace metals. ACS symposium series 835: 262–297
Berman M, Bartha R (1986) Levels of chemical versus biological methylation of mercury in sediments. Bull Environ Contam Toxicol 36:401–404
Bloom NS, Fitzgerald WF (1988) Determination of volatile mercury species at the picogram level by low temperature gas chromatography with cold-vapor atomic fluorescence detection. Anal Chim Acta 208:151–161
Bloom NS, Moretto LM, Scopece P, Ugo P (2004) Seasonal cycling of mercury and monomethyl mercury in the Venice Lagoon (Italy). Mar Chem 91:85–99
Chase ME, Jones SH, Hennigar P, Sowles J, Harding GCH, Freeman K et al (2001) Gulfwatch: monitoring spatial and temporal patterns of trace metal and organic contaminants in the Gulf of Maine (1991–1997) with the blue mussel, Mytilus edulis L. Mar Pollut Bull 42:491–505
Clarkson TW (1990) Human health risks from methyl mercury in fish. Eviron Toxicol Chem 9:957–961
Cline JD (1969) Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14:454–458
Couture RM, Gobeil C, Tessier A (2010) Arsenic, iron and sulfur co-diagenesis in lake sediments. Geochim Cosmochim Acta 74:1238–1255
Eckley CS, Hintelmann H (2006) Determination of mercury methylation potentials in the water column of lakes across Canada. Sci Total Environ 368:111–125
Eckley CS, Watras CJ, Hintelmann H (2005) Mercury methylation in the hypolimnetic waters of lakes with and without connection to wetlands in northern Wisconsin. Can J Fish Aquat Sci 62:400–411
Feng XB, Bai WY, Shang LH, He TR, Qiu GL, Yan HY (2011) Mercury speciation and distribution in Aha Reservoir which was contaminated by coal mining activities in Guiyang, Guizhou, China. Appl Geochem 26:213–221
Feyte S, Gobeil C, Tessier A, Cossa D (2012) Mercury dynamics in lake sediments. Geochim Cosmochim Acta 82:92–112
Fitzgerald WF, Clarkson TW (1991) Mercury and monomethylmercury: present and future concerns. Environ Health Perspect 96:159–166
Fleming EJ, Mack EE, Green PG, Nelson DC (2006) Mercury methylation from unexpected sources: molybdate-inhibited freshwater sediments and an iron-reducing bacterium. Appl Environ Microbiol 72(1):457–464
Gill GA, Bloom NS, Cappellino S, Driscoll CT, Dobbs C, Mcshea L et al (1999) Sediment-water fluxes of mercury in Lavaca Bay, Texas. Environ Sci Technol 33(5):663–669
Gilmour CC, Podar M, Bullock AL, Graham AM, Brown SD (2013) Mercury methylation by novel microorganisms from new environments. Environ Sci Technol 47:11810–11820
Guédron S, Huguet L, Vignati DAL, Liu B, Gimbert F, Ferrari BJD, Zonta R, Dominik J (2012) Tidal cycling of mercury and methylmercury between sediments and water column in the Venice Lagoon (Italy). Mar Chem 130–131:1–11
Hammerschmidt CR, Fitzgerald WF (2004) Geochemical controls on the production and distribution of methylmercury in near-shore marine sediments. Environ Sci Technol 38:1487–1495
Hammerschmidt CR, Fitzgerald WF, Balcom PH, Visscher PT (2008) Organic matter and sulfide inhibit methylmercury production in sediments of New York/New Jersey Harbor. Mar Chem 109:165–182
He TR, Feng XB, Guo YN, Giu GL, Li ZG, Liang L, Lu J (2008) The impact of eutrophication on the biogeochemical cycling of mercury species in a reservoir: a case study from Hongfeng Reservoir, Guizhou, China. Environ Pollut 154:56–67
Hines NA, Brezonik PL, Engstrom DR (2004) Sediment and porewater profiles and fluxes of mercury and methylmercury in a small seepage lake in northern Minnesota. Environ Sci Technol 38:6610–6617
Hollweg TA, Gilmour CC, Mason RP (2009) Methylmercury production in sediments of Chesapeake Bay and the mid-Atlantic continental margin. Mar Chem 114:86–101
Horvat M, Liang L, Bloom NS (1993) Comparison of distillation with other current isolation. methods for the determination of methyl mercury compounds in low level environmental samples. Part 2: water. Anal Chim Acta 282:153–168
Hurley JP, Watras CJ, Bloom NS (1991) Mercury cycling in a northern Wisconsin lake: the role of particulate matter in vertical transport. Water Air Soil Pollut 56:543–551
Jiang H (2005) Effects of hydroelectric reservoir on the biogeochemical cycle of mercury in the Wujiang River. Dissertation, Chinese Academy of Sciences
Kosolapov DB, Rogozin DY, Gladchenko IA, Kopylov AI, Zakharova EE (2003) Microbial sulfate reduction in a brackish meromictic steppe lake. Aquat Ecol 37:215–226
Li W, Wu FC, Liu CQ, Fu PQ, Wang J, Mei Y, Wang LY, Guo JY (2008) Temporal and spatial distributions of dissolved organic carbon and nitrogen in two small lakes on the Southwestern China Plateau. Limnol 9:163–171
Liang L, Horvat M, Feng XB, Shang LH, Li H, Pang P (2004) Re-evaluation of distillation and comparison with HNO3 leaching/solvent extraction for isolation of methylmercury compounds from sediment/soil samples. Appl Organometal Chem 18:264–270
Luo S (2003) The Indicators of Fe, Mn and S of the current sedimentation of lakes in Yunna–Guizhou Plateau. Dissertation, Chinese Academy of Sciences
Mauro JBN, Guimaraes JRD, Hintelmann H, Watras CJ, Haack EA, Coelho-Souza SA (2002) Mercury methylation in macrophytes, periphyton, and water-comparative studies with stable and radio-mercury additions. Anal Bioanal Chem 374:983–989
Mehrotra AS, Sedlak D (2005) Decrease in net mercury methylation rates following iron amendment to anoxic wetland sediment slurries. Environ Sci Technol 39:2564–2570
Method 1630 (2001) Methyl mercury in water by distillation, aqueous ethylation, purge and trap, and CVAFS. United States Environmental Protection Agency
Method 1631 (2002) Mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry (Method 1631, Revision E). United States Environmental Protection Agency
Method 1669 (1996) Sampling ambient water for trace metals at EPA water criteria levels. United States Environmental Protection Agency
Munthe J, Bodaly RA, Branfireun BA, Driscoll CT, Gilmour CC, Harris R et al (2007) Recovery of mercury-contaminated fisheries. Ambio 36:33–44
Regnell O, Ewald G (1997) Factors controlling temporal variation in methyl mercury levels in sediment and water in a seasonally stratified lake. Limnol Oceanogr 42(8):1784–1795
Regnell O, Hammar T, Helgée A, Troedsson B (2001) Effects of anoxia and sulfide on concentrations of total and methyl mercury in sediment and water in two Hg-polluted lakes. Can J Fish Aquat Sci 58(3):506–517
Schaefer JK, Morel FMM (2009) High methylation rates of mercury bound to cysteine by Geobacter sulfurreducens. Nat Geosci 2:123–126
Shao D, Kang Y, Wu S, Wong H (2012) Effects of sulfate reducing bacteria and sulfate concentrations on mercury methylation in freshwater sediments. Sci Total Environ 424:331–336
Song LT (2008) Iron isotope and sulfur isotope geochemistry in lake ecosystem-case studies of Hongfeng lake and Aha lake, Guizhou, China. Dissertation, Chinese Academy of Sciences
Sullivan KA, Mason RP (1998) The concentration and distribution of mercury in Lake Michigan. Sci Total Environ 213:213–228
Sunderland EM, Gobas FAPC, Heyes A, Branfireun BA (2004) Speciation and bioavailability of mercury in well-mixed estuarine sediments. Mar Chem 90:91–105
Ullrich SM, Tanton TW, Abdrashitova SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Critical Rev Environ Sci Technol 31(3):241–293
Verta M, Matilainen T (1995) Methylmercury distribution and partitioning in stratified Finnish forest lakes. Water Air Soil Poll 80:585–588
Wang FS (2003) Geochemical behavior of trace metal elements at sediment –water interface in seasonal anoxic lakes. Dissertation, Chinese Academy of Sciences
Wang JL (2004) Environmental micrology. Higher Education Press, Beijing
Warner KA, Roden EE, Bonzongo JC (2003) Microbial mercury transformation in anoxic freshwater sediments under iron-reducing and other electron-accepting conditions. Environ Sci Technol 37:2159–2165
Watras CJ, Bloom NS, Claas SA, Morrison KA, Gilmour CC, Craig SR (1995) Methylmercury production in the anoxic hypolimnion of a dimictic seepage lake. Water Air Soil Poll 80:735–745
Watras CJ, Morrison KA, Regnell O, Kratz TK (2006) The methylmercury cycle in Little Rock Lake during experimental acidification and recovery. Limnol Oceanogr 51(1):257–270
Zhang W (1999) Environmental characters and eutrophication in Hongfeng Reservoir and Baihua Reservoir. Guizhou Technological Publishing, Guiyang
Acknowledgments
This paper was financially supported by the Natural Science Foundation of China (41063006, 41363007, and 41273099), “211 Project” Key Discipline Construction (KST2009003), and the Guizhou Key Laboratory Construction Program (Qiankeheji-Z-[2012]4012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
He, T., Zhu, Y., Yin, D. et al. The impact of acid mine drainage on the methylmercury cycling at the sediment–water interface in Aha Reservoir, Guizhou, China. Environ Sci Pollut Res 22, 5124–5138 (2015). https://doi.org/10.1007/s11356-014-3864-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3864-x