Abstract
Antibiotics and other pharmaceuticals are contaminants of the environment because of their widespread use and incomplete removal by microorganisms during wastewater treatment. The influence of a mixture of ciprofloxacin (CIP), gentamicin (GM), sulfamethoxazole (SMZ)/trimethoprim (TMP), and vancomycin (VA), up to a final concentration of 40 mg/L, on the elimination of chemical oxygen demand (COD), nitrification, and survival of bacteria, as well as the elimination of the antibiotics, was assessed in a long-term study in laboratory treatment plants (LTPs). In the presence of 30 mg/L antibiotics, nitrification of artificial sewage by activated sludge ended at nitrite. Nitrate formation was almost completely inhibited. No nitrification at all was possible in the presence of 40 mg/L antibiotics. The nitrifiers were more sensitive to antibiotics than heterotrophic bacteria. COD elimination in antibiotic-stressed LTPs was not influenced by ≤20 mg/L antibiotics. Addition of 30 mg/L antibiotic mixture decreased COD removal efficiency for a period, but the LTPs recovered. Similar results were obtained with 40 mg/L antibiotic mixture. The total viable count of bacteria was not affected negatively by the antibiotics. It ranged from 2.2 × 106 to 8.2 × 106 colony-forming units per milliliter (CFU/mL) compared with the control at 1.4 × 106–6.3 × 106 CFU/mL. Elimination of the four antibiotics during phases of 2.4–30 mg/L from the liquid was high for GM (70–90 %), much lower for VA, TMP, and CIP (0–50 %), and highly fluctuating for SMZ (0–95 %). The antibiotics were mainly adsorbed to the sludge and not biodegraded.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In the GERMAP report of 2008, the total consumption of antibiotics in human medicine was estimated to account for 250–300 tons per year. Eighty-five percent were used in the outpatient sector. Between 2003 and 2007, for example, the oral consumption of cephalosporins and fluorochinolones in this sector increased by 30 % each. One major problem of this practice is the increasing resistance of pathogenic bacteria against different antibiotics, e.g., of Enterococcus faecium against aminopenicillins and glycopeptide antibiotics or of Escherichia coli against fluorochinolones and sulfamethoxazole (SMZ)/trimethoprim (TMP) (GERMAP 2008). Another problem is that most pharmaceuticals used in medicine, including antibiotics, are persistent or are metabolized only partially in the human body. The unchanged substances as well as their metabolites are then excreted with urine and faeces and enter wastewater treatment plants (WWTPs) by way of sewage. In addition, approximately 20–40 % of households in Germany dispose of expired or surplus pharmaceuticals by drainage into sewers (Al-Ahmad et al. 1999, 2009). Because different laboratory tests showed that most antibiotics, including sulfonamides, TMP, and fluorochinolones, are not or only to a minor extent biodegradable in aquatic environments (Al-Ahmad et al. 1999; Alexy et al. 2004; Halling-Sørensen et al. 2000; Junker et al. 2006; Lindberg et al. 2006), they pass WWTPs with or without influence on treatment processes and can be detected in the range of micrograms per litre in WWTP effluents, in surface water, and in groundwater (Benito-Pena et al. 2006; Kümmerer 2008). Some retention of antibiotics may be caused by adsorption. In the case of fluorochinolones, approximately 70 % of antibiotic from the aqueous phase are adsorbed to sludge during wastewater treatment (Golet et al. 2003; Lindberg et al. 2006). For this reason, the presence of antibiotics in raw sewage could also influence the different degradation processes in WWTPs, e.g., elimination of chemical oxygen demand (COD) and nitrification. Antibiotics should have a more drastic influence on nitrification than on COD elimination because the autotrophic nitrifiers are less numerous in activated sludge and grow slowly (Halling-Sørensen 1993). It is known that within nitrification, nitrite oxidation is particularly sensitive. Inhibition of nitratation leads to the accumulation of nitrite, a particularly toxic nitrogen compound, in WWTPs. This could not only be problematic during the treatment of sewage but also after discharging effluent to receiving waters. COD elimination could also be disturbed by increasing antibiotic concentrations, with the consequence that legally demanded boundary values for COD concentrations in the effluents of WWTPs cannot be maintained.
The aim of this work was to study the effects of increasing concentrations of equal amounts of the four antibiotics—ciprofloxacin (CIP), gentamicin (GM), SMZ/TMP, and vancomycin (VA)—on COD removal and nitrification during biological wastewater treatment in laboratory-scale treatment plants (LTPs). To prevent entry of other antibiotics from sewage, an artificial wastewater was used. The chosen antibiotics were added as a mixture, 25 % (w/v) of each antibiotic, with increasing concentrations ranging from 100 μg/L to 40 mg/L. The LTPs were run for 442 days to investigate the long-term impact of antibiotics on wastewater treatment.
Materials and Methods
WWTPs
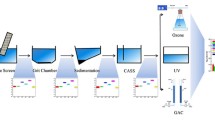
In this study, three continuously operated LTPs (model KA 1; Behr Labor-Technik GmbH, Düsseldorf, Germany) in accordance with DIN 38412 L24 (1981) and DIN 38412 L26 (1994) were run in parallel. One LTP, without addition of antibiotics, was used as a control, and the other two LTPs were operated with increasing concentrations of the antibiotics described later in the text. For long-term operation, aeration through sintered glass at the bottom of the aeration tank and sludge recirculation from the clarifier were performed with compressed air. Two separate peristaltic pumps, one for a concentrated artificial wastewater and one for dilution tap water as described later in the text, were used for providing artificial wastewater. A scheme of the modified LTPs is shown in Fig. 1. In the LTPs, the microbiological processes for carbon elimination and nitrification, how they take place in the aeration tank of a conventional WWTP, should be simulated. The downstream clarifier (1.5 L) should provide a constant sludge concentration in the aeration tank (3 L) by the sludge recirculation unit, each consisting of borosilicate glass. The effluent from the clarifier was continuously discharged. The LTPs were inoculated with activated sludge from the municipal WWTP of Karlsruhe (Germany).
Artificial Wastewater, Removal of COD, and Nitrification
Synthetic sewage differs from real wastewater by a defined composition with a constant carbon-to-nitrogen ratio. No other substances, such as pharmaceuticals, antibiotics, or microorganisms like in real sewage, enter the LTPs. Nine liters of artificial OECD (Organization for Economic Cooperation and Development) wastewater (80 mg/L peptone from casein, 80 mg/L peptone from meat, 110 mg/L meat extract, 30 mg/L urea, 28 mg/L K2HPO4, 7 mg/L NaCl, and 2 mg/L MgSO4·7 H2O) according to DIN 38412 L24 and OECD 303A (2001) were continuously fed every day to the aeration tanks until day 91. Because during operation with OECD medium, formation of scum and an increase in filamentous organisms due to nutrient limitations were observed, the concentration was slowly increased ≤threefold until day 142. For nutrient supply during continuous reactor operation, a 600fold concentrated solution of the OECD wastewater was prepared with demineralized water and autoclaved for 15 min at 121 °C. During steady state, 45 mL of this concentrate and 9 L tap water for dilution were continuously fed every day to the aeration tanks to finally obtain a threefold concentrated OECD medium. The hydraulic retention time (HRT) in the LTPs was 12 h.
To quantify carbon and nitrogen removal, efficiencies of COD elimination and nitrification were calculated. The COD concentrations of influent (approximately 350 mg/L) and effluent samples of the LTPs were determined as described later in the text using a calibration curve. The COD elimination (%) was calculated according to Eq. 1:
For nitrification, ammonia must be present in the respective sample. The OECD wastewater contained peptone, meat extract, and urea, and the total organic nitrogen content was approximately 57 mg/L. Total nitrogen content and ammonium–nitrogen (NH4 +–N), nitrite–nitrogen (NO2 −–N), and nitrate–nitrogen (NO3 −–N) concentrations in influent and effluent samples of the LTPs were determined. Nitrification efficiency (%) was calculated according to Eq. 2 under the assumption that all organic nitrogen compounds (=100 % N) could be hydrolyzed into NH4 +–N and subsequently oxidized to nitrate:
Antibiotics
The four tested antibiotics were CIP (Bayer HealthCare AG, Germany), GM (Caesar and Loretz GmbH, Germany), SMZ/TMP (ratio 5:1 = SXT) (Berlin-Chemie AG, Germany), and VA (Lyomark Pharma GmbH, Germany). They were selected from different chemical substance groups according to their mechanisms of action and their frequency of use. The main characteristics of the chosen antibiotics are listed in Table 1. The control LTP was operated without the addition of antibiotics. To the test LTPs 1 and 2, antibiotic mixtures of CIP, GM, SXT, and VA with 25 % (w/v) of each antibiotic were added at increasing concentrations from stock solutions: 0.1 mg/L starting at day 22, 0.2 mg/L starting at day 32, 0.4 mg/L starting at day 50, 0.8 mg/L starting at day 64, 1.2 mg/L starting at day 78, 2.4 mg/L starting at day 171, 3.6 mg/L starting at day 198, 7.2 mg/L starting at day 247, 14.4 mg/L starting at day 288, 20 mg/L starting at day 306, 30 mg/L starting at day 372, and 40 mg/L starting at day 456. At a concentration of 7.2 mg/L, addition of increasing amounts of antibiotic mixture was first performed only in LTP 1. If no serious collapses in the wastewater-relevant parameters were analyzed in LTP 1, the concentration in LTP 2 was increased.
Adsorption of Antibiotics to Activated Sewage Sludge
To determine adsorption of antibiotics, 120 mL activated sludge were transferred from the control LTP into 250-mL Schott bottles and autoclaved for 30 min at 121 °C to inactivate microorganisms. After cooling to room temperature, antibiotics were added from stock solutions to give a final concentration of 0.9 mg/L of each antibiotic. Stock solutions contained either 1 mg/L CIP, GM, or VA, 0.5 mg/L SMZ, or 0.1 mg/L TMP and were sterile filtrated with 0.2-μm filters. Five parts SMZ and 1 part TMP were added into the same bottle to simulate application conditions. Bottles were closed with rubber stoppers and incubated for 24 h at 27 °C on a shaker (120 rpm). After incubation, the content of the Schott bottles was transferred into centrifuge beakers and the sludge separated from liquid for 10 min at 11,400×g. Liquid and sludge were collected separately, and the antibiotic distribution was analyzed by Technologiezentrum Wasser (TZW) (Karlsruhe, Germany).
Nitrification–Inhibition Test
A method based on DIN EN ISO 9509 L38 (2007) was used to estimate the short-term inhibitory effect of the antibiotics on nitrifying activated sludge from the control LTP by measuring the decrease of ammonium. After centrifugation of activated sludge at 10,950×g for 15 min, the supernatant was discarded and the sludge pellet resuspended in the same volume of tap water. Centrifugation was repeated and the pellet homogenously resuspended to the initial volume with tap water. The total volume of each assay of the inhibition test was 50 mL in 110-mL serum bottles containing 25 mL washed activated sludge, 5 mL medium (5.04 g/L sodium hydrogen carbonate, 2.65 g/L ammonium sulfate), and an appropriate volume of the antibiotic stock solution to obtain the respective concentration plus the corresponding volumes of water to get 50 mL. The antibiotic stock solution contained 150 mg/L each of CIP, GM, SXT, and VA. In addition, a nitrification control assay was run without the addition of antibiotics and an inhibition control assay with 2 mL of known inhibitor allylthiourea (1.16 g/L). All assays were performed in duplicate and incubated at 20 °C for 6 h in the dark, aerated with compressed air, and mixed on a magnetic stirrer. Samples were taken at time t = 0 and 6 h and filtrated through a 0.45-μm filter (Roth, Karlsruhe, Germany). The ammonia concentration was measured using cuvette test LCK 303, which is suitable for 2–47 mg/L NH4 +–N (Hach Lange, Düsseldorf, Germany).
Analyses
The artificial OECD wastewater was used without further treatment for the determination of COD, the total Kjeldahl nitrogen (TKN) or total bound nitrogen (TNb), NH4 +–N, NO2 −–N, and NO3 −–N. The same parameters were also determined for the effluent of the LTPs, but NH4 +–N, NO2 −–N, and NO3 −–N were measured after filtration through a 0.45-μm filter. COD was determined according to the method of Wolf and Nordmann (1977). TKN was determined according to DIN EN 25663 H11 (1994). Alternatively, TNb was measured using the LATON cuvette test LCK 338 for the range of 20–100 mg/L total nitrogen (Hach Lange, Düsseldorf, Germany). NH4 +–N concentration was determined according to DIN 38406 E5 (1983). The concentration of NO2 −–N was determined according to DIN 38405 D10 (1993). NO3 −–N concentration was measured directly according to standard methods (American Public Health Association 1995). Single antibiotics as primary substances from the influent and effluents of the LTPs at different time intervals were determined by an external analytical laboratory by way of high-performance liquid chromatography–mass spectrometry (HPLC–MS) (TZW, Karlsruhe, Germany) according to Sacher et al. (2001). Elimination rates were calculated on the basis of the antibiotic concentrations measured in the influent and effluent of the LTP 1.
All solutions were prepared with MilliQ water (Millipore, Schwalbach, Germany). All chemicals were of analytical grade and purchased from Merck/VWR (Darmstadt, Germany), Sigma-Aldrich, Fluka (Taufkirchen, Germany), or Roth (Karlsruhe, Germany).
CFUs
At every sampling time, approximately 10–20 mL activated sludge were taken by a sludge pipette, put in an Erlenmeyer flask, and homogenized for 30 s with an Ultra Turrax (Janke and Kunkel, Staufen, Germany). Then a series of 10fold dilutions with sterile saline solution (0.9 % NaCl) was prepared, and 100 μL of each dilution were plated in duplicate on Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung (DEV) nutrient agar (10 g/L meat peptone, 10 g/L meat extract, 5 g/L NaCl, and 18 g/L agar–agar [pH 7.3]). After incubation for 48 h at 37 °C, grown colonies were counted and the CFUs/mL calculated.
Results
Short-Term Nitrification–Inhibition Test
In Fig. 2, results of the nitrification–inhibition test with activated sludge from the control LTP are listed. The only statistically significant decrease in ammonia-oxidation efficiency, approximately 18 %, was analyzed in the assay with 6 mg/L antibiotic mixture. Compared with the control assay, assays with 1.2, 2.4, 7.2, 12, 13.2, 15.6, and 40 mg/L antibiotic mixture showed a nonsignificantly increased ammonia-oxidation efficiency (maximally 12 %), and assays with 3.6, 4.8, 8.4, 9.6, 10.8, and 20 mg/L antibiotic mixture showed a nonsignificantly decreased ammonia-oxidation efficiency (maximally 18 %). Ammonia oxidation by activated sludge that was not previously stressed with antibiotics was not inhibited by increasing concentrations of the antibiotic mixture ≤40 mg/L in a short-term nitrification–inhibition test.
Nitrification in LTPs With Increasing Concentrations of Antibiotics
To calculate nitrification efficiency of the activated sludge according to Eq. 2, total bound nitrogen, ammonia–nitrogen, nitrite–nitrogen, and nitrate–nitrogen concentrations of the OECD wastewater and the effluents of the three LTPs were analyzed (Fig. 3a–c). Increasing amounts of the antibiotic mixture were added from day 22 onward to LTPs 1 and 2. From 7.2 mg/L, the antibiotic mixture in LTP 2 was added time delayed to that of LTP 1 to avoid failure of both LTPs at further increasing antibiotic concentrations. No influence of low antibiotic concentrations on nitrification was seen within 82 days. All ammonia was nitrified to nitrate. From days 82 to 142, the concentration of the OECD medium was tripled stepwise because the formation of scum and an increase in filamentous organisms, presumably due to nutrient limitation, were observed in all three LTPs independently of antibiotic addition. The more concentrated medium initially caused disturbances of the nitrification process. At a steady state with the threefold concentrated OECD wastewater (e.g., days 200–330) and at increasing antibiotic concentrations ≤14.4 mg/L, ammonia was no longer completely oxidized in the antibiotic stressed LTPs (Fig. 3b, c), but nitrate was still the only final product. In the presence of 20 to ≤30 mg/L antibiotic mixture, nitrification mainly led to nitrite, and only a little nitrate was formed. After inoculation, the nitrification efficiency of the activated sludge in the three LTPs fed with OECD wastewater stabilized quickly and reached values of 80–95 % until day 82 (Fig. 4a, b). Regardless of whether or not the four antibiotics were present, nitrification in all LTPs was rather unstable due either to the formation of scum and dominance of filamentous organisms (data not shown) or failure of sufficient oxygen supply (biofilm-clogged air diaphragm). Differences in nitrification activity during the experimental period from days 157 to 353 (e.g., days 162–199 or 290–334 [Fig. 4a] and days 297–353 [Fig. 4b]) were apparently not caused by the antibiotics or the carbon supply but rather by varying process conditions. Overall, nitrification efficiency in the three reactors at the greater loading rate (threefold concentrated OECD medium, 12 h HRT) was approximately 20–40 % lower than at the lower loading rate (OECD medium, 12 h HRT). When 20 mg/L antibiotics were supplied, nitrification apparently seemed to collapse. However, nitrite and a little nitrate were still formed (Fig. 3b, c).
Ammonia, nitrite, and nitrate concentrations in the effluent of the control LTP (a), the antibiotic stressed LTP 1 (b), and LTP 2 (c). LTPs were fed with OECD wastewater (days 1–91), which was continuously concentrated to threefold until day 142 and then supplied with this concentration onward during the experiment. A mixture of equal amounts of the four antibiotics was added to LTPs 1 and 2 to give final concentrations of 0.1 mg/L from day 22 onward, 0.2 mg/L from day 32 onward, 0.4 mg/L from day 50 onward, 0.8 mg/L from day 64 onward, 1.2 mg/L from day 78 onward, 2.4 mg/L from day 171 onward, and 3.6 mg/L from day 198 onward. The antibiotic concentration of LTP 1 was increased to 7.2 mg/L at day 247, to 14.4 mg/L at day 288, to 20 mg/L at day 306, and from day 372 onward to 30 mg/L. In LTP 2, the antibiotic concentration was increased to 7.2 mg/L time-delayed at day 288, 14.4 mg/L at day 306, 20 mg/L at day 325, and 30 mg/L at day 379
Elimination of COD in LTPs With Increasing Concentrations of Antibiotics
COD elimination was >80 % for both OECD wastewater and threefold concentrated OECD wastewater at 12 h HRT. There was no influence of ≤20 mg/L antibiotic mixture on COD removal. When the antibiotic concentration was increased to 30 mg/L, there was a short period of significantly decreased carbon removal efficiency, which recovered after several days. In the presence of 40 mg/L antibiotics, carbon removal decreased much further but recovered, also after a while, to approximately 70 % (data not shown). Carbon removal was less affected by increasing antibiotic concentrations than nitrification.
Total Viable Count in the LTPs With Increasing Concentrations of Antibiotics
As listed in Table 2, the total viable count of the activated sludge from the municipal wastewater treatment plant in Karlsruhe, which was used as an inoculum, decreased from 2.4 × 107 to 9.1 × 105 CFU/mL after 22 days of operation of the LTPs with OECD wastewater. The remaining population seemed to adapt to OECD wastewater because the total viable count increased later to 2.2 × 106 CFU/mL in the LTPs with antibiotics and to 3.5 × 106 CFU/mL in the control LTP and stabilized at this level until the end of the experiment. Increasing concentrations ≤30 mg/L antibiotic mixture had no effect on the total viable count of the LTPs despite the fact that the antibiotics should act either bacteriostatically or even bactericidally (Table 1).
Elimination of Single Antibiotics
As listed in Table 1, the water solubility of the antibiotics was much greater than the applied concentration. According to the log P values, GM and VA had hydrophilic and SMZ, TMP, and CIP hydrophobic properties. Furthermore, partitioning of the antibiotics between water phase and activated sludge was different (Table 3). Whereas SMZ, TMP, and CIP were quantitatively recovered in water and sludge, only 12–27 % of GM and VA were recovered in total, and <1 % of GM and approximately 6 % of VA seemed to be adsorbed (Table 3). Although recovery of GM and VA in the liquid was low, almost no GM and VA could be desorbed from sterilized or living sludge, suggesting either a tight adsorption and/or chemical conversion. Adsorption of SMZ, TMP, and CIP was in the range of 8–27 %, and the rest was dissolved in water. GM was eliminated from the water phase by >90 % at low antibiotic concentrations and to an extent of >60 % at high antibiotic concentrations by living (Fig. 6) or sterilized sludge (Table 3). In contrast, TMP was almost not eliminated in the LTP (Fig. 6), whereas approximately 25 % was adsorbed to sterilized sludge (Table 3). Elimination of CIP in the LTPs was, maximally, 40 %. More than 20 % of the antibiotic adsorbed to sterilized sludge. SMZ was recovered quantitatively with 8 % being adsorbed to sterilized sludge. Elimination of SMZ in the LTPs varied between very low and very high values (Fig. 6). Due to the inhomogeneous suspension of activated sludge, achieving a quantitative balance of antibiotics that were dissolved in the wastewater or absorbed on sludge particles was difficult. The sum of antibiotic concentrations in liquid and sludge (Table 3) apparently added up to 93–95 % for TMP, SMZ, and CIP, indicating at least no degradation of the antibiotics. Recoveries in liquid plus sludge were much lower for GM (20–27 %) and VA (12–19 %), which might have been due to chemical or microbial degradation.
Discussion
The experimental setup chosen for the present study should simulate conditions resembling those in a WWTP. An artificial OECD wastewater was used despite the concern that only specific bacteria might be selected; however, there was certainty that no other antibiotics were entering the LTPs. With respect to reported antibiotic concentrations in WWTP effluents, which were in the range of lower micrograms per litre (Kümmerer 2004; Ternes 2001), the final antibiotic concentration of 40 mg/L in this study may seem to be high. However, to inhibit one or more important metabolic processes of biological wastewater treatment by antibiotics, a stepwise increase of the concentration of a mixture of CIP, GM, SXT, and VA ≤ 40 mg/L in the incoming wastewater was necessary. Such high concentrations of pharmaceuticals were found in Patancheru near Hyderabad, India, in the effluent of a WWTP where a major production site of generic drugs for the world market is located. The WWTP is used by approximately 90 bulk drug manufacturers, and concentrations of CIP, the most abundant drug, at 14 to ≤31 mg/L have been reported (Fick et al. 2009; Larsson et al. 2007). In other wastewater from a pharmaceutical company, 45 mg/L nalidixic acid, a quinolone-type antibiotic, were detected (Sirtori et al. 2009).
The influence of 1.2–40 mg/L antibiotic mixture containing equal amounts of CIP, GM, SXT, and VA on nitrification of the population of an activated sludge system that was fed with OECD wastewater was tested in batch assays. The results (Fig. 2) indicated neither promoting nor inhibiting conditions of ammonia oxidation in the presence of the antibiotics. VA should have no effect on Gram-negative, ammonia-oxidizing bacteria (AOB) because of its mode of action on cell wall biosynthesis (Table 1). A possible reason why the other antibiotics showed no effect may be the short test duration of 6 h. Generation times of AOB vary from 8 h for Nitrosomonas sp. (Prosser 1986), to 23 h for Nitrosolobus spec., and 20–138 h for Nitrosospira sp. (Belser and Schmidt 1978; Jiang and Bakken 1999). Therefore, inhibition of cell division or protein biosynthesis may be observed only after test duration of several days. Furthermore, single bacteria or complex biomass and sludge have reserves of growth factors (e.g., folic acid) that may prevent inhibition of growth by SMZ and TMP, which are both inhibitors of folic acid biosynthesis (Table 1).
Nitrification of ammonia from hydrolyzed peptone and meat extract of the artificial wastewater, which was used for all three LTPs, showed obvious fluctuations during the experiment. In the first 80 days, nitrification was relatively stable with efficiencies >70 % (Fig. 4a, b). The following strong fluctuations were the result of an enhanced development of filamentous bacteria under low organic loading conditions, associated with the formation of scum in all three LTPs, independently of antibiotic addition (data not shown). By increasing organic loading through addition of threefold-concentrated OECD wastewater, the problems could be overcome, and nitrification efficiencies finally stabilized after day 199. From days 162 to 199, both LTPs were rather instable. The decreased nitrification efficiency in LTP 1 was more obvious (Fig. 4a) than that of LTP 2 (Fig. 4b), but the reasons for this decrease were different in both LTPs. In LTP 1, increased antibiotics caused an increase of nitrite concentration in the effluent −51 mg/L (Fig. 3b), whereas the same increase of antibiotic concentration caused an accumulation of ammonia ≤61 mg/L in LTP 2 (Fig. 3c). Nitrification efficiency recovered within 28 days to 72.6 % (LTP 1) and 62.6 % (LTP 2). When 20 mg/L antibiotics were added for 28 days (Fig. 4a, b), a dramatic decrease of nitrification efficiency in LTPs 1 and 2 occurred at 2.5 and 6.3 %, respectively. In LTPs 1 and 2, the oxidation of ammonia to nitrite was still working, but the oxidation of nitrite to nitrate was disturbed (Fig. 3b, c). Dokianakis et al. (2004) reported that SMZ and ofloxacin, a member of the fluoroquinolone antibiotics, inhibited nitrite oxidation of isolated nonadapted nitrite oxidizing bacteria within 5 h at concentrations of 6 and 10 mg/L. This phenomenon could also be observed in our long-term experiment. In LTP 1, nitrification efficiency recovered to 24 % by recovering the oxidation of nitrite to nitrate, whereas this was not the case in LTP 2. A further increase ≤30 mg/L antibiotics in LTP 1 led to complete failure of nitrification in both LTPs with a time delay in LTP 1 (Fig. 4a). The development of antibiotic resistance by nitrifiers would not matter in this study because nitrification was completely inhibited at an antibiotic concentration of 40 mg/L (data not shown) and hitherto, to our knowledge, no such studies have been published. At the beginning of the experiment, all three LTPs were inoculated with activated sludge from a full-scale WWTP. LTPs 1 and 2 obtained the same concentrations of the four antibiotics and thus should have behaved similarly in the parallel assays. The nonsimilar behavior of the complex biomass was not surprising if one takes into consideration that Kaewpipat and Grady (2002) showed with denaturing gradient gel electrophoresis that replicate complex microbial communities in activated sludge systems were not identical. Answers to small changes might be considered chaotic (Kooi et al. 1997). Two initially identical systems operated under identical conditions can diverge in their behavior to a disturbance and ultimately exhibit different characteristics.
The COD removal efficiencies of all three LTPs were more effective and stable compared to the nitrification efficiencies. The strong fluctuations from days 82 to 157 (change to threefold concentrated artificial wastewater) occurring in the nitrification efficiencies of the LTPs operated with antibiotics were not observed because the two processes occur separately and are catalyzed by different groups of microorganisms. The LTPs operated with antibiotics showed decreased COD elimination from day 380 (after the increase to 30 mg/L antibiotics) onward, which probably might have been due to the addition of antibiotics or due to nitrite that was not oxidized to nitrate (Fig. 3b, c) because the control LTP was stable at the same time (Fig. 5a, b). Both LTPs recovered until day 416, and even the addition of 40 mg/L antibiotic mixture to LTP 1 had no effect on COD elimination (data not shown). One possible explanation could be the different action of the species involved because carbon removal is performed by the more robust and fast growing heterotrophic bacteria compared with the slow-growing chemoautotrophic nitrifiers. The heterotrophs possibly might have adapted to the high nitrite concentrations that were present because of the breakdown of the nitratation (Fig. 3b, c).
High concentrations of antibiotics did not influence COD elimination and therefore growth and activity of heterotrophs. Colonies on DEV nutrient agar plates at different time intervals, representing increasing concentrations of antibiotics, showed no decrease of CFUs/mL in the LTPs with and without antibiotics (Table 2). This is surprising if we take into account the bacteriostatic or bactericidal action of the pharmaceuticals, but drugs can only act if they are dissolved in water and thus bioavailable. One possibility that could decrease the bioavailability of antibiotics and thus minimize or even neutralize their impact are extracellular polymeric substances (EPSs), which consist mainly of carbohydrates, proteins, and humic substances. These polymers are excreted by bacteria of activated sludge and are major components of sludge flocs, which could be defined as a “swimming biofilm.” It is known that the EPS-network inside the flocs is capable of adsorbing pollutants (Comte et al. 2007). This is one reason why bacteria in biofilms were more resistant to antibiotics and other harmful substances than single, freely floating bacteria (Goldberg 2002; Peng et al. 2002). EPS might also have some relevance for our results in that it adsorbs antiobiotics and decreases their toxicity. However, adsorption seemed to have a limiting effect because the nitrifiers in deeper layers of the sludge flocs were apparently inhibited by addition of 20 mg/L antibiotics. This was taken as an indication that toxic concentrations of the antibiotics were present.
In addition to COD degradation, elimination of antibiotics was also determined (Fig. 6). The elimination of GM was relatively stable between 64 to 95 % over the entire tested range of antibiotic concentrations. Adsorption tests with autoclaved activated sludge showed that only little GM could be found in the liquid after 24 h of adsorption, but the missing GM could also not be extracted from the sludge, similarly to VA (Table 3). This may mean that GM adsorbs strongly and irreversibly to the sludge and thus was eliminated by abiotic influences and not by biodegradation. Autoclaving might destroy some components of EPS and de-arrange the polymeric network of activated sludge flocs that had adsorbed pollutants due to coagulation of, e.g., protein components. Hydrolysis of EPS by heating was shown by Karapanagiotis et al. (1989) and by Comte et al. (2007). If the sludge that was used in our experiments was autoclaved, the floc structure changed. The gel-like sludge flocs appeared more compact, presumably to denatured protein components (data not shown). However, we do not think that adsorption phenomena of antibiotics would change drastically. Josephson et al. (1979) showed that GM adsorbed substantially to the surface of glass containers, probably due to electrostatic interactions between negative charges on the surface of the glass and the positively charged GM. This also could have happened in our experiment because the LTPs were made of borosilicate glass. Elimination of VA was highest at 7.2 and 30 mg/L antibiotics (30–60 %, respectively). This behavior does not indicate that the complex structure of the primary substance VA is biodegraded. One possible explanation of the “high and low elimination” are highly sensitive adsorption and desorption processes to the sludge. Biological degradation seems also unlikely for SMZ because of subsequent high and low concentrations in the liquid (Fig. 6). Al-Ahmad et al. (1999) already showed that SMZ was not biodegraded after 40 days in a closed bottle test at a concentration of 3.8 mg/L. Drillia et al. (2005) investigated aerobic biodegradation of SMZ by an enriched consortium, but only when carbon or nitrogen were depleted in the feed medium was SMZ degraded to undetectable levels. Nutrient limitation could be excluded in the present study, and adsorption to sludge followed by later desorption is also no explanation because preliminary adsorption tests with inactivated sludge showed that approximately 87 % of SMZ was found in water phase (Table 3). Negligible adsorption of SMZ has also been reported in other studies (Li and Zhang 2010; Wu et al. 2009). Elimination of TMP started at 30 mg/L antibiotic mixture to an extent of 35.4 %, so biodegradation after adaption might be possible (Fig. 6). In contrast, Lindberg et al. (2006) found approximately the same concentration of TMP in the final effluent of a WWTP as in the raw sewage. Biological degradation of CIP seems also unlikely, and this is supported by literature. For example, Al-Ahmad et al. (1999) showed that CIP was not biodegraded after 40 days in a closed bottle test at a concentration of 3.5 mg/L, whereas Lindberg et al. (2006) found that CIP sorbed to the used sludge, which could also be an explanation for the finding in this study. Except for GM, the tested antibiotics showed a fluctuating elimination from water phase, which indicated elimination primarily by adsorption rather than biodegradation.
Conclusion
With increasing concentrations of the antibiotics CIP, GM, SXT, and VA to totally 30 mg/L, nitrification of ammonia stopped at nitrite, whereas nitrification was completely inhibited in the presence of 40 mg/L antibiotic mixture. The degradation of organic compounds was less affected by 30 mg/L antibiotic mixture than was nitrification. COD elimination >70 % was still observed. Under conditions with real municipal wastewater, which contains antibiotics in the range of ng/L to μg/L, biological processes in a WWTP should not be influenced by antibiotics. The fluctuating higher and lower antibiotic concentrations in the effluent found at increasing concentrations during the long-term experiment indicated abiotic influences, such as adsorption/desorption processes, rather than biodegradation. Therefore, activated sludge treatment seems not to be an appropriate process for antibiotic elimination from sewage. Other treatment methods, such as membrane technologies, might be necessary.
References
Al-Ahmad A, Daschner FD, Kümmerer K (1999) Biodegradability of cefotiam, ciprofloxacin, meropenem, penicillin G, and sulfamethoxazole and inhibition of waste water bacteria. Arch Environ Contam Toxicol 37:158–163
Al-Ahmad A, Haiß A, Unger J, Brunswick-Tietze A, Wiethan J, Kümmerer K (2009) Effects of a realistic mixture of antibiotics on resistant and nonresistant sewage sludge bacteria in laboratory-scale treatment plants. Arch Environ Contam Toxicol 57:264–273
Alexy R, Kümpel T, Kümmerer K (2004) Assessment of degradation of 18 antibiotics in the closed bottle test. Chemosphere 57:505–512
American Public Health Association (1995) Standard methods for the examination of water and wastewater. APHA, Washington, DC
Belser LW, Schmidt EL (1978) Serological diversity within a terrestrial ammonia-oxidizing population. Appl Environ Microbiol 36:589–593
Benito-Pena E, Partal-Rodera AI, Leon-Gonzalez ME, Moreno-Bondi MC (2006) Evaluation of mixed mode solid phase extraction cartridges for the preconcentration of beta-lactam antibiotics in wastewater using liquid chromatography with UV-DAD detection. Anal Chim Acta 556:415–422
Comte S, Guibaud G, Baudu M (2007) Effect of extraction method on EPS from activated sludge: an HPSEC investigation. J Hazard Mater 140:129–137
DIN 38405 D10 (1993) In: DIN German Standards Institute e.V. (ed) German standard methods for water, wastewater and sewage analysis. Berlin [in German]
DIN 38406 E5 (1983) In: DIN German Standards Institute e.V. (ed) German standard methods for water, wastewater and sewage analysis. Berlin [in German]
DIN 38412 L24 (1981) In: DIN German Standards Institute e.V. (ed) German standard methods for water, wastewater and sewage analysis. Berlin [in German]
DIN 38412 L26 (1994) In: DIN German Standards Institute e.V. (ed) German standard methods for water, wastewater and sewage analysis. Berlin [in German]
DIN EN 25663 H11 (1994) In: DIN German Standards Institute e.V. (ed) German standard methods for water, wastewater and sewage analysis. Berlin [in German]
DIN EN ISO 9509 L38 (2007) In: DIN German Standard Institute e.V. (ed) German standard methods for water, wastewater and sewage analysis. Berlin [in German]
Dokianakis SN, Kornaros ME, Lyberatos G (2004) On the effect of pharmaceuticals on bacterial nitrite oxidation. Water Sci Technol 50:341–346
Drillia P, Dokianakis SN, Fountoulakis MS, Kornaros M, Stamatelatou K, Lyberatos G (2005) On the occasional biodegradation of pharmaceuticals in the activated sludge process: the example of the antibiotic sulfamethoxazole. J Hazard Mater 122:259–265
DrugBank (2005) Open data drug and drug target database. http://www.drugbank.ca. Accessed 14 March 2011
Fick J, Söderström H, Lindberg RH, Phan C, Tysklind M, Larsson DG (2009) Contamination of surface, ground, and drinking water from pharmaceutical production. Environ Toxicol Chem 28:2522–2527
GERMAP (2008) Antibiotika-Resistenz und–Verbrauch. In: BVL, PEG, Division of Infectious Diseases (eds) Bericht über den Antibiotikaverbrauch und die Verbreitung von Antibiotikaresistenzen in der Human und Veterinärmedizin in Deutschland [in German]. Rheinbach
Goldberg J (2002) Biofilms and antibiotics resistance: a genetic linkage. Trends Microbiol 10:264
Golet EM, Xifra I, Siegrist H, Alder AC, Giger W (2003) Environmental exposure assessment of fluorochinolone antibacterial agents from sewage to soil. Environ Sci Technol 37:3243–3249
Halling-Sørensen B (1993) Biological nitrification and denitrification. In: Halling-Sørensen B, Jorgensen SE (eds) The removal of nitrogen compounds from wastewater. Elsevier, Amsterdam, pp 41–53
Halling-Sørensen B, Lützhoft HCH, Andersen HR, Ingerslev F (2000) Environmental risk assessment of antibiotics: comparison of mecillinam, trimethoprim and ciprofloxacin. J Antimicrob Chemother 46:53–58
Jiang QQ, Bakken LR (1999) Comparison of Nitrosospira strains isolated from terrestrial environments. FEMS Microbiol Ecol 30:171–186
Josephson L, Houle P, Haggerty M (1979) Stability of dilute solutions of gentamicin and tobramycin. Clin Chem 25:298–300
Junker T, Alexy R, Knacker T, Kümmerer K (2006) Biodegradability of C-14-labeled antibiotics in a modified laboratory scale sewage treatment plant at environmentally relevant concentrations. Environ Sci Technol 40:318–324
Kaewpipat K, Grady CPL Jr (2002) Microbial population dynamics in laboratory-scale activated sludge reactors. Water Sci Technol 46:19–27
Karapanagiotis NK, Rudd T, Steritt R, Lester J (1989) Extraction and characterization of extracellular polymers in digested sewage sludge. J Chem Technol Biotechnol 44:107–120
Kooi BW, Boer MP, Kooijman SALM (1997) Complex dynamic behavior of autonomous microbial food chains. J Math Biol 36:24–40
Kümmerer K (2004) Resistance in the environment. J Antimicrob Chemother 54:311–320
Kümmerer K (2008) Pharmaceuticals in the environment. Sources, fate, effects and risk, 3rd edn. Springer, Berlin
Larsson DGJ, de Pedro C, Paxeus N (2007) Effluent from drug manufacturers contains extremely high levels of pharmaceuticals. J Hazard Mater 148:751–755
Li B, Zhang T (2010) Biodegradation and adsorption of antibiotics in the activated sludge process. Environ Sci Technol 44:3468–3473
Lindberg RH, Olofsson U, Rendahl P, Johansson MI, Tysklind M, Andersson BAV (2006) Behavior of fluorochinolones and trimethoprim during mechanical, chemical, and active sludge treatment of sewage water and digestion of sludge. Environ Sci Technol 40:1042–1048
OECD 303A (2001) Guidelines for the testing of chemicals, section 3: degradation and accumulation. http://www.oecd-ilibrary.org. Accessed 27 April 2009
Peng JS, Tsai WC, Chou CC (2002) Inactivation and removal of Bacillus cereus by sanitizer and detergent. Int J Food Microbiol 77:11–18
Prosser JI (1986) Nitrification. Society for General Microbiology special publication 20, Oxford
Sacher F, Lange FT, Brauch H-J, Blankenhorn I (2001) Pharmaceuticals in groundwaters analytical methods and results of a monitoring program in Baden-Württemberg, Germany. J Chromatogr A 938:199–210
Sirtori C, Zapata A, Oller I, Gernjak W, Agüera A, Malato S (2009) Decontamination industrial pharmaceutical wastewater by combining solar photo-Fenton and biological treatment. Water Res 43:661–668
Ternes TA (2001) Analytical methods for the determination of pharmaceuticals in aqueous environmental samples. Trends Anal Chem 20:419–434
Wolf P, Nordmann W (1977) Eine Schnellmethode zur Bestimmung des chemischen Sauerstoffbedarfs in Abwasser [A study-method for measuring COD of wastewater]. Korrespondenz Abwasser 24:277–281
Wu C, Spongberg AL, Witter JD (2009) Sorption and biodegradation of selected antibiotics in biosolids. J Environ Sci Health A 44:454–461
Acknowledgments
This study (IGF 15830N/2) of the research association DECHEMA e.V. was funded by the AiF under the program of encouragement of industrial research and development (IGF) by the Federal Ministry for Economics and Technology (BMWi) based on a decision of the German Bundestag. We are grateful to Bayer HealthCare AG, Caesar and Loretz GmbH, Berlin-Chemie AG, and Lyomark Pharma GmbH, Germany, for providing the huge amounts of antibiotics used in this project. We thank A. Morio for technical and analytical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmidt, S., Winter, J. & Gallert, C. Long-Term Effects of Antibiotics on the Elimination of Chemical Oxygen Demand, Nitrification, and Viable Bacteria in Laboratory-Scale Wastewater Treatment Plants. Arch Environ Contam Toxicol 63, 354–364 (2012). https://doi.org/10.1007/s00244-012-9773-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-012-9773-4