Abstract
Industrial effluent treatment plants (ETPs) are very important in protecting the environment and different life forms from harmful industrial waste. Hence, the efficiency of ETPs must be regularly monitored, particularly after major repair or replacement work. Present study evaluated the performance of an ETP over a period of 4 months, during which aeration tank (T1) of the activated sludge unit was replaced with a new one (T2). System had to be maintained operational during this transition, which warranted close monitoring of the system performance due to the daily load of hazardous industrial wastewater. Analysis showed that the raw wastewater was highly variable in composition and contained many hazardous organic and inorganic pollutants, such as heavy metals, bisphenol A and cyanoacetylurea. It showed significant toxicity against HepG2 cells in vitro. However, the ETP was found to successfully treat and detoxify the wastewater. Denaturing gradient gel electrophoresis (DGGE) analysis showed large temporal fluctuations in the ETP microbial community, which is consistent with the variable composition of wastewater. It indicated that functional stability of the ETP was not associated with stability of the microbial community, probably due to high microbial biodiversity and consequently high functional redundancy. In conclusion, the CETP showed consistent level of detoxification and microbial community dynamics after switching to T2, indicating successful development, acclimatization and commissioning of T2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industries produce large amount of effluent, which contains many harmful chemicals, such as acids, bases, metals and organic pollutants, depending on the nature of the industries. Many of these chemicals are persistent and toxic and exert a variety of health effects, such as endocrine disruption, genotoxicity, bioaccumulation and ecotoxicity (Patel and Pandey 2012). The increasing burden of these contaminants in the environment is a great concern all over the world. Hence, the industrial effluent needs to be treated to an acceptable degree before release into the environment (Gardner et al. 2012). In this regard, industrial effluent treatment plant (ETP) offers a great economical and convenient option. It utilizes combination of physical, chemical and biological processes for the treatment of effluents from diverse industries at a single place. However, ETP is faced with the difficulty of treating complex and variable mixtures of organic and inorganic pollutants from different industries (Moosvi and Madamwar 2007). At times, the toxic chemicals or sudden shock loading in the effluent can inhibit microbial activity, which adversely affects the performance of ETP. Such failure can lead to serious environmental contamination (de Melo et al. 2013).
The biological process is an eco-friendly method that utilizes diverse metabolic capabilities of microorganisms, and hence is a very important component of ETP (Gentile et al. 2007). It generally involves activated sludge process (ASP), where a seed sludge is adapted and selected for specific effluents (Li and Jin 2009). During the acclimatization period, the microbial community undergoes dramatic variation, which decides the success/failure of the unit. Hence, microbial community composition of the ASP is of paramount importance (Mikesková et al. 2012). Many studies have reported that functional stability of microbial communities is key to the performance of ASP (Moura et al. 2009) and can give important clues for the improvement of ETP operation (Yang et al. 2011). The ASP unit is highly sensitive to shock loading, foam formation, operational parameters, biomass concentration and viability, and its malfunctioning can decrease the ETP performance or even lead to system shutdown (de Melo et al. 2013; Moura et al. 2009). Further, continuous long-term operation involves repairing and maintenance of the ASP unit, which may disrupt the active microbial community. Hence, long-term ETP operation requires careful monitoring of the biological step, particularly during system commissioning, maintenance shutdown or replacements, as the microbial communities in the biological treatment are highly responsive to immediate environmental changes (Chong and Chen 2007). Polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE) analysis of 16S rRNA genes provides a powerful tool for molecular fingerprinting of microbial community in ASP (Rathod and Archana 2013).
Present study focuses on an industrial ETP in India, where a new aeration tank was being commissioned due to the malfunctioning of aerators in the existing ASP. The new tank was also required for capacity enhancement of the ETP. The system was kept operational during this transition due to the routine wastewater load. Performance of the ETP during this transition period was monitored in terms of microbial community dynamics, wastewater quality and composition, toxicity and correlation between these parameters. Such studies have been performed only at laboratory scale and with synthetic wastewater so far. Schmidt et al. (2012) studied the disruption of activated sludge community in three laboratory-scale reactors after addition of antibiotics. It resulted in inhibition of the nitrifiers but not the heterotrophs, and the wastewater treatment efficiency recovered during long-term incubation. However, the response and recovery pattern were different between identically treated reactors, indicating that replicate microbial communities in the reactors might have diverged due to small disturbances. Similarly, the microbial community was found to determine the susceptibility of laboratory-scale methanogenic reactors to organic shock loading. Reactors lacking bacteria from Fen cluster did not survive organic shock loading and had to be restarted by addition of fresh inoculum (Steinberg and Regan 2011). However, these controlled studies cannot be extrapolated to the field due to several operational uncertainties such as wastewater quality, shock loading, microbial inhibition and climatic fluctuations. Field-scale studies have been carried out on steadily operating ETPs only (Tocchi et al. 2012) and have not addressed the microbial dynamics during perturbations as described in this study. Start-up is known to be a very critical step in ASP because the development of microbial communities is highly unpredictable and establishment of wrong microorganisms can lead to failure of the ETP (Gardner et al. 2012). Instead of restoring a failure, detecting early deviations and taking corrective measure are more sustainable strategies. Therefore, it is important to monitor the process parameters including the microbial community during process start-up or after breakdown (Wagner et al. 2002). Hence, the development of microbial community in the new tank was closely followed in the current study. Such knowledge-driven treatment of an ETP perturbation has not been reported so far. Furthermore, the microbial diversity depends on both wastewater composition and geographic location, and no systematic study has been reported from the current ETP/location. Additionally, this is the first study to evaluate success of the ETP maintenance operation by a diverse array of parameters. It can be used as a framework for evaluation of other ETPs, as routine maintenance procedures, which can drastically affect the ETP performance, are very common.
Material and methods
ETP operation
The ETP was located in an industrial zone in India. The ETP had the capacity of 5,000 m3 day−1 wastewater and received wastewater from diverse sources, like food, textile, dying and chemical industries. The wastewater was mostly dark black coloured with variable composition, due to high diversity of the source industries. The wastewater quality parameters are given in Supplementary Tables 1 and 2. The broad treatment operations of the ETP are shown in Fig. 1. Mixed liquid suspended solids (MLSS) in the aeration tank was controlled at 3 g L−1. A new aeration tank (T2) was being commissioned in the ETP due to malfunctioning of the aerators in the old aeration tank (T1). Microbial community in T2 was developed by addition of biomass from T1 along with jaggery and cattle dung. Once desired MLSS was achieved, biomass was acclimatized by gradually diverting the wastewater flow to T2 and incrementally increasing the load over a period of 2 months. Finally, all the wastewater was routed through T2, and T1 was completely decommissioned for maintenance work. It also provided the ETP an opportunity of capacity expansion by simultaneous operation of T1 and T2 in future.
Collection of samples
Sampling was done during the transition from T1 to T2 over a period of 4 months, i.e. December 2011 to March 2012. This included the first 2 months where the wastewater was incrementally loaded in T2, followed by monitoring of T2 for additional 2 months. Thus, sampling involved only T1 on 0 day, both T1 and T2 during 15th–60th day, and only T2 during 75th–120th days. Samples of 5-L volume were collected every 15 days from different treatment steps, namely, raw wastewater, ASP-treated water and the final treated water. The collected wastewater samples were brought to laboratory at 4 °C and stored according to the nature of analysis to be carried out (APHA 2005). They were analyzed for physico-chemical parameters, organic pollutants, heavy metals, toxicity, bacterial count and molecular diversity.
Analytical methods
The physico-chemical analysis of wastewater samples was performed according to the standard methods for the examination of water and wastewaters (APHA 2005). The parameters measured were pH, total suspended solids (TSS), total dissolved solids (TDS), chemical oxygen demand (COD), NH3-N, PO4-P, sulphate, sulphide and oil and grease (O&G) content. For metal analysis, samples were digested with HNO3 and HCl in Ethos 900 microwave (Milestone Srl, Italy) and analyzed on iCAP 6300 DUO inductively coupled plasma spectrophotometer (Thermo Fisher, USA). Al, As, B, Be, Cd, Cr, Cu, Fe, Mg, Mn, Ni, Pb and Zn were estimated using multi-element standard VI (Merck, USA).
Gas chromatography-mass spectrometry (GC-MS) analysis was carried out on Clarus 680 GC-Clarus 600C quadrapole MS (PerkinElmer, USA) using DB-5 column (30 m, 0.25 mm, 0.25 μm, Agilent, USA). One litre of each sample was extracted thrice with ethyl acetate. Then, the extracted aqueous phase was adjusted to pH 2, and the extraction procedure was repeated. Resulting solvent extracts were pooled and reduced to 5 mL using a rotary evaporator. The concentrated extract was passed through Na2SO4 column before GC-MS analysis. Each sample of 1 μL was injected using helium as the carrier gas at 1 mL min−1. The injector was set at 280 °C, and the oven program was 60 °C for 2 min, increase to 150 °C at 10 °C min−1 and hold for 2 min, increase to 200 °C at 5 °C min−1 and hold for 2 min, and a final increase to 300 °C at 5 °C min−1 and hold for 10 min. Transfer line and ion source temperature were 200 and 150 °C, respectively. Ionization was carried out in EI + mode at electron energy of 70 eV. The mass scan range was 50 to 600 m/z, and data was collected using the TurboMass software. Peaks were identified by searching mass spectra in the NIST library. However, they could not be quantified due to lack of analytical standards, and results were expressed qualitatively only.
Toxicity evaluation
Cytotoxicity of the samples was determined on HepG2 cells using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Žegura et al. 2009). Sample preparation was similar to that for GC-MS analysis, except that the final extract was completely evaporated, dissolved in dimethyl sulfoxide (DMSO) and filter sterilized. HepG2 cells were obtained from National Centre for Cell Sciences (NCCS), Pune, India. Cells were seeded in 96-well plate at 1 × 104 cells per well in DMEM medium with 10 % fetal bovine serum. The plate was incubated at 37 °C and 5 % CO2 for 24 h to allow attachment of the cells to plate surface. The samples of 50 μL were added to the cells, and the plate was incubated further for 24 h. DMSO was added as negative control, while H2O2 was used as the positive control. Then, 20 μL of MTT solution (5 mg mL−1) was added in every well, and incubation was continued for 1 h. The MTT-containing medium was removed and replaced with 200 μL DMSO. After proper mixing, the plates were read on Infinite 200 Pro microtiter plate reader (Tecan, Switzerland) at 550 nm. The assay was done in triplicate, and toxicity was expressed as percent inhibition of MTT reduction as compared to negative control. The greater the inhibition, the lesser is the cell viability, and the greater is the cytotoxicity.
Microbiological analysis
The samples were processed for microbial enumeration immediately upon arrival at laboratory. They were serially diluted in phosphate-buffered saline (PBS) and plated on M9 medium (composition in g L−1: Na2HPO4, 10.2; KH2PO4, 3; NaCl, 0.6; NH4Cl, 20; glucose, 5; agar, 15) and nutrient agar (composition in g L−1: peptone, 5; beef extract, 1.5; yeast extract, 1.5; NaCl, 5; agar, 15). Nutrient agar was employed to count fast-growing and fastidious bacteria, while M9 minimal medium supported the growth of slow-growing bacteria with simple nutritional requirements (Bafana 2013). The plates were incubated at 25 °C for a week before colony counting.
Molecular biodiversity analysis
For DNA extraction, 50 mL of each sample was centrifuged at 12,000g for 10 min at 4 °C, immediately upon arrival at laboratory. Resulting pellet was washed with PBS and stored at −80 °C till further processing. DNA extraction was then performed using the soil microbe DNA isolation kit (Zymo Research, USA) according to the manufacturer’s protocol. V3 region of the bacterial 16S rRNA gene was amplified from the DNA samples using the universal primers 338F-GC-clamped (5′-CGCCC GCCGC GCGCG GCGGG CGGGG CGGGG GCACG GGGGG ACTCC TACGG GAGGC AGCAG-3′) and 518R (5′-ATTAC CGCGG CTGCT GG-3′) (Muyzer et al. 1993). The PCR conditions were 94 °C for 5 min, 35 cycles of 94 °C for 30 s, 56 °C for 30 s and 72 °C for 1 min, followed by a final extension of 72 °C for 7 min. The resulting samples were analyzed by DGGE using D-Code universal mutation detection system (Bio-Rad, USA). Polyacrylamide gel of 8 % with a denaturing gradient of 30–60 % (100 % denaturant consisted of 7 M urea and 40 % formamide) in 1X TAE buffer (pH 8.3) was used. Electrophoresis was carried out at 100 V and 60 °C for 16 h, and the gel was stained with 50 μg mL−1 of ethidium bromide for 15 min. The DGGE profiles were analyzed using Quantity One software (Bio-Rad, USA) and represented in the form of binary matrix. A distance matrix was calculated based on Dice coefficient, and dendrogram was obtained by unweighted pair group method with arithmetic mean (UPGMA). The distance matrix was also used for moving window analysis, which showed the temporal variation in the microbial community (Wittebolle et al. 2008). The average change in the community was represented as the mean ± standard deviation of the moving window curve data points. The microbial community dynamics was graphically visualized by principal component analysis (PCA) and canonical correspondence analysis (CCA) using SAS Version 8 (SAS Institute Inc., USA).
Statistical analysis
All parameters were analyzed in triplicate. t test and ANOVA were carried out using SPSS 16 (IBM, USA) at the significance level of 0.05.
Results and discussion
Wastewater treatment by ETP
The study involved sampling during all periods, i.e. before, during and after commissioning of T2. Various physico-chemical parameters and the microbial biodiversity were estimated to establish the baseline ETP performance and detect any shift from it. Figure 2 shows the variation in the water quality parameters of raw and final treated wastewater. ANOVA analysis showed significant variation among the raw wastewater samples for all the parameters (p < 0.05), indicating a largely fluctuating wastewater quality. Such operational constraints were not present in the controlled laboratory studies reported so far, which highlights the significance of this study. The raw wastewater was highly coloured and turbid on all days, while the treated water was always clear. Further, the treated water complied with the stipulated limits for all the parameters analyzed on all days. A t test showed that there was significant reduction in all the analyzed wastewater constituents after treatment (p < 0.05). The treated wastewater showed stable values of COD and TSS despite the large fluctuations in these parameters in the raw wastewater. The average COD removal efficiency was about 81 % with average residual COD of 235 mg L−1 in the treated wastewater (Supplementary Table 1). Similarly, the removal efficiencies of the NH3-N and TSS were also very high. There was no indication of any effect from the replacement of ASP unit. The ETP was functionally stable with consistent removal efficiency throughout the study period. It indicated that T2 had successfully developed the requisite microbial population, which was further confirmed by DGGE analysis as described below.
Removal of inorganic and organic pollutants
Variation in the metal concentrations of raw and ASP-treated wastewater is shown in Fig. 2. The values represent total metal concentrations as metal ion speciation was not studied. However, it should be noted that oxidation state of several metals is known to greatly influence their toxicity. Out of the analyzed metals, As, Be, B, Cd, Cu, Ni and Pb were absent, while Al, Cr, Fe, Mg, Mn and Zn were present in significant amount. ANOVA analysis showed huge fluctuations in the load of metals, especially Fe (p < 0.05). Still, ASP along with primary clarifier showed significant removal efficiency (p < 0.05) and yielded stable level of all metals in the treated water (Supplementary Table 2). The presence of organic pollutants in the effluent was investigated by GC-MS analysis. Table 1 shows a compilation of the hazardous compounds predominantly detected on different days, although they all were not present together in a single sample. Majority of these compounds were biodegraded in the ASP, and hence disappeared from the ASP-treated water (Table 1). Some new products of biodegradation, which were absent in the raw wastewater, also appeared. For example, decanoic acid in the ASP-treated water probably arised from the biodegradation of higher chain length fatty acids present in the raw wastewater. This confirmed the biodegradation process in the ASP. Some compounds, like diisooctylphthalate, n-hexadecanoic acid, 5-methyl-5-phenyl-2,4-imidazolidinedione, passed unchanged in the ASP-treated water, but their GC-MS peaks significantly decreased indicating a reduction in the concentration.
It can be seen that the raw wastewater contained many hazardous organic and inorganic pollutants, and was highly variable in terms of composition and water quality parameters (Fig. 2). This was because of the diverse nature and variable wastewater output of the source industries. Similar diversity of wastewater quality has been reported for other ETPs earlier (Moura et al. 2009). Compounds, such as bisphenol A, phthalic esters and phenols, have earlier been reported to be present in industrial effluents, where they can pose serious ecological threat (Fürhacker et al. 2000). Despite this variability, the ASP efficiently removed most of the organic pollutants and metals on all days. The suggested mechanism of action of activated sludge involves biodegradation of organic pollutants and bioaccumulation of metals (Chandra et al. 2011). This resulted in significant reduction in the COD, TSS, nitrogen and phosphorus load of the wastewater, and the treated wastewater met the environmental discharge limits (Supplementary Table 1). Further, the replacement of ASP did not have any discernible effect on the removal of pollutants.
Assessment of wastewater detoxification
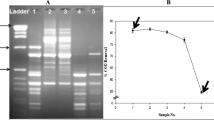
It has been shown that the application of MTT assay with HepG2 cells is highly sensitive in monitoring cytotoxicity of wastewaters and surface waters (Žegura et al. 2009). Hence, it was used to determine detoxification of wastewater in this study. Results showed that raw wastewater consistently had very high level of cytotoxicity, which was significantly reduced (p < 0.05) by the ASP unit (Fig. 3). The average (±standard deviation) inhibition of MTT reduction was 91 ± 2.3 and 30 ± 9 % for raw and ASP-treated wastewater, respectively. The average reduction in toxicity due to ASP treatment was 61 ± 10 %. The treated wastewater toxicity was slightly higher on day 30 (p < 0.05), which might be due to the disturbance in microbial diversity of ASP as discussed below. The toxicity removal by ASP then recovered back with further acclimatization of the biomass in T2. Interestingly, physico-chemical parameters of the treated wastewater were within the stipulated limits on day 30 (Fig. 2), indicating that they can underestimate the potential hazard posed by the wastewater to the environment and human health. Unfortunately, toxicity assays are seldom used to estimate the efficiency of wastewater treatment in ETPs. This study highlights the importance of involving toxicity assays along with the traditional physico-chemical parameters in assessing the wastewater quality. Apart from day 30, the reduction in toxicity was continuously stable even after replacement of the ASP unit, confirming the functionality of the newly commissioned unit. Comparable level of detoxification has been reported by other researches. A combined treatment with ozonation/white rot fungus at laboratory scale caused an abatement of toxicity of industrial dye effluent by 70 % in MTT assay with Caco-2 cells (Vanhulle et al. 2008).
Microbial community analysis
The biomass in ASP tank was estimated by culture-dependent method. For this, the samples were plated on M9 and nutrient agar plates, and the viable bacteria were counted in the form of colony-forming units (cfu) mL−1. Nutrient agar and M9 minimal medium were employed to count both fast-growing and slow-growing bacteria. Figure 4 shows that the biomass was stable in both T1 and T2 throughout the study period (p > 0.05). Also, the bacterial count in T2 was comparable to that in T1 (p > 0.05), indicating satisfactory biomass level in the new ASP tank. The stability of biomass indicated absence of shock loading during the study period. Hence, the effect of shock loading, which is a potential disruptor of ETPs, could not be addressed in this study.
a Microbial biomass in the aeration tanks T1 and T2 determined on nutrient agar (NA) and M9 medium (M9). Error bars represent standard deviation from triplicate experiment, b moving window analysis showing the temporal variation of DGGE-based microbial community in T1 and T2, where each point represents difference between two consecutive sampling days in terms of Dice coefficient. T1/T2 shows pattern of difference between T1 and T2 communities on each day
The stability of the microbial community composition was further determined by culture-independent PCR-DGGE analysis of the 16S rRNA gene. A dendrogram constructed from the DGGE profiles showed that the communities in T1 and T2 were inter-related, and no distinction could be drawn between the two. CCA analysis showed that the microbial diversity was highly correlated to O&G, Al and COD removal efficiency (p < 0.05). Detailed observation revealed that the COD removal efficiency of the activated sludge was at its minimum (around 76 %) on days 30, 60 and 75, which coincided with drastic fluctuations in the microbial community (Fig. 5). Interestingly, the wastewater detoxification was also below average on day 30 (Fig. 3). Thus, the significant variation in diversity on these days was in accordance with the prediction from CCA and demonstrated the importance of microorganisms in smooth functioning of an ETP. In agreement with this, PCA analysis also showed huge variation in the microbial communities on days 60 and 75, and no distinction between T1 and T2 communities on other days (Fig. 5). The variation in microbial community could not be observed with simple determination of biomass on agar plates, highlighting the important insights that can be provided by DGGE.
Moving window analysis also showed high temporal variation in the microbial community of both T1 and T2 (Fig. 4). The average (±standard deviation) % variation between two consecutive samplings was 80 ± 16 and 58 ± 24 for T1 and T2, respectively. Careful examination revealed that the microbial community in T2 initially showed very high variation due to increase in the wastewater loading. The variability then slowly decreased, and the population became more stable as the wastewater loading reached the maximum of 100 %, indicating successful acclimatization (Fig. 4). Li et al. (2010) also reported a similar change in microbial community of activated sludge due to gradual increase in the wastewater concentration. The microbial communities in T1 and T2 during the period of simultaneous operation also showed high variation relative to each other. The relative average % variation between T1 and T2 was 58 ± 32.
The microbial community in the aeration tank was highly dynamic, which probably reflected a large variation in the raw wastewater composition and the diverse nature of the source industries. Li and Jin (2009) have reported that change in the bacterial community diversity is due to the integrated effects of all the contaminants in the wastewater. Despite the microbial variation, the ASP unit could effectively remove pollutants on all the days (except day 30, due to potential reasons discussed above). This is in accordance with Miura et al. (2007) who showed that the microbial population can vary significantly without affecting the treatment efficiency. Thus, the community variation probably represented successful adaptation of the microbial population to the wastewater variability. Further, the replacement of ASP tank did not have much effect on the treatment efficiency. Although the reduction in toxicity was relatively low on day 30, it improved gradually, and high level of toxicity removal was achieved by the end of T2 commissioning. Thus, the new tank T2 had developed an efficient microbial population specific for the incoming wastewater, which was phylogenetically diverse, but functionally stable. The operation of commissioning of the new ASP tank could be concluded to be successful.
Conclusions
Present study investigated the success of replacement of ASP unit of an ETP. Results showed that the raw wastewater was highly variable and contained many hazardous organic and inorganic constituents. However, the ASP unit successfully removed the pollutants and detoxified the wastewater for safe discharge. Although the detoxification efficiency transiently decreased during the acclimatization of new ASP tank, it gradually recovered with time. The study highlighted the importance of toxicity bioassays in evaluating the success of ASP replacement operation. Both old and new ASP units were characterized by very highly variable, but comparable microbial communities. The replacement operation did not affect the performance of ETP, indicating successful commissioning of the new ASP unit. The study can be used as a framework to conduct data-driven evaluation of other ETPs during maintenance operation.
References
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington
Bafana A (2013) Diversity and metabolic potential of culturable root-associated bacteria from Origanum vulgare in sub-Himalayan region. World J Microbiol Biotechnol 29:63–74
Chandra R, Bharagava RN, Kapley A, Purohit HJ (2011) Bacterial diversity, organic pollutants and their metabolites in two aeration lagoons of common effluent treatment plant (CETP) during the degradation and detoxification of tannery wastewater. Bioresour Technol 102:2333–2341
Chong NM, Chen YS (2007) Activated sludge treatment of a xenobiotic with or without a biogenic substrate during start-up and shocks. Bioresour Technol 98:3611–3616
de Melo ED, Mounteer AH, Leão LH, Bahia RC, Campos IM (2013) Toxicity identification evaluation of cosmetics industry wastewater. J Hazard Mater 244–245:329–334
Fürhacker M, Scharf S, Weber H (2000) Bisphenol A: emissions from point sources. Chemosphere 41:751–756
Gardner M, Comber S, Scrimshaw MD, Cartmell E, Lester J, Ellor B (2012) The significance of hazardous chemicals in wastewater treatment works effluents. Sci Total Environ 437:363–372
Gentile ME, Nyman JL, Criddle CS (2007) Correlation of patterns of denitrification instability in replicated bioreactor communities with shifts in the relative abundance and the denitrification patterns of specific populations. ISME J 1:714–728
Li J, Jin Z (2009) Effect of hypersaline aniline-containing pharmaceutical wastewater on the structure of activated sludge-derived bacterial community. J Hazard Mater 172:432–438
Li J, Jin Z, Yu B (2010) Changes in the structure and diversity of bacterial communities during the process of adaptation to organic wastewater. Can J Microbiol 56:352–355
Mikesková H, Novotný Č, Svobodová K (2012) Interspecific interactions in mixed microbial cultures in a biodegradation perspective. Appl Microbiol Biotechnol 95:861–870
Miura Y, Hiraiwa MN, Ito T, Itonaga T, Watanabe Y, Okabe S (2007) Bacterial community structures in MBRs treating municipal wastewater: relationship between community stability and reactor performance. Water Res 41:627–637
Moosvi S, Madamwar D (2007) An integrated process for the treatment of CETP wastewater using coagulation, anaerobic and aerobic process. Bioresour Technol 98:3384–3392
Moura A, Tacão M, Henriques I, Dias J, Ferreira P, Correia A (2009) Characterization of bacterial diversity in two aerated lagoons of a wastewater treatment plant using PCR–DGGE analysis. Microbiol Res 164:560–569
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Patel H, Pandey S (2012) Evaluation of physical stability and leachability of Portland Pozzolona Cement (PPC) solidified chemical sludge generated from textile wastewater treatment plants. J Hazard Mater 207–208:56–64
Rathod J, Archana G (2013) Molecular fingerprinting of bacterial communities in enriched azo dye (Reactive Violet 5R) decolorising native acclimatised bacterial consortia. Bioresour Technol 142:436–444
Schmidt S, Winter J, Gallert C (2012) Long-term effects of antibiotics on the elimination of chemical oxygen demand, nitrification, and viable bacteria in laboratory-scale wastewater treatment plants. Arch Environ Contam Toxicol 63:354–364
Steinberg LM, Regan JM (2011) Response of lab-scale methanogenic reactors inoculated from different sources to organic loading rate shocks. Bioresour Technol 102:8790–8798
Tocchi C, Federici E, Fidati L, Manzi R, Vincigurerra V, Petruccioli M (2012) Aerobic treatment of dairy wastewater in an industrial three-reactor plant: Effect of aeration regime on performances and on protozoan and bacterial communities. Water Res 46:3334–3344
Vanhulle S, Trovaslet M, Enaud E, Lucas M, Taghavi S, van der Lelie D, van Aken B, Foret M, Onderwater RCA, Wesenberg D, Agathos SN, SchneiderYJ CAM (2008) Decolorization, cytotoxicity, and genotoxicity reduction during a combined ozonation/fungal treatment of dye-contaminated wastewater. Environ Sci Technol 42:584–589
Wagner M, Loy A, Nogueira R, Purkhold U, Lee N, Daims H (2002) Microbial community composition and function in wastewater treatment plants. Antonie Van Leeuwenhoek 81:665–680
Wittebolle L, Vervaeren H, Verstraete W, Boon N (2008) Quantifying community dynamics of nitrifiers in functionally stable reactors. Appl Environ Microbiol 74:286–293
Yang C, Zhang W, Liu R, Li Q, Li B, Wang S, Song C, Qiao C, Mulchandani A (2011) Phylogenetic diversity and metabolic potential of activated sludge microbial communities in full-scale wastewater treatment plants. Environ Sci Technol 45:7408–7415
Žegura B, Heath E, Černoša A, Filipič M (2009) Combination of in vitro bioassays for the determination of cytotoxic and genotoxic potential of wastewater, surface water and drinking water samples. Chemosphere 75:1453–1460
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOC 34 kb)
Supplementary Table 2
(DOC 30 kb)
Rights and permissions
About this article
Cite this article
Bafana, A., Kumar, G., Kashyap, S.M. et al. Dynamics of effluent treatment plant during commissioning of activated sludge process unit. Environ Sci Pollut Res 22, 3538–3546 (2015). https://doi.org/10.1007/s11356-014-3597-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3597-x