Abstract
Microbial processes play a vital important role in the removal of contaminants in constructed wetland (CW). However, the microbial physiology and community structure can be influenced by environmental conditions. In this study, four pilot-scale integrated vertical-flow constructed wetlands (IVCWs) were employed to treat domestic and nitrified wastewaters. The microbial properties, along with their response to wastewater quality characteristics and seasonal variation, were determined. The results showed higher Shannon–Weiner diversity (H) and evenness (E) index of fatty acids (FAs), and relative abundances of signature FAs in down-flow cells and in the systems fed with domestic wastewater (DW). The relative abundances of fungi and gram-negative and aerobic bacteria were greater in up-flow cells. The dominant anaerobic bacteria found in most cells might be accounted for the prevailing anaerobic environment within the wetland beds, which could mean that the system fed with nitrified wastewater (NW) should perform better in nitrogen removal. The redundancy analysis (RDA) showed that pollutant concentrations, especially organic matter, influence the FA compositions greatly, and the most significant difference of microbial community structures was detected in down-flow cells fed with DW and up-flow ones with NW. The branched FAs, which could be used to represent anaerobic bacteria, were observed in down-flow cells treating DW and had a significant positive correlation with chemical oxygen demand (COD) concentration, probably suggesting the important role of anaerobic bacteria in organic matter degradation in the IVCWs. Seasonal variation, however, did not greatly influence the microbial community structure in the IVCWs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Owing to some well-recognized advantages, such as low cost for construction, operation, and maintenance; acceptable and sustainable performance; easy management; environmental friendliness, etc., constructed wetlands (CWs) have been applied more and more frequently for treatment of various wastewater sources (Kadlec and Knight 1996; Zhi and Ji 2012). The function of a CW tightly depends on interactions of substrates, plants, and associated microorganisms, which are primarily responsible for water quality improvement (Kadlec and Knight 1996; Faulwetter et al. 2009). Among them, microorganisms, with high variability due to a range of microbial habitats in CW, are generally believed to be the key determinant of pollutant transformation and degradation, and microbial community plays a fundamental role in regulating these processes (Kadlec and Knight 1996; Ahn et al. 2007). Therefore, explorations on microbial community and its influencing factors could reveal the “black boxes” of pollutant removal mechanisms in CW.
To date, the knowledge on microorganisms in CW is still largely derived from assumptions and indirect evidences (Faulwetter et al. 2009; Zhang et al. 2010). Furthermore, microbial community can be very sensitive to environmental variations, and its structure can shift in concert with the alterations of some environmental factors, such as pH, temperature, dissolved oxygen (DO), substrate, vegetation, availability of organic carbon, etc. (Arroyo et al. 2013; Zhao et al. 2010). Therefore, microbial community structure is a good indicator for the status of the CW ecosystem and may be employed to monitor and assess biotic processes (Pronk et al. 2009). Indeed, the microbial diversity and plasticity of a CW system can provide a buffer against fluctuations in environmental conditions and may be critical for its proper function and maintenance (Ibekwe et al. 2003). Thus, evaluating the microbial compositions and their alteration can provide an insight into the responses of a CW system to variations of operational and environmental conditions.
Lipids are the primary components of cellular membranes, which are composed of a number of compounds with structural and functional diversities, and their composition can be applied to characterize microbial community structure and assess the physiological conditions experienced by microorganisms (Alef and Nannipieri 1995; Zelles 1999a). As a sensitive and reproducible biochemical method, fatty acid (FA) analysis is usually employed to detect environmental status and stress in an ecosystem (Zelles 1999a). Alterations of microbial physiological parameters and community composition caused by environmental variation can be reflected in FA profiles, which is useful to quantify responses of microorganisms to environmental stress (Steer and Harris 2000; Zelles et al. 1994; Zelles 1999b). The FA method has been commonly applied to analyze microbial community composition in soils (Frostegård et al. 2011) and has been successfully used to assess the characteristics of microbial communities in CWs in recent years (Zhou et al. 2009; Weaver et al. 2012; Tao et al. 2012; Wu et al. 2013).
Domestic wastewater and nitrified wastewater (e.g., effluent from activated sludge treatment plant, agricultural runoff, etc.) are two typical wastewaters with different characteristics that are frequently treated using CWs in China (Wu et al. 2008). The microbial features in CWs dealing with these wastewaters may be distinct due to the influence of water quality properties; however, available information is very limited.
Accordingly, in this study, four pilot-scale integrated vertical-flow constructed wetlands (IVCWs) were established to treat domestic and nitrified wastewaters. The main purposes include (1) comparing temporal and spatial variations of microbial features and community structures in the IVCWs and (2) evaluating the effect of the wastewater quality properties on the microbial ecology in the IVCWs.
Materials and methods
Experimental systems and the influent
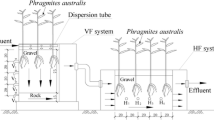
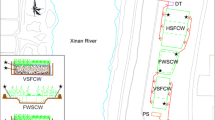
The study was carried out in four pilot-scale IVCWs located near Donghu Lake (30° 33′ N, 114° 23′ E), Wuhan, China. The structure diagram of the IVCW has been presented in Chang et al. (2012, 2013).
In April 2011, Arundo donax var. versicolor and Canna indica were transplanted into down-flow and up-flow cells, respectively, at a density of 9 stem m−2. After planting, domestic wastewater (DW) and nitrified wastewater (NW) were intermittently and evenly distributed into the IVCWs at a hydraulic loading rate of 12.5 cm day−1. The water quality parameters of these two wastewaters are summarized in Table 1.
Sample collection
The experiment was carried out from May 2011 to February 2012. Water samples were taken approximately weekly from inlet and outlet of each IVCW system, and the substrate sampling campaigns were conducted on four dates: August 22, 2011 (Aug.), October 25, 2011 (Oct.), December 22, 2011 (Dec.), and January 5, 2012 (Jan.). Five substrate subsamples were collected from the top 5–10 cm in each cell and placed in a sterile plastic bag after removing root and macrofauna by hand, then homogenized thoroughly by kneading and shaking the plastic bag (ca. 500×g). The samples were immediately transported to the laboratory and stored at −20 °C until analysis.
Sample analysis
Water quality analysis
Physico-chemical parameters of the wastewater including pH, dissolved oxygen (DO), temperature (T), and electric conductivity (EC) were measured in the field immediately after collection with an Orion 5-star portable multimeter (Thermo Fisher Scientific Company, USA). In the laboratory, total nitrogen (TN), nitrate (NO3 −), ammonium (NH4 +), nitrite (NO2 −), and total phosphorus (TP) were analyzed according to standard methods (APHA 1998). Chemical oxygen demand (COD) was determined using a spectrophotometer (DR/2010, Hach Co., Loveland, CO, USA).
Fatty acid analysis
FA analysis was conducted by ester-linked method described by (Schutter and Dick 2000). Briefly, 5 g of substrate (fresh weight) was incubated with 15 ml 0.2 M methanolic KOH at 37 °C in Teflon-capped centrifuge tubes for 1 h. During this period, the sample was swirled for 30 s every 10 min and ester-linked FAs were released and methylated. After incubation, 3 ml of 1 M acetic acid was added to neutralize the pH of the suspension, and 5 ml of hexane was then added to extract the fatty acid methyl esters (FAMEs) twice. The sample was centrifuged at 4,800×g for 10 min to separate the organic and aqueous phases every time, and the organic phases were carefully combined, transferred to a clean glass tube, and evaporated off under N2. The FAMEs were dissolved in 0.5 ml of 1:1 hexane:methyl-tert butyl ether for analysis. All instruments involved were washed with methanol and hexane three times, and all reagents used were chromatography grade. FAMEs were separated using an Agilent 6890 Series capillary gas chromatograph (Agilent Technologies), identified by MIDI peak identification software (MIDI Inc., Newark, DE, USA) according to their chromatographic mass spectral comparison and retention times, and quantified using a set of standard 26-component qualitative Bacterial Acid Methyl Ester (BAME) Mix (Supelco, USA) that ranged from C11 to C20. FAs with a chain length of more than 20 were not analyzed because they usually do not originate from microorganisms (Zelles et al. 1992).
For each sample, FAME extraction and analysis were carried out in triplicate. The nomenclature of fatty acid was described by Frostegård et al. (1993), and the abundance of individual FA is expressed to be its mole percentage (% mol) in all FAs in each sample.
Diversity and equitability of the FAs
To assess the diversity of FAs, the ubiquitous Shannon–Weiner index (H) was applied:
where n is the number of FAs detected and pi is the content percentage of each FA (%) calculated by dividing the peak height of one FA to the sum the ones detected in one sample.
The equitability of the FAs was evaluated using Pielou’s evenness index (E) as follows:
where H is the Shannon–Weiner diversity index and S is the total number of FAs identified in the sample.
Statistical analysis
The average values of water quality and microbial characteristics of the two replicate IVCW systems were used for calculation. Values of H and E and ratios of biomarker FAs of different samples were compared using one-way univariate analysis of variance (ANOVA) followed by least significant difference (LSD) test using SPSS 17.0 software package for Windows, and the difference was regarded to be significant when p ≤ 0.05.
In order to evaluate the distinction of microbial community structure in samples and identify the influence of wastewater quality characteristics, the linear model of ordination, redundancy analysis (RDA), was implemented. The analysis was performed by the software package Canoco for Windows with version 4.5. In the analysis, species were referred to the relative abundance of individual FA in samples and environmental variables involved quantitative (water qualities) and dummy (plant species) variables.
Results
Wastewater quality characteristics and pollutant removal efficiencies
The water quality characteristics and treatment performance of the IVCW systems on the four substrate sampling dates are shown in Table 1.
Effective removal for organic substance in DW was achieved even in January with water temperature being just around 5 °C, while the lower removal efficiencies obtained for NW might be due to low influent concentrations. The removal of nitrogen and phosphorus was more efficient for NW than that for DW except in January.
FAME compositions
Almost all the 26 kinds of FAs were detected in the substrate (Fig. 1).
As a common and ubiquitous FA, palmitic acid (16:0) showed the highest proportion, especially in up-flow cells. Besides, FAs 14:0, i15:0, a15:0, 16:1ω9, and 18:0 were also enriched. These dominant FA species accounted for 60.4–71.8 % of the total FAs in down-flow cells fed with DW, 74.3–81.5 % in down-flow cells fed with NW, 77.4–87.8 % in up-flow cells fed with DW, and 82.5–90.8 % in up-flow cells fed with NW, respectively. Relative abundances of FAs 16:0 and 18:0 in down-flow cells fed with DW were significantly lower than those in other cells.
The Shannon–Weiner diversity index (H) of FAs ranged from 1.44 to 2.51 (Fig. 2), with the highest value observed in the down-flow cells treating DW, while the lowest value in the up-flow cells treating NW. Concerning the wastewater type, higher H values for FAs were attained in the IVCWs fed with DW.
Values of 0.44 to 0.77 for evenness index (E) of FAs were obtained. Similarly, higher equitability was observed in the down-flow cells and systems dealing with DW. However, no consistent variations with operation time were observed for the values of H and E.
The FAs could be grouped according to their chemical structures, and the relative amounts of FA groups are depicted in Fig. 3.
As seen in Fig. 3, saturated FAs were present at the highest percentages in all samples, followed by branched and monounsaturated FAs. Just small amounts of cyclopropane, hydroxyl, and polysaturated FAs were identified, with the relative abundances of 0.5–4.0, 0.1–2.8, and 0.1–2.0 %, respectively.
The proportion of odd-numbered saturated FAs in down-flow cells fed with DW, constituting 17.0–22.5 % of the total FA content, was significantly higher than that in other cells. On the other hand, the even-numbered saturated FAs had the lowest abundance, which, on the contrary, were predominantly present in up-flow cells treating NW. The down-flow cells had a larger relative amount of branched and cyclopropane FAs than the up-flow ones. Noticeably, similar compositions of FA groups were observed for up-flow cells fed with DW and down-flow ones fed with NW.
Signature FAs
Signature FAs can be used to characterize and diagnose different groups of microorganisms, and their ratios can characterize the relative dominance of microbial groups and metabolic status of microorganisms (Findlay et al. 1990; Zelles 1999b). Among these FAs detected, 18:2ω9c can be used as fungal signature FA, and 12:0 2OH, 12:0 3OH, i15:0, a15:0, 15:0, 14:0 3OH, i16:0, 16:1ω9, i17:0, cy17:0, 17:0, 18:1ω9c, 18:1ω9t, and cy19:0 are indicative of bacteria according to previous references (Tunlid et al. 1989; Frostegård et al. 1993). Regarding diagnostic FAs of bacteria, branched FAs i15:0, a15:0, i16:0, and i17:0 are employed as general indicators of Gram-positive (G+) bacteria (Rajendran et al. 1997), while 12:0 2OH, 12:0 3OH, 14:0 3OH, 16:1ω9, cy17:0, 18:1ω9c, 18:1ω9t, and cy19:0 are primarily associated with Gram-negative (G−) bacteria (Wollenweber and Rietschel 1990; Zelles 1999b). In addition, monounsaturated fatty acids (MUFAs) are typically contained in aerobic bacteria, whereas branched FAs are primarily contained in anaerobic bacteria; therefore, the ratio of MUFAs/branched FAs can be indicative of the relative amounts of aerobic to anaerobic bacteria (Rajendran et al. 1992; Rajendran et al. 1997). The environmental stress experienced by microorganisms was commonly indicated by the ratio of trans to cis FAs (18:1ω9t/18:1ω9c; Guckert et al. 1986) due to the isomerization of cis to trans FAs was one of the adaptive mechanisms to increase microbial membrane fluidity induced by environmental stress (Heipieper et al. 1996); thus, a higher ratio indicated stronger stress.
The ratios of signature FAs of microorganisms in the IVCWs are depicted in Fig. 4.
As shown in Fig. 4, the ratios of fungi/bacteria ranged between 0.001 and 0.078, and the values obtained in up-flow cells were significantly higher than those in down-flow ones. Regarding the distinction derived from wastewater type, higher relative abundance of fungi was mostly found in units fed with NW. The ratios in all cells varied with operation time similarly, such as declined in Dec. and increased in Jan., demonstrating the impact of seasonal variation.
In down-flow cells, the relative abundances of G+ biomarker FAs were all higher than those of G−, indicating a predominance of G+ bacteria (Fig. 4b), while the G− bacteria prevailed over G+ bacteria in up-flow cells in most cases. In addition, the ratios of G+ to G− bacterial FAs almost decreased markedly with the operation time, suggesting a shift in composition to less G+-dominated or G−-dominated bacterial community with the decreasing temperature.
Most of the ratios of MUFAs to branched FAs were found to be less than 1.0, which demonstrating a larger proportion of anaerobic bacteria in the IVCWs, especially in down-flow cells. Higher values observed in down-flow cells compared to up-flow ones probably indicated more anaerobic bacteria-dominated microbial communities. However, no consistent and significant differences were observed between the ratios of the IVCWs fed with different wastewaters. Moreover, these ratios almost showed an ascending trend with the operation time.
The ratios of 18:1ω9t to 18:1ω9c ranged from 0.58 to 3.22, with the lowest values mostly found in up-flow cells treating NW.
Microbial community structure in the IVCWs
Different FA compositions with biological specificity were derived from various biochemical metabolisms of different microbial groups; thus, microbial community structure can be characterized by the compositions of signature FAs (Zelles 1999a; Green and Scow 2000).
Four functional groups were classified by Findlay et al. (1990) according to the presence of biomarker FAs in microorganisms. Polyunsaturated FAs were biomarkers for microeukaryotes (group І), while monounsaturated FAs were indicative of aerobic prokaryotes (group II). Additionally, branched FAs from C14–C16 represented Gram-positive and anaerobic prokaryotes (group III), whereas branched and cyclopropane FAs from C17–C19 were associated with sulfate-reducing bacteria (SRB) and other anaerobic prokaryotes (group IV). Their compositions in the IVCWs at different sampling times are shown in Fig. 5.
In down-flow cells, group III made up the highest proportion, ranging from 25.0 to 35.2 % of the total FAs for IVCWs treating DW and 16.2–35.0 % for the ones treating NW, respectively, then followed by group II, which contributed percentages of 9.0–18.3 and 9.2–19.1 % to the total FAs. Nevertheless, in some cases, the relative abundances of group II were higher than those of group III in up-flow cells. Relative low contents of group IV were detected, with the range of 1.1–5.2 %. Very little magnitudes of group І were obtained, especially in down-flow cells. Noticeably, the proportions of the FAs as biomarkers for microbial functional groups in the total FAs were the highest in the down-flow cells fed with DW, which were greater than 46.0 %, while they were nearly the lowest in the up-flow ones fed with NW, with the values of less than 22.0 %.
Effects of wastewater quality characteristics on microbial community structure
The results of redundancy analysis (RDA) of FAME data are shown in Fig. 6 and Table 2.
As seen from Fig. 6, the first axis of the RDA explained 81.4 % of the variance in FA species and the second explained 9.3 %. The microbial community structures in different cells varied with the wastewater quality properties, and the samples collected at different operation times were roughly separated into four groups according to the location. The correlations between species and environment parameters were significant (Table 2). The microbial communities in samples were effectively distinguished by the first axis, with the most significant difference detected between those in down-flow cells dealing with DW and up-flow ones with NW. Pollutant concentrations in wastewater, especially COD, showed remarkable influences on microbial community. The COD concentration was more closely associated with branched FAs, which more characterized the samples from down-flow cells fed with DW. The samples of up-flow cells fed with NW were confined to the left quadrant and were characterized by only polysaturated and some hydroxyl and saturated FAs, which correlated with concentrations of NO3 −, NO2 −, and DO. In addition, DO concentration was also a crucial determinant for microbial community structure, and limited effect of plant species and slight effect of water temperature were found.
Discussions
The variation of major microbial groups and metabolic status of the microorganisms could be partly reflected by the ratios of biomarker FAs (Findlay et al. 1990; Amir et al. 2008). In this study, a remarkably better TN removal performance was achieved for NW except in January despite the low influent COD/N, while an unsatisfactory NH4 + removal for DW suggested that denitrification was relatively liable to proceed in the IVCWs. This could have resulted from the prevailing anaerobic environment within the wetland beds indicated by the larger proportion of anaerobic bacteria and caused primarily by the intermittent feeding mode and continuous saturated condition. DO concentration could significantly influence microbial community structure (Fig. 6), and lower ratios of aerobic/anaerobic bacteria in down-flow cell (Fig. 4) might suggest a more prevailing anaerobic condition. The ratios of MUFAs/branched FAs obtained in this trial were largely lower than those observed in a membrane bioreactor (Chang et al. 2011) and small-scale vertical-flow CWs established with PVC columns (Li et al. 2010) in which aerobic bacteria were dominantly present, but comparable to those recorded in other IVCW systems treating eutrophic lake water (Zhou et al. 2009) or simulated domestic wastewater (Wu et al. 2013), and a little lower than those reported by Tao et al. (2012) treating domestic sewage employing IVCW with intermittent aeration addition. The elevated relative abundance of aerobic bacteria in January might have partly resulted from the increased DO level (Table 1), which was beneficial to nitrification process but unfavorable to nitrate removal, and could partly explain the less drastic decrease of TN removal efficiency for DW as compared to NW at a low temperature (Table 1).
Very low relative amounts of fungi were observed in the IVCWs, especially in down-flow cells, probably due to the higher pollution level and prevailing anaerobic condition within the IVCW systems which were unfavorable to the growth of fungi (Rajendran et al. 1992; Rajendran et al. 1997). Moreover, almost all organic compounds in the synthetic wastewater were easily decomposable, facilitating the growth of bacteria but not fungi (Küsel et al. 1999). Nevertheless, the greatly low proportion of fungi compared with bacteria in these systems agreed with those references obtained in other IVCWs (Zhou et al. 2009; Tao et al. 2012).
The relative abundance of G+ bacteria in down-flow cells was significantly greater than that in up-flow ones, which agrees with Kamaludeen et al. (2003) who stated that the G− signature FAs increased with wastewater purification process.
As for the ratio of trans FA to cis FA (18:1ω9t/18:1ω9c), much higher values than those determined in soils and aquifers (Green and Scow 2000) and in CWs treating municipal tertiary effluent (Jin and Kelley 2007) were obtained, demonstrating much greater physiological stress suffered by microorganisms in the IVCWs, which might have probably resulted from the considerably higher pollution level and prevailing anoxia within the wetland beds (Guckert et al. 1986; Rajendran et al. 1992; Rajendran et al. 1997). However, these ratios were similar to those observed in IVCWs treating domestic sewage (Tao et al. 2012) and a little lower than those in non-planted vertical flow CWs treating mixed wastewater of eutrophic lake water and septic tank sewage (Li et al. 2010).
The microbial community structure in the IVCWs was significantly influenced by the water quality characteristics. Higher diversity and evenness of FAs and proportions of FAs as biomarkers for microbial functional groups in down-flow cells in comparison to up-flow ones might be owing to the higher nutrient availability. Among the pollutants, COD concentrations showed the most notable effect on FA compositions (Fig. 6). The highest diversity and evenness of FAs obtained in down-flow cells of IVCW treating DW probably resulted from the markedly greater easily biological available organic substance contained in the wastewater. Actually, available carbon source has been proven to be able to affect microbial community structure greatly (Marschner et al. 2003; Wu et al. 2009).
However, it was notable that the COD concentration was more related to branched FAs which can be indicative of anaerobic bacteria and more characterized samples from down-flow cells treating DW (Fig. 6), probably suggesting the significance of anaerobic decomposition in COD removal for DW in the IVCWs. This was consistent with Wu et al. (2013) who also dealt with DW using IVCWs and could probably explain the efficient COD removal of the IVCW systems despite the low DO level (Table 1).
Conclusions
-
(1)
The Shannon–Weiner diversity and evenness index of FAs and the abundance of biomarker FAs in the IVCWs fed with DW and in down-flow cells were markedly higher than those in systems with NW and in up-flow cells.
-
(2)
The relative abundances of fungi and G− and aerobic bacteria were higher in up-flow cells than in down-flow ones.
-
(3)
Anaerobic bacteria were dominantly present in most cells, which could partly explain the better TN removal performance for NW.
-
(4)
Pollutant concentrations, especially COD, imposed crucial effects on the FA compositions.
References
Ahn C, Gillevet PM, Sikaroodi M (2007) Molecular characterization of microbial communities in treatment microcosm wetlands as influenced by macrophytes and phosphorus loading. Ecol Indic 7:852–863
Alef K, Nannipieri P (1995) Methods in applied soil microbiology and biochemistry. Academic, London
Amir S, Merlina G, Pinelli E, Winterton P, Revel JC, Hafidi M (2008) Microbial community dynamics during composting of sewage sludge and straw studied through phospholipids and neutral lipid analysis. J Hazard Mater 159:593–601
APHA (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Arroyo P, Ansola G, de Miera LES (2013) Effects of substrate, vegetation and flow on arsenic and zinc removal efficiency and microbial diversity in constructed wetlands. Ecol Eng 51:95–103
Chang J-J, Liang W, Xiao E-R, Wu Z-B (2011) Effect of intermittent aeration on the microbial community structure of activated sludge in a submerged membrane bioreactor. Water Environ J 25:214–218
Chang J, Wu S, Dai Y, Liang W, Wu Z (2012) Treatment performance of integrated vertical-flow constructed wetland plots for domestic wastewater. Ecol Eng 44:152–159
Chang J, Wu S, Dai Y, Wu ZB, Liang W (2013) Nitrate removal from tail water by integrated vertical-flow constructed wetlands at a high hydraulic loading rate. Desalin Water Treat 51:6031–6037
Faulwetter JL, Gagnon V, Sundberg C, Chazarenc F, Burr MD, Brisson J, Camper AK, Stein OR (2009) Microbial processes influencing performance of treatment wetlands: a review. Ecol Eng 35:987–1004
Findlay RH, Trexler MB, White DC (1990) Response of a benthic microbial community to biotic disturbance. Mar Ecol Prog Ser 62:135–148
Frostegård Å, Tunlid A, Bååth E (1993) Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl Environ Microbiol 59:3605–3617
Frostegård Å, Tunlid A, Bååth E (2011) Use and misuse of PLFA measurements in soils. Soil Biol Biochem 43:1621–1625
Green CT, Scow KM (2000) Analysis of phospholipid fatty acids (PLFA) to characterize microbial communities in aquifers. Hydrogeol J 8:126–141
Guckert JB, Hood MA, White DC (1986) Phospholipid ester-linked fatty-acid profile changes during nutrient deprivation of Vibrio cholerae—increases in the trans cis ratio and proportions of cyclopropyl fatty-acids. Appl Environ Microbiol 52:794–801
Heipieper HJ, Meulenbeld G, van Oirschot Q, de Bont J (1996) Effect of environmental factors on the trans/cis ratio of unsaturated fatty acids in Pseudomonas putida S12. Appl Environ Microbiol 62:2773–2777
Ibekwe AM, Grieve CM, Lyon SR (2003) Characterization of microbial communities and composition in constructed dairy wetland wastewater effluent. Appl Environ Microbiol 69:5060–5069
Jin G, Kelley TR (2007) Characterization of microbial communities in a pilot-scale constructed wetland using PLFA and PCR-DGGE analyses. J Environ Sci Health A 42:1639–1647
Kadlec RH, Knight RL (1996) Treatment wetlands. Lewis Publishers, Boca Raton, Florida
Kamaludeen SPB, Megharaj M, Naidu R, Singleton I, Juhasz AL, Hawke BG, Sethunathan N (2003) Microbial activity and phospholipid fatty acid pattern in long-term tannery waste-contaminated soil. Ecotoxicol Environ Saf 56:302–310
Küsel K, Wagner C, Drake HL (1999) Enumeration and metabolic product profiles of the anaerobic microflora in the mineral soil and litter of a beech forest. FEMS Microbiol Ecol 29:91–103
Li M, Zhou Q, Tao M, Wang Y, Jiang L, Wu Z (2010) Comparative study of microbial community structure in different filter media of constructed wetland. J Environ Sci 22:127–133
Marschner P, Kandeler E, Marschner B (2003) Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol Biochem 35:453–461
Pronk M, Goldscheider N, Zopfi J (2009) Microbial communities in karst groundwater and their potential use for biomonitoring. Hydrogeol J 17:37–48
Rajendran N, Matsuda O, Imamura N, Urushigawa Y (1992) Variation in microbial biomass and community structure in sediments of eutrophic bays as determined by phospholipid ester-linked fatty-acids. Appl Environ Microbiol 58:562–571
Rajendran N, Matsuda O, Rajendran R, Urushigawa Y (1997) Comparative description of microbial community structure in surface sediments of eutrophic bays. Mar Pollut Bull 34:26–33
Schutter ME, Dick RP (2000) Comparison of fatty acid methyl ester (FAME) methods for characterizing microbial communities. Soil Sci Soc Am J 64:1659–1668
Steer J, Harris JA (2000) Shifts in the microbial community in rhizosphere and non-rhizosphere soils during the growth of Agrostis stolonifera. Soil Biol Biochem 32:869–878
Tao M, He F, Xu H, Zhou Q, Xu D, Zhang L, Wu Z (2012) Changes in microbial community structure in constructed wetland under aeration condition. J Agro-Environ Sci 31:1195–1202 (In Chinese)
Tunlid A, Hoitink HAJ, Low C, White DC (1989) Characterization of bacteria that suppress rhizoctonia damping-off in bark compost media by analysis of fatty-acid biomarkers. Appl Environ Microbiol 55:1368–1374
Weaver MA, Zablotowicz RM, Krutz LJ, Bryson CT, Locke MA (2012) Microbial and vegetative changes associated with development of a constructed wetland. Ecol Indic 13:37–45
Wollenweber HW, Rietschel ET (1990) Analysis of lipopolysaccharide (lipid-a) fatty-acids. J Microbiol Methods 11:195–211
Wu ZB et al (2008) Integrated vertical-flow constructed wetland. Science Press, Beijing (In Chinese)
Wu YP, Ma B, Zhou L, Wang HZ, Xu JM, Kemmitt S, Brookes PC (2009) Changes in the soil microbial community structure with latitude in Eastern China, based on phospholipid fatty acid analysis. Appl Soil Ecol 43:234–240
Wu S, Chang J, Dai Y, Wu Z, Liang W (2013) Treatment performance and microorganism community structure of integrated vertical-flow constructed wetland plots for domestic wastewater. Environ Sci Pollut Res 20:3789–3798
Zelles L (1999a) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterization of microbial communities in soil: a review. Biol Fertil Soils 29:111–129
Zelles L (1999b) Identification of single cultured micro-organisms based on their whole-community fatty acid profiles, using an extended extraction procedure. Chemosphere 39:665–682
Zelles L, Bai QY, Beck T, Beese F (1992) Signature fatty acids in phospholipids and lipopolysaccharides as indicators of microbial biomass and community structure in agricultural soils. Soil Biol Biochem 24:317–323
Zelles L, Bai QY, Ma RX, Rackwitz R, Winter K, Beese F (1994) Microbial biomass, metabolic activity and nutritional status determined from fatty acid patterns and poly hydroxybutyrate in agriculturally managed soils. Soil Biol Biochem 26:439–446
Zhang L, Wang M-H, Hu J, Ho Y-S (2010) A review of published wetland research, 1991-2008: ecological engineering and ecosystem restoration. Ecol Eng 36:973–980
Zhao Y, Liu B, Zhang W, Hu C, An S (2010) Effects of plant and influent C: N: P ratio on microbial diversity in pilot-scale constructed wetlands. Ecol Eng 36:441–449
Zhi W, Ji G (2012) Constructed wetlands, 1991-2011: a review of research development, current trends, and future directions. Sci Total Environ 441:19–27
Zhou Q, He F, Zhang L, Wang Y, Wu Z (2009) Characteristics of the microbial communities in the integrated vertical-flow constructed wetlands. J Environ Sci 21:1261–1267
Acknowledgments
This work was supported by grants from the Major Science and Technology Program for Water Pollution Control and Treatment (2011ZX07303-001), Jiangsu Youth Fund (BK20130430, 51408593, BY2011193), and National Natural Science Foundation of China (51179184, 41272272).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Chang, Jj., Wu, Sq., Liang, K. et al. Comparative study of microbial community structure in integrated vertical-flow constructed wetlands for treatment of domestic and nitrified wastewaters. Environ Sci Pollut Res 22, 3518–3527 (2015). https://doi.org/10.1007/s11356-014-3594-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3594-0