Abstract
Ongoing and widespread use of triclosan (TCS) negatively affects the environment and human health. The objective of this study was to investigate the fate of TCS in activated sludge systems under pulsed electric field stimulation and to explore the role of biodegradation and adsorption in its removal. Two parallel PEF-SBRs activated sludge systems were constructed with sodium acetate (R1) and glucose (R2) as carbon sources respectively to illustrate the effects of different carbon source treatment systems on the performance, extracellular polymer substances (EPS) secretion characteristics, and microbial communities of TCS-containing wastewater. TCS inhibited ammonia removal, with the inhibition becoming more pronounced as the TCS concentration (range of 0.5–5 mg/ L) increases, but the inhibition gradually faded as the sludge was acclimatized, and the continuous increase of TCS concentration had almost no effect on the removal effect. The EPS contents increased when TCS was added at 5 mg/L but decreased significantly at 10 mg/L TCS. The main removal pathway for TCS in both reactors was biodegradation, but the degradation efficiency of TCS was significantly higher in R2 than in R1. High throughput sequencing data showed that Thauera was dominant in R1, while Pseudomonas had the highest proportion of relative abundance in R2. This study further illustrated the effect of TCS on activated sludge systems and microbial communities under the influence of pulsed electric fields.
Graphical Abstract

Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Triclosan (C12H7Cl3O2, 5-chloro-2-(2',4'-dichlorophenoxy) phenol, TCS), with antibacterial, deodorizing, disinfecting, and antiseptic effects, was widely used in industrial and personal care products such as textiles, medical devices, children's toys, toothpaste, soap and face wash (Ruszkiewicz et al., 2017; Zhao et al., 2012). As triclosan is lipid soluble, it binds to fatty acid ligases to form trimeric FAS-NAD + -TCS complexes which hinder lipid synthesis and thus inhibit bacterial cell growth (Schweizer, 2001). Triclosan, therefore, dominated the ingredient list of most care products, causing it to enter municipal sewage treatment plants with domestic sewage. The ordinary municipal wastewater treatment process did not achieve the complete elimination of TCS, which was subsequently discharged into the natural environment (Chen et al, 2014; Guerra et al., 2019; van Wijnen et al., 2018). For example, wastewater (10.9–86200 ng/L) (Kookana et al., 2011; Senthil et al., 2010; Zhao et al., 2010), surface water (0.16–2300 ng/L) (Yavuz et al., 2015), groundwater (0–0.03 μg/L) (Raji & Packialakshmi, 2022; Sorensen, et al., 2015), sediment (28–37,189 μg/kg) (Cantwell et al., 2010), and drinking water (0.2–14.5 ng/L) (Benotti et al., 2009) were detected. Animals and plants exposed to the TCS environment for long periods were damaged and eventually enter the human body through nature's biological chain, causing irreparable damage to the body. A large number of toxicological studies have shown that, as an environmental hormone substance, the endocrine-disrupting effect of TCS will not only produce various toxic effects and carcinogenic effects on mammals but also have an important impact on the growth, development, and reproduction of algae and fish in the water (Hao et al., 2019; Nandikes et al., 2022). Meanwhile, TCS could affect microbial activity in the environment and might cause changes in the structure or function of the microbial community in the wastewater treatment system. Given the high ecological risk of triclosan, an effective elimination method of TCS was required.
Residues of TCS and its related compounds in the environment have been of increasing concern in recent years. To date, various technologies have been developed for the removal of TCS. Activated sludge (ASP) is a universally used method for aerobic biological treatment of wastewater. However, the partial treatment of TCS in conventional ASP proved that TCS is refractory to biodegradation (Chen et al., 2011). A study had shown that extending the hydraulic retention time (HRT), sludge retention time (SRT), and temperature could enhance the removal efficiency of TCS, but excessive prolongation of the HRT or SRT may decrease the treatment ability and lead to sludge expansion (Armstrong et al., 2018). The sequencing batch reactors (SBRs) had a good effect on the removal of TCS, while the adsorption of TCS by the biomass in the system also contributed to the removal of TCS from the effluent (Wang et al., 2020). Previous studies (Stasinakis et al., 2007) have shown that the addition of 0.5 mg/L TCS initially inhibited the nitrification of the no-acclimatized sludge, but the inhibition diminished as domestication progressed, and an increase in TCS dosing had no impact on the removal of ammonia. Similarly, the effect of different concentrations of TCS on the continuously activated sludge system was found to have no significant adverse effect on the removal of chemical oxygen demand (COD), biochemical oxygen demand (BOD), and ammonia nitrogen (NH4+-N) (Federle et al., 2002). Analysis of the effect of functional bacteria in activated sludge on the removal of TCS showed that 2 mg/LTCS was almost completely removed at an SRT of 7–8 days, with the contribution of AOB (36–42%), unknown degrading bacteria (59%) and KCY1 (8.5%), respectively (Lee et al., 2015). Nitrosomonas europaeal in activated sludge systems was able to biodegrade 0.5—2 mg/L TCS in mineral salt medium (Hyungkeun et al., 2009). Stasinakis et al. (2007) used synthetic wastewater as a substrate to construct an activated sludge system to investigate the effect of SRT on the degradation capacity of TCS, and the results showed that the degradation capacity increased with the increase of SRT. According to Holzem et al., (2018), TCS reduced the abundance and diversity of denitrifying bacteria in aged biosolids. What’s more, EPS facilitate the adhesion and aggregation of the microorganism and contribute to the establishment and stabilization of microbial community structures (Hashem et al., 2021; Miao et al., 2018). In addition, EPS was secreted by microbial cells to defend infringement from the severe environment and different organic pollutants could be adsorbed by EPS through complex interactions (Yan et al., 2019). Loosely bound EPS (LB-EPS) banded more strongly to TCS than tightly bound EPS (TB-EPS) in a neutral environment. The hydrophobic zone in the sludge EPS was found to adsorb a wide range of organic pollutants, including phenanthrene and benzene (Liu et al., 2001; Sheng et al., 2008). The organic components of EPS could effectively adsorb sulfamethazine through hydrophobic partitioning and hydrogen bonding to remove organic pollutants (Xu et al., 2013; Ismail et al., 2022).
Pulsed electric field (PEF), as the representative technology of electric field effect on microorganisms, has great research value and application prospects in food processing, medicine, biotechnology, and other fields (Lee & Chang, 2014). It had been shown that PEF had a sublethal effect on microorganisms and that microorganisms were stimulated rather than inactivated when low electric field strengths based on sublethal levels were applied to them. Therefore, the technology of using PEF to stimulate microorganisms to treat pollutants has attracted more and more attention. A study comparing two means of sludge pretreatment, PEF and ultrasonic, showed that PEF treated sludge significantly more efficiently than ultrasonic pretreatment (Rittmann et al., 2009). Our previous research proved that denitrification processes were facilitated when the applied voltage was reduced from the working voltage for microbial inactivation (kilovolts) to only a few volts in the presence of a pulsed electric field and a periodically reversed electric field (Qian et al., 2017, 2018; Zhang et al., 2019).
Herein, two PEF-SBRs coupling systems with different carbon sources were operated to explore the effects of different TCS concentrations on system performance, including organic loading and nitrification, etc. The concentrations of TCS and EPS were determined at different phases and mass balance was performed to estimate the role of adsorption and biodegradation. High-throughput sequencing was carried out to profile the composition of the microbial community within the reactors. Combined with the analysis of microbial community information and the fluctuation of COD, NH4+-N, NO3−-N, and NO2−-N content, the key role of functional bacteria in the process of stabilizing the activated sludge system containing TCS and degrading TCS was fully explained.
2 Materials and Methods
2.1 Chemicals
Triclosan (C12H7Cl3O2, ≧ 97% purity) was purchased from Sigma-Aldrich (China). Acetonitrile (C2H3N, ACN), and methanol (MeOH) high-performance liquid chromatography (HPLC) grade chemicals were supplied by Rankem Chemical, China. Triclosan stock solution was prepared by dissolving 10 mg of triclosan standard in methanol solvent (100 mg/L). The configured stock solution was stored in the dark at 4℃.
2.2 Wastewater Sampling and Characteristic
Seed sludge was obtained from a conventional activated sludge municipal wastewater treatment plant (WWTP) located in Shenyang. The samples were collected in brown glass containers and refrigerated at 4℃ before being transported to the laboratory for characterization and processing. Chemical oxygen demand (COD), total organic carbon (TOC), pH, conductivity, and color were measured to characterize the physicochemical properties of the samples. The details of the samples were showed in Table S1.
Artificial wastewater consisted of 4.25 g glucose; 0.50 g ammonium chloride (NH4Cl); 0.0075 g sodium chloride (NaCl); 0.002 g calcium chloride hexahydrate (CaCl2・6H2O); 0.04 g magnesium sulfate heptahydrate (MgSO4・7H2O); 0.016 g dipotassium hydrogen phosphate (K2HPO4), and 0.04 g mono-potassium phosphate (KH2PO4) in 1Lde-ionized water.
2.3 Continuous-Flow Reactors Construction
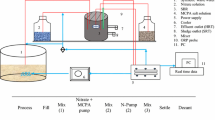
Two identical cylindrical lab-scale sequencing batch reactors (SBRs) with serrated grooves were used in this study, and each chamber had a 1.5 L working volume. The outer surface of the chambers was wrapped with tin foil to exclude the effect of photodegradation. According to previous studies, iron and carbon plates were inserted as anode and cathode respectively in the grooves with a pulsed power supply connected to the top. The operating parameters of the pulsed power supply were 0.01 A pulse current, 1000 Hz pulse frequency, 6 cm plate spacing, and 50% duty cycle. Porous ceramic diffusers and air flow meters were used to aerate the reactors to ensure that the dissolved oxygen level in the chambers stabilized at 2 mg/L-3 mg/L. Wastewater and solids recycling were driven by peristaltic pumps. Two cylindrical plastic tanks with a liquid volume of 50 L were used to provide nutrients for continuous feeding and to drain discharge respectively. The reactors operated for four cycles per day, and each cycle consisted of 5 min of feeding with artificial wastewater, 330 min of aerobic nitrification, 20 min of sludge settling, 5 min of effluent discharging, and idle periods. According to pre-experiment optimization results (Fig. S1), HRT was 10 h, and SRT was maintained for 10 days. Meanwhile, the mixed liquor suspended solid (MLSS) ranged between 3000 ± 500 mg/L, the MLSS determination method has been added to the supplementary material. The configuration of the reactor was shown in Fig. 1.
Two continuous-flow PEF-SBRs systems were run in parallel. All chambers were operated at 20 ± 1.5 ℃ in the dark, pH was kept at 7.5 ± 0.2. R1 and R2 were added sodium acetate and glucose, respectively. Two reactors were run for 98 days with four phases. The first 7 days (phase I) were aimed to set up the reactors and keep the system operation stable, all systems were fed with artificial municipal wastewater to keep the characteristics of influent wastewater were COD 300 ± 20 mg/L, NH4+-N 50 ± 5 mg/L, and NO3−-N < 5 mg/L. 0.5, 5, and 10 mg/L TCS solution was added to the last three phases of R1 and R2, respectively. The second phase and third phases of R1 and R2 were run for 28 days, while the last phase was run for 35 days, a total of 98 days. TCS mass balance was calculated at the end of each stage. The concentration of TCS used in this work was higher than that found in municipal wastewater, aiming to explore the degradation produced by TCS. Seven sludge samples were withdrawn, including one seed sludge sample and six experimental samples obtained from the end of each phrase in R1 and R2 for subsequent analysis.
2.4 Mass Balance of Triclosan
The proportion of biosorption and biodegradation in the TCS removal process was assessed by mass balance calculations. At the end of each stage after the addition of TCS, samples of the influent, effluent, and sludge from the system were collected and the concentration and quality of the TCS were measured. TCS mass balance was calculated by measuring the amounts of TCS in the liquid and sludge phase at the end of the last idle period using the following equations (Jia et al., 2020):
where \(M_{biodegradation}\) was the amount of TCS biodegradation, \(M_{in}\) was the amount of TCS added, \({M}_{eff}\) was the amount of TCS remained in the liquid phase, \(M_{adsorption}\) was the amount of TCS remained in the sludge phase, \(C_{in}\) was the concentration of TCS influent, \(V_{in}\) was the volume of influent injected in this cycle, \({C}_{l}\) was the concentration of TCS in the liquid phase, \({C}_{li}\) was the concentration of TCS in the liquid phase in the last idle period, \({V}_{l}\) was the volume of liquid in this cycle, \({V}_{li}\) was the volume of liquid in the last idle period, \({C}_{s}\) was the concentration of TCS in the sludge phase, \({C}_{si}\) was the concentration of TCS in the sludge phase in the last idle period, \({V}_{s}\) was the volume of sludge in this cycle, \({V}_{si}\) was the volume of sludge in the last idle period.
2.5 Microbial Community Analysis
To obtain sufficient biomass for the identification of the microbial community, samples needed to be collected and concentrated several times at the same moment. Samples must be washed three times with PBS buffer after collection. Samples were treated with a cell crusher for 10 min to completely break down the microbial cells for DNA extraction. The Genomic DNA kit (Sangon Biotech, Shanghai, China) had been used for extracting DNA from the samples. The obtained DNA was sent to polymerase chain reaction (PCR) amplification of the V3-V4 region of the bacterial 16S rRNA gene. 20μL of the mixture was used for PCR reactions. The PCR products were quantitated by QuantiFluor™-ST fluorometer (Promega, USA) and sequenced on Illumina MiSeq 2*150 platform according to the criteria.
The fastq files were de-multiplexed and quality-filtered by Quantitative Insights Into Microbial Ecology (QIIME, version 2.0). The operational units (OTUs) were clustered (97% similarity cut-off) by UPARSE, and UCHIME was applied to take out the identified chimeric sequences. The RDP classifier was used to classify 16 S rRNA gene sequences with a 70% confidence threshold against the silva (SSU115) 16 S rRNA database. The relative abundances at the phylum, order, and genus levels were calculated by taxonomic data. The alpha diversity was exhibited by the values of Chao1, Shannon, and Simpson index. A principal coordinate analysis was performed to analyze the variability of the microbial community among the different samples.
The abundance of involved bacteria, Ammonia—Oxidizing Archaea (AOA) and Ammonia—Oxidizing Bacteria (AOB), and Nitrite—Oxidizing Bacteria (NOB) of the 13 sludge samples were quantified by quantitative-polymerase chain reaction (qPCR). The plasmid DNA containing the marker genes was diluted from 108 to 101 copies to determine the standard curve. The primers and composition of the mixture used in this study were shown in supplemental materials. Samples and standard plasmids in triplicate.
2.6 Analytical Methods
To control the operation of continuous-flow systems, monitors of influent and effluent COD, NH4+-N, NO3−-N, and NO2−-N were performed every two days using Standard Methods (APHA, 2005). All the samples were filtered with 0.45 μm pore size Millipore filters (Jinteng, Tianjin) before testing. The concentration of DO was monitored by a portable DO meter (HQ30D, Hach, China). Temperature and pH values were determined daily by portable instruments. The ultrasonic heating method was applied to withdraw EPS from the obtained samples as supplemental materials. The Polysaccharides (PS) in EPS were tested by anthrone-sulfate spectrophotometry, with glucose as the prototype for the standard curve. The protein (PN) using the Folin—phenol reagent method for determination of bovine serum albumin as the prototype for the standard curve. The experiments were repeated three times in parallel and the mean values were taken for analysis (Li & Yang, 2007). Triclosan concentration was detected by HPLC (Agilent 1200 Series) equipped with a C18 column (Zorbax Eclipse Plus, 4.6 × 150 mm, 5 μm). The measurement wavelength was 230 nm. The column temperature was set at 45℃. TCS separation was achieved with a mobile phase of A-70% water and B-30% acetonitrile at a flow rate of 1.0 mL/min. The injection volume was 2 μL.
Statistical analysis and correlation analysis were analyzed by SPSS software (version 23.0). The data were compared with the mean ± standard deviation. Additionally, a one-way analysis of variance (ANOVA) was used to test the variance of the biodegradation efficiency of TCS. P-value < 0.05 was used as a criterion for judging whether the data were statistically significant.
3 Results and Discussion
3.1 The Removal Efficiencies of Parameters in a Continuous System
3.1.1 Effect of TCS on COD Removal
The PEF-SBRs systems were operated steadily for 98 days. The removal performance of R1 and R2 were depicted in Fig. 2a and b, during the start-up phase of the experiment (phase I), similar data of different parameters were observed in both systems. The average removal efficiencies of COD were 85.83%, and 88.34%, respectively. To further explore the fate and influence of the domestic sludge process, TCS was added to the influent wastewater according to the above experimental design. The addition of TCS led to a small increase in the influent concentration of COD, the average influent concentration was 320 ± 10 mg/L. No deterioration of COD removal efficiency was observed when 0.5 and 5 mg/L TCS were injected into reactors. The effluent COD of R1 and R2 ranged around 30 mg/L during phases II and III, which indicated that 0.5 and 5 mg/L TCS had little adverse effect on the reduction of COD. On the contrary, when 10 mg/L TCS was loaded to the two reactors, in the first few days of the fourth stage, the removal efficiencies of COD dropped slightly compared with the first three-phase, averaging about 77.58%. However, from the 12th day of the fourth stage, the COD removal rate in both reactors gradually increased, and then gradually recovered to more than 90%, especially the removal rate of reactor R2, which could reach 96.38%. This phenomenon may be due to the toxic interference caused by the amount of TCS in the reactors exceeding the tolerance value of activated sludge, and after a period of acclimation and culture, the tolerance capacity of the activated sludge increased and then survived. The overall removal capacity of R2 was slightly higher than that of R1, possibly because glucose was more readily utilized by microorganisms in activated sludge.
3.1.2 Effect of TCS on Nitrogen Removal
The variations of nitrogen removal in two reactors were shown in Fig. 2c and d. In phase I, average removal efficiencies of NH4+-N in R1 and R2 both reached about 95%, on the contrary, the effluent concentration NO3−-N and NO2−-N were increased with the reduction of NH4+-N. With 0.5 mg/L TCS added to reactors, NH4+-N removal efficiencies were sharply reduced to 63.78% and 65.99%, respectively. In the next experiment, the removal rate of NH4+-N gradually increased and by the end of phase II, it was similar to that observed in phase I, demonstrating that microorganisms in activated sludge gradually adapted to TCS and recovered the system. Further increase of TCS injection (5 and 10 mg/L) did not significantly affect the degradation capacity of NH4+-N. Similar to COD removal, compared with R2, R1 owned a relatively lower removal ammonia ability, it might be due to the difference in carbon source. The nitrification reaction occurred when TCS was added, the production of NO2−-N might be the inhibition of NOB by TCS (Jia et al., 2020). But with the increase of NO3−-N in the effluent, the concentration of NO2−-N decreased from the initial 38.78 mg/L to almost 3.66 mg/L in the first phase. From the second stage of R1 to the end of the reaction operation, the average effluent NO3−-N and NO2−-N concentrations remained stable at 52.48 mg/L and 0.76 mg/L, respectively. The variation trend of parameter concentration in the R2 reactor was similar to R1. The concentration of NO2−-N was 43.78 mg/L at the initial stage of reactor operation, but soon dropped to 8.3 ± 0.5 mg/L. And at the next three phases of the reaction, the average effluent NO3−-N and NO2−-N concentrations remained stable at 47.53 mg/L and 0.48 mg/L, respectively. These results proved that TCS had an inhibitory effect on nitrogen removal, but after a period of acclimation, the inhibitory effect was weakened. The conversion of NO3−-N to NO2−-N recovered faster than the conversion of NO2−-N to N2. Overall, R2 had stronger resistance to TCS than R1.
To assess the nitrification inhibition of TCS, nitrification rates were calculated at different experimental stages. The average nitrification rate was calculated by Eq. (5). It was assumed that the nitrogen required for cell growth was NH4+-N in this work.
where qN was the average nitrification rate, mg Ng/VSS/h; \(Inf.{{NH}_{4}}^{+}-N\) was the influent content of NH4+-N, mg/h; \(Eff.{{NH}_{4}}^{+}-N\) was the effluent content of NH4+-N, mg/h; \(N_{synthesis}\) was the nitrogen required for cell growth, mg/h, \(N_{synthesis}\) was obtained based on the daily biomass removed by the activated sludge system and 12% of the nitrogen biomass (Rittmann et al., 2009); V was the reactor volume, L; MLVSS was the concentration of volatile suspended solids in the reactors, g/L.
The effect of TCS on nitrification rates was similar to the previously reported effect on NH4+-N removal (Stasinakis et al., 2007). The addition of 0.5 mg/L TCS resulted in a significant decrease in qN, from 1.35 mg Ng/VSS/h (phase I) to 0.58 mg Ng/VSS/h (phase II) in R1 and from 1.66 mg Ng/VSS/h (phase I) to 0.62 mg Ng/VSS/h (phase II) in R2. The further increase in TCS content did not significantly affect the nitrogen removal capacity.
3.1.3 The Removal Efficiencies of TCS in Continuous PEF-SBRs Systems
TCS removal efficiencies of R1 and R2 were shown in Fig. 3a. The effluent concentration of TCS of R1 and R2 were 0.26 mg/L and 0.03 mg/L at the end of phase II, respectively. And at the end of phase III, 2.11 mg/L and 0.25 mg/L TCS were observed in effluent. At the end of the experiment, 4.88 mg/L and 0.78 mg/L TCS remained in the systems. Mass balance analyses were performed to further explore the fate of TCS in continuous systems. According to Fig. 3b and c, in the second phase of R1 (0.5 mg/L TCS addition), the average removal efficiency of TCS was only 80%, with biodegradation accounting for 63.78% and adsorption for 16.22%, and the remainder all discharged. When 3 mg/L TCS was added to phase III, 86.88% of TCS average removal efficiency (80% of biodegradation and 6.88% of adsorption) was observed. Nevertheless, after adding 10 mg/L TCS into phase IV, the average TCS removal efficiency was only 63.33%, including 57.35% of biodegradation and 5.98% of adsorption. Moreover, in the second phase of R2 (0.5 mg/L TCS addition), the average removal efficiency of TCS was 95.55%, with biodegradation accounting for 90% and adsorption for 5.55%, and the remainder all discharged. When 3 mg/L TCS were added to phase III in R2, 97.88% of TCS average removal efficiency (95.89% of biodegradation and 1.99% of adsorption) was observed. Nevertheless, after adding 10 mg/L TCS into phase IV, the average TCS removal efficiency in R2 was only 82.12%, including 80% of biodegradation and 2.12% of adsorption.
The results demonstrated that the main removal pathway of TCS was biodegradation in both reactors, while the proportion of biodegradation and adsorption was different. The degradation efficiency of TCS in R2 was significantly higher than that in R1, and the proportion of biodegradation in R2 was higher than that in adsorption. This might be the reason for the difference in the performance of the two systems, and the difference in the removal mechanism could be due to the difference in the abundance of microorganisms degrading TCS in the systems (Wang et al., 2020). The lowest removal efficiency occurred at phase III of R1 probably due to the toxic effect of the TCS addition on the activated sludge in the system and the high hydrophobicity of TCS negatively influencing the removal. EPS and cell structure are the main contributors to the process of pollutant adsorption by activated sludge. It has been shown that EPS plays a crucial role in the adsorption and removal of micropollutants and that the characteristics of the organic fraction (especially the protein/polysaccharide ratio, i.e. PN/PS) were the main influencing factors (Zhang et al., 2018). The hydrogen bonds and hydrophobic interaction was regarded as the dominant factor of the TCS binding to proteins in EPS. Meanwhile, the interaction between TCS and amides promotes the electrostatic interaction between the -COO- (C = O) and the -NH3+(N–H), which further enhanced the adsorption of TCS by activated sludge. Hydrophobic interactions were the main mechanism of adsorption between log Kow larger compounds and hydrophobic adsorbents. Polysaccharides were considered to be good natural adsorbents and the hydroxyl and carboxyl groups could act as ligands and reaction sites for organic substances. Meanwhile, polysaccharides were able to bind to organic pollutants through electron donor–acceptor interactions and bridging interactions. What’s more, the presence of hydrogen donors (OH groups) in sludge and hydrogen acceptors (Cl−, -NH or -COO-) in the analytical structure of organic matter could facilitate the formation of hydrogen bonds, thereby promoting activated sludge adsorption.
3.2 The Effect of TCS on EPS in Activated Sludge
Most microorganisms activate their self-protection mechanism by secreting EPS when exposed to toxic substances (Wang et al., 2018). To reveal the defense mechanism of the activated sludge system against TCS, EPS content was monitored. TB-EPS and LB-EPS generated by R1 and R2 at different TCS concentrations were depicted in Fig. 4. The TB-EPS content of the seed was 59.88 mg/g VSS, including 31.24 mg/g VSS of PN and 5.89 mg/g VSS of PS. When 0.5 mg/L TCS was present, TB-EPS concentration in R1 (R1-1) was 79.13 mg/g VSS (66.25 mg/g VSS of PN and 18.35 mg/g VSS of PS), and that in R2 (R2-1) was 102.42 mg/g VSS (85.35 mg/g VSS of PN and 25.48 mg/g VSS of PS). With the increasing amount of TCS, EPS content in the two systems also increased, but under the condition of the presence of the same TCS concentration, EPS concentration in R2 was higher than that in R1. In R1 phase III and IV, TB-EPS increased to 101.88 mg/g VSS (R1-2) and 122.66 mg/g VSS (R1-3), respectively. Similarly, TB-EPS increased to 125.52 mg/g VSS (R2-2) and 148.86 mg/g VSS (R2-3) in phases III and IV of R2, respectively. As shown in Fig. 4b, the LB-EPS concentration in R1 was increased from 5.21 mg/g VSS (seed) to 8.32 mg/g VSS (R1-1), 13.48 mg/g VSS (R1-2), and 9.38 mg/g VSS (R1-3), respectively. Obviously, the LB-EPS content in R2 was significantly higher than that in R1. The LB-EPS content of R2-1(19.88 mg/g VSS) was 2.39 times that of R1-1, that in R2-2 and R2-3 were 14.98 mg/g VSS and 17.58 mg/g VSS. Different from TB-EPS, the content of LB-EPS first increased and then decreased with the increase in TCS concentration.
EPS contains a variety of organic functional groups, so activated sludge can provide the electrostatic force required for biological adsorption in the face of various pollutants, effectively improving the removal efficiency. With the continuous operation of the systems, EPS contents were increasing, and the increase was mainly PN. When the concentration of TCS increased, the contents of LB-EPS and TB-EPS initially decreased to varying degrees and then increased rapidly. This was because TCS inhibited the metabolic activity of sludge microorganisms, thus reducing the secretion of EPS. These results indicated that in the face of TCS stress, microorganisms secrete a large amount of PN to resist the toxicity of TCS and form a protective barrier, and extracellular proteins can participate in the biodegradation process of TCS as enzymes. Moreover, the non-growth-related energy generated when the microorganisms degraded TCS was provided to the microorganisms producing EPS, which promoted PN increase. During the entire period of system operation, the rise of TB-EPS was more significant than that of LB-EPS. In the final stage of the reaction, TB-EPS reached 148.86 mg/g VSS in R2, among which, PN increased nearly 4 times compared to the initial, while LB-EPS was only 17.58 mg/g. It showed that the contribution of PN to the particle stability in TB-EPS was greater than that in LB-EPS, indicating that the higher the TB-EPS was, the more advantages it has in combating the toxicity of TCS. Compared with the changes of PN/PS in the two groups of reactors (Table 1), the PN/PS of LB-EPS fluctuated between 1 and 6 without obvious rule, while the PN/PS of TB-EPS gradually increased with the increase of activated sludge particle size, up to 9. Higher PN/PS indicated higher hydrophobicity and lower surface charge on the sludge surface, which contributed to increased tolerance to TCS.
3.3 Variations of Functional Genes and Related Denitrifying Bacteria in PEF-SBRs Systems
The abundance of total bacteria, AOA (amoA gene), AOB (amoB gene), and NOB involved in microbial reactions in the system were shown in Fig. 5. The abundance of bacteria in seed sludge was 5.12 × 1012 copies/g SS. For R1, the abundance of bacteria first dropped to 5.80 × 1010 copies/g SS in phase II and then gradually increased to 4.03 × 1012 copies/g SS (phase III) and 4.80 × 1012 copies/g SS (phase IV). Meanwhile, the bacterial abundance in R2 had the same trend, but the overall abundance was higher than that in R1. The bacteria abundance was 6.30 × 1011 copies/g SS (phase II), 4.50 × 1012 copies/g SS (phase III), and 5.26 × 1012 copies/g SS (phase IV), respectively. The abundance in phase IV in R2 was a little higher than that of the seed. The results were in line with the finding reported by Dai et al., 2022. In addition, in Fig. 5b, in both R1 and R2, the abundance of AOA remained low after an extremely rapid decline from the abundance of seed sludge (7.68 × 107 copies/g SS) in the second stage. The results demonstrated that the addition of TCS had a strong inhibitory effect on AOA, and the inhibition could not be recovered with the increase of TCS content. For AOB, the abundance in R1 showed a gradual upward trend from 1.87 × 108 copies/g SS (seed) to 3.76 × 109 copies/g SS (phase II), 4.97 × 109 copies/g SS (phase III), and 5.04 × 109 copies/g SS (phase IV), respectively. In R2, the abundance of AOB recovered to 3.96 × 108 copies/g SS in phase III after dropping sharply to 7.87 × 106 copies/g SS in phase II and continued to increase to 4.25 × 109 copies/g SS (phase IV). The data indicated that compared to R1, AOB was inhibited with the addition of 0.5 mg/L TCS in the R2 system (glucose as substrate), but AOB abundance was gradually recovered when TCS addition was gradually increased, indicating that the AOB gene was able to be acclimated. As shown in Fig. 5d, when 0.5 mg/L TCS addition, the abundance of NOB of R1 and R2 dropped from 1.96 × 107 copies/g SS (seed) to 6.88 × 106 copies/g SS and 9.35 × 106 copies/g SS. The abundance of NOB increased gradually with increasing TCS concentration, with average abundances of 2.17 × 108 copies/g SS (R1) and 2.85 × 108 copies/g SS (R2) at the latter two phases. In brief, the addition of TCS had a transient inhibitory effect on AOB and NOB, which was weakened with increasing TCS concentration, but the inhibitory effect on AOA persisted.
3.4 Microbial Community Structure Analysis
The diversity index of samples Alpha were shown in Table 2. The larger values of the alpha diversity index, the higher the species abundance (Shi et al., 2020). The OTUs number, Chao1 index, and Shannon index showed a declining trend in R1, indicating that the first addition of triclosan had an obvious inhibitory effect on microorganisms, leading to a decrease in microbial diversity. In R1, the number of OTUs dropped from 1576 to 388, and the Chao1 index values decreased from 1816.33 to 466.43, the value of the Shannon index reduced from 6.88 to 3.15. On the contrary, the number of OTUs, Chao1, and Shannon value showed a trend of decreasing first and then increasing in R2, which was different from that of R1. The number of OTUs dropped from 1576 to 435 at the end of phase II, and then increased to 828 at the end of phase IV. Similarly, the values of Chao1 and Shannon of R2 reduced from 1816.33 and 6.88 to 431.78 and 3.28 after 0.5 mg/L TCS addition, and then rose to 816.85 and 5.21 at the end of the whole run, respectively. It could be inferred that the augment of TCS could influence the biodiversity of the R2 system. In this process, the degradation efficiency of TCS decreased first and then increased, demonstrating that some triclosan degraders were enriched.
The changes in microbial community structure at the phylum level and genus level in the two systems were shown in Fig. 6a. At the phylum level, Proteobacteria was the dominant class in both R1 and R2 and the relative abundance was all above 80%. Proteobacteria could remove nitrogen and phosphorus and were common dominant bacteria in activated sludge systems (Sun et al., 2021). When 0.5 mg/L TCS was added, the relative abundance of Proteobacteria in the two systems decreased to 63.68% and 66.88%. In phase III, the relative abundance of Proteobacteria with nitrification function in the two systems recovered to more than 95%. The final dominance of the microbial community in R1 and R2, with relative abundances of 98.12% and 99.25%, respectively, indicated that Proteobacteria in the PEF-SBR were more resistant to TCS than others.
At the level of genus classification, Haliangium, Defluviicoccus, and Dermatophilus were mainly observed in seed samples in R1 systems Fig. 6b. The relative abundance of these bacteria genera decreased gradually with the operation of the system, indicating that the physiological tolerance of bacteria had a significant impact on their response to adverse environmental changes (Xu et al., 2020), so the non – tolerant bacteria were eventually removed from R1. Thauera was the dominant genus in the R1 system after 0.5 mg/L TCS addition (phase II), and its relative abundance was 74.88%. Thauera was a microorganism with EPS secretion and nitrogen removal function. Acidovorax (3.22%), Ottowia (5.98%), and Sphingopyxis (3.38%) appeared in phase II, which may be potential TCS degraders similar to Thauera. Sodium acetate as a carbon source promoted the growth of Acidovorax, which was capable of cracking TCS to release nitrogen. When the TCS concentration increased to 5 mg/L, the relative abundance of Thauera, Ottowia, Acidovorax, and Sphingopyxis increased to 79.86%, 7.31%, 4.11%, and 3.88%, respectively. This was precisely in line with the phenomenon of higher pollutant degradation capacity at this stage. In phase IV (10 mg/L TCS loaded), the relative abundance of Thauera was dropped to 53.89%, indicating that TCS inhibited the functional colonies in the system. However, this bacterium was an important denitrification functional bacterium, which also explained that the NH4+-N removal efficiency of the system decreased with the increase of TCS concentration and led to the accumulation of nitrate nitrogen.
As for R2, Hydrogenophaga, Dechloromonas, and Brevundimonas showed a declining trend, while Pseudomonas, Paracoccus, Zoogloea, and Flavobacterium increased steadily. Initially, Pseudomonas was predominant, the relative abundance was 72.86% at the end of phase I, and Pseudomonas played a vital role in denitrification. After adding 0.5 mg/L TCS (phase II), Pseudomonas remained in high abundance (78.66%), and Paracoccus (3.13%), Zoogloea (5.66%), and Flavobacterium (5.89%) appeared. Due to the addition of TCS, Paracoccus, Zoogloea, and Flavobacterium were stimulated to secrete more EPS for self-protection. Paracoccus was closely related to the digestion and absorption of carbon and nitrogen. Zoogloea was resistant to adverse external conditions and enhanced microbial cohesion. In phase III, several dominant bacteria genera in phase II still maintained the leading position, and their relative abundance increased to varying degrees. However, the proportion of dominant bacteria decreased in phase IV, which may be due to the toxic effect of TCS addition on bacteria, which reduced the activity of bacteria. Overall, the results implied that TCS could influence microbial communities in nitrification systems. Likewise, it was the presence and relative abundance of dominant bacteria with TCS degradation capabilities in the activated sludge system that allows for a high degradation efficiency of TCS.
4 Conclusion
This work aimed to investigate the behavior of TCS in activated sludge systems and to study the role of biodegradation and adsorption. The main mechanism for TCS removal was found to be biodegradation. PEF-SBRs system performance was inhibited by TCS initial addition, but with increased TCS injection inhibition could be eliminated. The R2 reaction system with glucose as a carbon source showed stronger resistance to inhibition than the R1 reaction system with sodium acetate as a carbon source. TCS had a relatively weak effect on COD. The results of the EPS showed that the TB-EPS content was higher than LB-EPS and the PN content was always higher than PS. The microbial community analysis showed that Thauera was dominant in R1 and Pseudomonas was the predominant genus in the R2 system. The addition of TCS had a short-term inhibitory effect on AOB and NOB, but with increasing TCS concentrations, AOB and NOB abundances gradually recovered or even became more abundant than that in the seed samples. However, the inhibitory effect of TCS on AOA was irreversible.
Data Availability
Data will be made available on request.
References
American Public Health Association (APHA) (2005) Standard Methods for the Examination of Water and Wastewater, 21st ed. American Water Works Association (AWWA) & Water Environment Federation (WEF), Washington D.C.
Armstrong, D. L., Lozano, N., Rice, C. P., Ramirez, M., & Torrents, A. (2018). Degradation of triclosan and triclocarban and formation of transformation products in activated sludge using benchtop bioreactors. Environmental Research, 161, 17–25. https://doi.org/10.1016/j.envres.2017.10.048
Benotti, M. J., Trenholm, R. A., Vanderford, B. J., Holady, J. C., Stanford, B. D., & Snyder, S. A. (2009). Pharmaceuticals and endocrine disrupting compounds in. U.S. drinking water. Environmental Science and Technology, 43, 597–603. https://doi.org/10.1021/es801845a
Cantwell, M. G., Wilson, B. A., Jun, Z., Wallace, G. T., King, J. W., Olsen, C. R., Burgess, R. M., & Smith, J. P. (2010). Temporal trends of triclosan contamination in. dated sediment cores from four urbanized estuaries: Evidence of preservation and accumulation. Chemosphere, 78, 347–352. https://doi.org/10.1016/j.chemosphere.2009.11.021
Chen, X., Nielsen, J. L., Furgal, K., Liu, Y., Lolas, I. B., & Bester, K. (2011). Biodegradation of triclosan and formation of methyl-triclosan in activated sludge under aerobic conditions. Chemosphere, 84(4), 452–456. https://doi.org/10.1016/j.chemosphere.2011.03.042
Chen, Z. F., Ying, G. G., Liu, Y. S., Zhang, Q. Q., Zhao, J. L., Liu, S. S., Chen, J., Peng, F. J., Lai, H. J., & Pan, C. G. (2014). Triclosan as a surrogate for household biocides: An investigation into biocides in aquatic environments of a highly urbanized region. Water Research, 58, 269–279. https://doi.org/10.1016/j.watres.2014.03.072
Dai, H. H., Gao, J. F., Li, D. C., Wang, Z. Q., Zhao, Y. F., & Cui, Y. C. (2022). Polyvinyl chloride microplastics changed risks of antibiotic resistance genes propagation by enhancing the removal of triclosan in partial denitrification systems with different carbon source. Chemical Engineering Journal, 429, 132465. https://doi.org/10.1016/j.cej.2021.132465
Federle, T. W., Kaiser, S. K., & Nuck, B. A. (2002). Fate and effects of triclosan in activated sludge. Environmental Toxicology and Chemistry, 21, 1330–1337. https://doi.org/10.1002/etc.5620210702
Guerra, P., Teslic, S., Shah, A., Albert, A., Gewurtz, S. B., & Smyth, S. A. (2019). Occurrence and removal of triclosan in Canadian wastewater systems. Environmental Science and Pollution Research International, 26(31), 31873–31886. https://doi.org/10.1007/s11356-019-06338-w
Hao, Z., Wu, Q., Li, Z., Li, Y., Li, Q., Lai, X., Liu, H., Zhang, M., Yang, T., Chen, J., Tang, Y., Miao, J., Xu, H., Li, T., & Hu, R. (2019). Maternal exposure to triclosan constitutes a yet unrecognized risk factor for autism spectrum disorders. Cell Research, 29(10), 866–869. https://doi.org/10.1038/s41422-019-0220-1
Hashem, M. A., Payel, S., Hasan, M., Momen, A., & Sahen, S. M. (2021). Green preservation of goatskin to deplete chloride from tannery wastewater. HighTech and Innovation Journal, 2(2), 99–107. https://doi.org/10.28991/HIJ-2021-02-02-03
Holzem, R. M., Gardner, C. M., Stapleton, H. M., & Gunsch, C. K. (2018). Using laboratory generated biosolids to evaluate the microbial ecotoxicity of triclosan in a simulated land application scenario. Environmental Science and Pollution Research, 25, 11084–11099. https://doi.org/10.1007/s11356-017-1147-z
Hyungkeun, R., Nethra, S., Fuman, Z., Chang-Ping, Y., Justin, S., & Kung-Hui, C. (2009). Biodegradation potential of wastewater micropollutants by ammonia-oxidizing bacteria. Chemosphere, 77, 1084–1089. https://doi.org/10.1016/j.chemosphere.2009.08.049
Ismail, W. N. W., Syah, M. I. A. I., Muhet, N. H. A., Bakar, N. H. A., Yusop, H. M., & Samah, N. A. (2022). Adsorption behavior of heavy metal ions by hybrid inulin-teos for water treatment. Civil Engineering Journal, 8(9), 1787–1798. https://doi.org/10.28991/CEJ-2022-08-09-03
Jia, J. X., Gao, J. F., Dai, H. H., Zhang, W. Z., Zhang, D., & Wang, Z. Q. (2020). DNA-based stable isotope probing identifies triclosan degraders in nitrification systems under different surfactants. Bioresource Technology, 302, 122815. https://doi.org/10.1016/j.biortech.2020.122815
Kookana, R. S., Ying, G.-G., & Waller, N. J. (2011). Triclosan: Its occurrence, fate and. effects in the Australian environment. Water Science and Technology, 63, 598–604. https://doi.org/10.2166/wst.2011.205
Lee, J. S., & Chang, I. S. (2014). Membrane fouling control and sludge solubilization using high voltage impulse (HVI) electric fields. Process Biochemistry, 49(5), 858–862. https://doi.org/10.1016/j.procbio.2014.03.001
Lee, D. G., Cho, K. C., & Chu, K. H. (2015). Removal of triclosan in nitrifying activated sludge: Effects of ammonia amendment and bioaugmentation. Chemosphere, 125, 9–15. https://doi.org/10.1016/j.chemosphere.2014.12.085
Li, X. Y., & Yang, S. F. (2007). Influences of loosely bound extracellular polymeric substance (EPS) on the flocculation, sedimentation and dewaterability of activated sludge. Water Research, 41(5), 1022–1030. https://doi.org/10.1016/j.watres.2006.06.037
Liu, A., Ahn, I. S., & Mansfield, C. (2001). Phenanthrene desorption from soil in the presence of bacterial extracellular polymer: Observations and model predictions of dynamic behavior. Water Research, 35(3), 835–843. https://doi.org/10.1016/S0043-1354(00)00324-9
Miao, L., Zhang, Q., Wang, S., Li, B., Wang, Z., Zhang, S., Zhang, M., & Peng, Y. (2018). Characterization of EPS compositions and microbial community in an Anammox SBBR system treating landfill leachate. Bioresource Technology, 249, 108–116. https://doi.org/10.1016/j.biortech.2017.09.151
Nandikes, G., Pathak, P., Razak, A. S., Narayanamurthy, V., & Singh, L. (2022). Occurrence, environmental risks and biological remediation mechanisms of triclosan in wastewaters: challenges and perspectives. Journal of Water Process Engineering, 49, 103078. https://doi.org/10.1016/j.jwpe.2022.103078
Qian, G. S., Hu, X. M., Li, L., et al. (2017). Effect of iron ions and electric field on nitrification process in the periodic reversal bioelectrocoagulation system. Bioresource Technology, 244, 382–390. https://doi.org/10.1016/j.biortech.2017.07.155
Qian, G. S., Ye, L. L., Li, L., et al. (2018). Influence of electric field and iron on the denitrification process from nitrogen-rich wastewater in a periodic reversal bio-electrocoagulation system. Bioresource Technology, 258, 177–186. https://doi.org/10.1016/j.biortech.2018.02.112
Raji, V. R., & Packialakshmi, S. (2022). Assessing the wastewater pollutants retaining for a soil aquifer treatment using batch column experiments. Civil Engineering Journal, 8(7), 1482–1491. https://doi.org/10.28991/CEJ-2022-08-07-011
Rittmann, B. E., Lee, H. S., Zhang, H. (2009). Full-scale application of focused-pulsed pre-treatment for improving biosolids digestion and conversion to methane. Biological data mining in protein interaction networks. Medical Information Science Reference. 1895–1901. https://doi.org/10.2166/wst.2008.547
Ruszkiewicz, J. A., Li, S., & Aschner, M. (2017). Is triclosan a neurotoxic agent? Journal of Toxicology and Environmental Health. Part B, Critical Reviews, 20, 104–117. https://doi.org/10.1080/10937404.2017.128118
Schweizer, H. P. (2001). Triclosan: A widely used biocide and its link to antibiotics. FEMS Microbiology Letters, 202, 1–7. https://doi.org/10.1111/j.1574-6968.2001.tb10772.x
Senthil, K. K., Mahalakshmi, P. S., Peck, A. M., & Sajwan, K. S. (2010). Mass loadings of triclosan and triclocarbon from four wastewater treatment plants to three rivers and landfill in Savannah, Georgia, USA. Archives of Environmental Contamination and Toxicology, 58, 275–285. https://doi.org/10.1007/s00244-009-9383-y
Sheng, G. P., Zhang, M. L., & Yu, H. Q. (2008). Characterization of adsorption properties of extracellular polymeric substances (EPS) extracted from sludge. Colloids & Surfaces B-Biointerfaces, 62(1), 83–90. https://doi.org/10.1016/j.colsurfb.2007.09.024
Shi, J., Xu, C., & Han, Y. (2020). Enhanced anaerobic degradation of nitrogen heterocyclic compounds with methanol, sodium citrate, chlorella, spirulina, and carboxymethylcellulose as co-metabolic substances. Journal of Hazardous Materials, 384, 121496. https://doi.org/10.1016/j.jhazmat.2019.121496
Sorensen, J. P. R., Lapworth, D. J., Nkhuwa, D. C. W., Stuart, M. E., Gooddy, D. C., Bell, R. A., Chirwa, M., Kabika, J., Liemisa, M., & Chibesa, M. (2015). Emerging contaminants in urban groundwater sources in Africa. Water Research, 72, 51–63. https://doi.org/10.1016/j.watres.2014.08.002
Stasinakis, A. S., Petalas, A. V., Mamais, D., Thomaidis, N. S., Gatidou, G., & Lekkas, T. D. (2007). Investigation of triclosan fate and toxicity in continuous-flow activated sludge systems. Chemosphere, 68, 375–381. https://doi.org/10.1016/j.chemosphere.2007.01.047
Sun, F. L., Xu, Z. T., & Fan, L. L. (2021). Response of heavy metal and antibiotic resistance genes and related microorganisms to different heavy metals in activated sludge. Journal of Environmental Management, 300, 113754. https://doi.org/10.1016/j.jenvman.2021.113754
van Wijnen, J., Ragas, A. M. J., & Kroeze, C. (2018). River export of triclosan from land to sea: A global modelling approach. Science of the Total Environment, 621, 1280–1288. https://doi.org/10.1016/j.scitotenv.2017.10.100
Wang, L., Li, Y., Wang, L., Zhu, M., Zhu, X., Qian, C., & Li, W. (2018). Responses of biofilm microorganisms from moving bed biofilm reactor to antibiotics exposure: Protective role of extracellular polymeric substances. Bioresource Technology, 254, 268–277. https://doi.org/10.1016/j.biortech.2018.01.063
Wang, Z. Q., Gao, J. F., Li, D. C., Dai, H. H., & Zhao, Y. F. (2020). Co-occurrence of microplastics and triclosan inhibited nitrification function and enriched antibiotic resistance genes in nitrifying sludge. Journal of Hazardous Materials, 399, 123049. https://doi.org/10.1016/j.jhazmat.2020.123049
Xu, J., Sheng, G. P., & Ma, Y. (2013). Roles of extracellular polymeric substances (EPS) in the migration and removal of sulfamethazine in activated sludge system. Water Research, 47(14), 5298–5306. https://doi.org/10.1016/j.watres.2013.06.009
Xu, W., Zhao, H., & Cao, H. (2020). New insights of enhanced anaerobic degradation of refractory pollutants in coking wastewater: role of zero-valent iron in metagenomic functions. Bioresource Technology, 300, 122667. https://doi.org/10.1016/j.biortech.2019.122667
Yan, Z., Meng, H., Yang, X., Zhu, Y., Li, X., Xu, J., & Sheng, G. (2019). Insights into the interactions between triclosan (TCS) and extracellular polymeric substance (EPS) of activated sludge. Journal of Environmental Management, 232, 219–225. https://doi.org/10.1016/j.jenvman.2018.11.059
Yavuz, M., Oggioni, M., Dilek, F. B., & Yetis, U. (2015). Biocides in drinking water. system of Ankara, Turkey. Desalination and Water Treatment, 53, 3253–3262. https://doi.org/10.1080/19443994.2014.933626
Zhang, H., Jia, Y., Khanal, S. K., Lu, H., Fang, H., & Zhao, Q. (2018). Understanding the role of extracellular polymeric substances on ciprofloxacin adsorption in aerobic sludge, anaerobic sludge, and sulfate-reducing bacteria sludge systems. Environmental Science and Technology, 52(11), 6476–6486. https://doi.org/10.1021/acs.est.8b00568
Zhang, C., Li, L., Wang, Y. J., et al. (2019). Enhancement of the ANAMMOX bacteria activity and granule stability through pulsed electric field at a lower temperature (16 ± 1°C). Bioresource Technology, 292, 121960. https://doi.org/10.1016/j.biortech.2019.121960
Zhao, J. L., Ying, G. G., Liu, Y. S., Chen, F., Yang, J. F., & Wang, L. (2010). Occurrence and risks of triclosan and triclocarban in the Pearl River system, South China: From source to the receiving environment. Journal of Hazardous Materials, 179, 215–222. https://doi.org/10.1016/j.jhazmat.2010.02.082
Zhao, F., Rezenom, Y. H., Russell, D. H., & Chu, K. H. (2012). Biodegradation of triclosan by a wastewater microorganism. Water Research, 46, 4226–4234. https://doi.org/10.1016/j.jenvman.2018.11.059
Acknowledgements
This research was supported by Fundamental Research Funds for Chinese Central Universities (No. N2101001).
Author information
Authors and Affiliations
Contributions
Xuejie Li: Writing- Original draft preparation, Methodology, and Software Analysis. Xiaomin Hu: Conceptualization, Supervision, Validation. Xin Zhao: Reviewing Editing and Supervision. Yan Zhao: Conceptualization, Editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. The rise of TCS inhibited denitrification, but had no major effect on COD removal.
2. TB-EPS played more crucial roles in the removal process compared to LB-EPS.
3. Thauera dominanted in R1, while Pseudomonas was the dominant genus in R2.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Hu, X., Zhao, X. et al. Investigation of Triclosan Fate and Performance in Continuous-Flow Activated Sludge Systems. Water Air Soil Pollut 234, 525 (2023). https://doi.org/10.1007/s11270-023-06552-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06552-w