Abstract
In order to investigate the treatment performance and microorganism mechanism of IVCW for domestic wastewater in central of China, two parallel pilot-scale IVCW systems were built to evaluate purification efficiencies, microbial community structure and enzyme activities. The results showed that mean removal efficiencies were 81.03 % for COD, 51.66 % for total nitrogen (TN), 42.50 % for NH4 +-N, and 68.01 % for TP. Significant positive correlations between nitrate reductase activities and TN and NH4 +-N removal efficiencies, along with a significant correlation between substrate enzyme activity and operation time, were observed. Redundancy analysis demonstrated gram-negative bacteria were mainly responsible for urease and phosphatase activities, and also played a major role in dehydrogenase and nitrate reductase activities. Meanwhile, anaerobic bacteria, gram-negative bacteria, and saturated FA groups, gram-positive bacteria exhibited good correlations with the removal of COD (p = 0.388), N (p = 0.236), and TP (p = 0.074), respectively. The IVCW system can be used to treat domestic wastewater effectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen and phosphorus pollution from domestic wastewater has become an urgent problem in the world, especially in developing countries (Li et al. 2009; Tsihrintzis and Gikas 2010; Mina et al. 2011). Constructed wetland, as an ecological engineering technology, has been proved to be able to remove nitrogen and phosphorus effectively (Vymazal 2002; Zhang et al. 2009; Tsihrintzis and Gikas 2010; Saeed and Sun 2011). In recent years, treatment performance of various types of constructed wetland for domestic wastewater has been studied intensively (van de Moortel et al. 2009; Kotti et al. 2010; Saeed and Sun 2011; Wang et al. 2011; Gikas et al. 2011; Stefanakis and Tsihrintzis 2012).
Zhang et al. (2009) have reported that the most limiting factors of Constructed wetlands application in China is the limited land resources. Integrated vertical-flow constructed wetland (IVCW), as a new, land saving, efficient wetland technology, has been successfully applied in China and Europe (Perfler et al. 1999; Liang et al. 2003; Zhou et al. 2009; Wu et al. 2011). Until now, most of this application has been focused on treatment of polluted water bodies, and researches on long-term treatment performance of IVCWs for domestic wastewater were rarely reported.
Numerous studies showed that wastewater purification in the constructed wetlands are mainly attributed to the metabolism of microbes and enzyme activities in substrate (Martens et al. 1992; Liang et al. 2003; Duarte et al. 2008; Weaver et al. 2012). In this study, two parallel pilot-scale IVCW systems were employed to investigate treatment performance, microbial community structure, and enzyme activities for domestic wastewater and the results will give references for future design, application, and operation management of the constructed wetland. Specific tasks are: (1) evaluate treatment performance at a hydraulic loading rate of 125 mm/day; (2) investigate yearly microbial community structure change; (3) identify the correlation among microbial community structure, enzyme activities, and pollutant removal efficiencies.
Materials and methods
Experimental setup
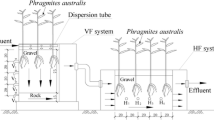
Two parallel pilot-scale IVCW systems were set up near Donghu Lake in Wuhan, China (30° 33′N, 114° 23′E; elevation, 27 m). Each with a down-flow chamber (1 m × 1 m × 1 m) in series with an up-flow chamber (1 m × 1 m × 1 m). The two chambers were separated by a wall but connected at the bottom, hence the water flow could be induced automatically from down-flow chamber to up-flow chamber and then out of the wetlands once a new inflow was added. Consequently, the chambers were saturated during the operation; the wastewater can make the most of limited land resources (Fig.1a). Gravel of 10–20 mm diameter was filled to a depth of 50 and 40 cm for the down-flow chamber and up-flow chamber, respectively, followed by a 35-cm-thick layer of 2–10 mm diameter gravel. The porosity of the substrate was estimated to be 0.40 and the effective volume of the wetland bed was 0.6 m3.
Based on our previous study, two species of macrophytes, Arundo donax and Canna indica, were planted at a density of 6 plants/m2 in the down-flow and up-flow chambers, respectively. The plants were fully grown during the experiments until the winter (Fig. 1 b, c, d).
Operation conditions
The influent was induced intermittently in every IVCW unit per day to yield a hydraulic loading rate of 125 mm/day, and the theoretical hydraulic retention time (HRT) was 2.4 days.
The study was carried out from 23rd April 2011 to 23rd February 2012, with an average ambient temperature of 22.03 °C. In order to minimize variability in the experiment, simulated domestic wastewater was used in the experiment. The influent characteristics are summarized in Table 1.
Samples collected
The influent and effluent water samples were collected from 23rd April 2011 to 23rd February 2012. The sampling frequency was scheduled for once every 2 days during the first 20 days, once every 4 days during the middle 30 days, and once a week during the last 250 days.
The horizontal substrate samples from five representative sites of the IVCW systems (as shown in Fig. 2) were collected and composited once every 2 months from 1st May 2011 to 1st January 2012 (Zhou et al. 2009; Zhang et al. 2011). All litter was removed and the samples were taken to the laboratory in sealed polypropylene bags and stored in a freezer at −20 °C until analysis of microbial community structure, or at 4 °C until analysis of enzyme activities.
Water quality analysis
pH, electrical conductivity (EC), dissolved oxygen (DO), and temperature were determined using Orion 5-star portable pH/conductivity/DO multimeter (Thermo Fisher Scientific Company, USA). Chemical oxygen demand (COD) (DRB 200, Hach, USA), total nitrogen (TN), ammonium nitrogen (NH4 +-N), nitrate nitrogen (NO3 −-N), nitrite nitrogen (NO2 −-N) and total phosphorus (TP) were measured according to the standard methods (SEPA, 2002).
Pollutant removal efficiency was calculated by the percentage of deduction in concentration for each pollutant as follows: removal efficiency = (1 − C eff/C inf) × 100 %, where C inf and C eff are influent and effluent concentrations in milligrams per liter.
Microbial community structure analysis
Fatty acid methyl esters (FAME) of substrate samples were analyzed using a mild alkaline methanolysis method (Schutter and Dick 2000). Fifteen milliliters of 0.2 M potassium hydroxide (KOH) in methanol was added into a 35-mL centrifuge tube containing 3 g of freeze-dried substrate. The contents of the tubes were mixed and incubated at 37 °C for an hour, during which ester-linked fatty acids were released and methylated. The tubes were vortexed every 10 min during the incubation period. Then, 3 mL of 1.0 M acetic acid was added to neutralize the pH of the tube contents. EL-FAMEs were partitioned into an organic phase by adding 10 mL hexane followed by centrifugation at 4,500 rpm for 15 min. After the hexane layer was transferred into a clean glass tube, the hexane was evaporated under N2 steam. In the final step, FAMEs were dissolved in three aliquots of 200 μL of hexane: methyl-tert butyl ether (1:1) and transferred to an amber vial for gas chromatography (GC) analysis. The MIDI peak identification software (MIDI, Inc., Newark, DE) was used to identify individual fatty acids. Fatty acid peaks were identified using 26-component bacterial acid methyl ester mix (Supelco, USA).
Fatty acids (FAs) are designated by the total number of carbon atoms, followed by a colon and the number of double bonds. Then a “ω” and a number show the position of the initial double bond from the methyl end of the chain, sometimes followed by a “c” or “t” for cis or trans configuration, respectively. The prefixes “i” and “a” refer to methyl branching at the iso- and anteiso- positions, respectively. Cyclopropane FAs have the prefix “cy” (Zelles 1999).
Different microbial groups, including Gram-positive and Gram-negative bacteria, characterized by typical fatty acids with different chain structures, can be used as biomarkers for these groups (Zelles 1999). In this study, fatty acids i15:0, a15:0, 15:0, i16:0, 16:1ω9, i17:0, 17:0, cy17:0, 18:1ω9c, 18:1ω9t, and cy19:0 were chosen to represent total bacteria (Pankhurst et al. 2001). Monounsaturated FAs (MUFAs), including 16:1ω9, 18:1ω9c and 18:1ω9t, were used as indicators of Gram-negative bacteria; branched FAs, including i15:0, a15:0, i16:0 and i17:0, were used to indicate Gram-positive; Cyclopropyl FAs, including cy17:0 and cy19:0 were used as indicators of Anaerobic bacteria (Zelles et al. 1992; Wilkinson 1988; D’Angelo et al. 2005).
Enzyme activity analysis
The dehydrogenase activity in the substrate was determined by Tabatabai (1994) method. The urease activity was analyzed according to Klose and Tabatabai (2000). The nitrate reductase activity was analyzed using KNO3 as a substrate (Abdelmagid and Tabatabai 1987). The phosphatase activity was analyzed by the Schinner and von Mersi (1990) method.
Statistical analysis
The experiments data were summarized and reported as mean values ± standard deviation (SD). Comparison of the averages was carried out by one-way ANOVA followed by the least significant difference (LSD) test, using the software of SPSS 16.0 (SPSS Inc., Chicago, IL, USA), significant differences was set at p < 0.05.
The effects of microbial communities and environmental parameters on enzyme activities and pollutants removal were tested using redundancy analysis (RDA). The RDA was performed using Canoco 4.5 software (Center for Biometry, Wageningen, the Netherlands) which was fully discussed in ter Braak (1994) and Leps and Smilauer (2003).
Results
Removal efficiencies for pollutants
The mean concentrations in the effluent and removal efficiency of pollutants during the experimental period are shown in Table 1 and Figs. 3 and 4.
COD
The average COD concentrations were reduced from 106.94 ± 17.98 mg/L to 19.74 ± 8.60 mg/L, with average COD removal efficiency of 81.03 ± 10.16 %. During the experimental period, the COD removal increased early, with the highest removal of 87.25 % occurred in September, and then decreased gradually.
N
In general, TN and NH4 +-N concentrations decreased from 11.54 ± 1.55 mg/L and 8.34 ± 1.70 mg/L to 5.53 ± 1.47 mg/L and 4.80 ± 1.03 mg/L, respectively. Accordingly, mean removal efficiencies for TN and NH4 +-N were 51.66 ± 13.96 % and 40.50 ± 16.83 %, respectively. As for NO3 −-N and NO2 −-N, the average effluent concentrations were 0.24 and 0.02 mg/L, with removal rates of 92.56 and 97.86 %, respectively.
TN removal was stable from April to September, and the average removal efficiency was 58.14 %, then decreased sharply (Fig. 2b), especially after November. Meanwhile, the NH4 +-N removal increased early, and then declined dramatically, with the maximum removal efficiency of 55.33 % occurred in September (Fig. 2c). At the end of the experiment, the poor efficiency of TN and NH4 +-N was observed probably due to lower temperature and withered plants.
TP
The average effluent TP concentration during the experimental period was 0.33 ± 0.18 mg/L, with a mean removal efficiency of 67.52 %. The TP removal was relatively stable from April to October, and then declined gradually from November.
Enzyme activities
The activities of dehydrogenase, urease, nitrate reductase and phosphatase in the down-flow chamber and up-flow chamber of the IVCWs are summarized in Fig. 5.
During the experimental period, dehydrogenase, urease, nitrate reductase and phosphatase activities showed a single-peak pattern (November). Except phosphatase, the average enzyme activities in the down-flow chamber were significantly higher than those in the up-flow chamber (p < 0.05).
Microbial community structure
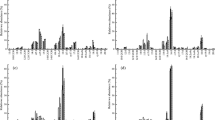
The microbial community structure during the experimental period is shown in Figs. 6 and 7.
The relative abundance of saturated FAs were dominated in the FAME profiles (45.34∼38.34 %) (Fig. 7), with the highest abundance occurred in May (p < 0.05) (Fig. 6), followed by branched FAs (Gram-positive bacteria) and monounsaturated FAs (Gram-negative bacteria), but no significant difference (p > 0.05) was observed between them. During the experimental period, branched FAs decreased from 24.78 ± 0.75 % to 19.66 ± 0.27 %, while monounsaturated FAs increased gradually from 21.67 ± 6.64 % to 27.13 ± 5.51 %. Abundance of cyclopropyl FAs (anaerobic bacteria) were the lowest one (4.13∼6.34 %), with the lowest abundance in May.
Discussion
Evaluation of treatment performance for pollutants
During the whole operation period, the average COD removal efficiencies were 81.03 ± 10.16 %. It was much higher than the previous studies reported by Fountoulakis et al. (2009), in which freewater surface constructed wetland, horizontal subsurface flow CW, and rotating biological contactor, packed bed filter were applied to treat domestic wastewater with mean inlet COD of 99.6 ± 49.4 mg/L under the same HLR of 125 mm/day; but similar to the result of Zhao et al. (2011), in which two-stage combinations of subsurface vertical down-flow and up-flow constructed wetland systems were applied; meanwhile, lower than the removal capacity for a mean influent COD of 510 mg/L (140 g COD/m2 day) by using ten pilot-scale vertical-flow constructed wetlands of different design and operation characteristics operated continuously for 3 years under Mediterranean climatic conditions (Stefanakis and Tsihrintzis 2012).
The removal efficiency of TN in this study was similar to the result of Wang et al. (2011) and Wu et al. (2011), but lower than Akratos and Tsihrintzis (2007), Kotti et al. (2010) and Gikas and Tsihrintzis (2012) for the similar size. The removal rates for NH4 +-N was similar to Chang et al. (2012), while lower than Kotti et al. (2010), Wu et al. (2011) and Xiong et al. (2011). This may be attributed to different HRT (Huang et al. 2000; Akratos and Tsihrintzis 2007; Kotti et al. 2010; Stefanakis and Tsihrintzis 2012).
The TN and NH4 +-N removal efficiencies were decreased from November, gradually decreased temperature was the main reason (Akratos and Tsihrintzis 2007; Tsihrintzis and Gikas 2010). As shown in Fig. 8, the N removal efficiency positively related to temperatures of the effluent, demonstrating temperatures had a significant impact on N removal. Kuschk et al. (2003) and Wu et al. (2011) have reported that nitrification was inhibited when the temperature was below 10 °C and decreased sharply below 6 °C, which was in agreement with our result.
The removal mechanisms of phosphorus from wastewater in constructed wetlands were mainly through substrate adsorption, iron exchange, and plant uptake (Billore et al. 1999). During the whole experiment, the TP removal efficiency remained relatively steady, and mean TP removal efficiencies (68.01 ± 17.45 %) was similar to the results of Chung et al. (2008), Wu et al. (2011) and Gikas and Tsihrintzis (2012), but lower than others (Xiong et al. 2011; Dong et al. 2011; Wang et al. 2011).
Relationships between substrate enzyme activities, pollutants removal, and operation time
The relationships among substrate enzyme activities, pollutants removal, and operation time are shown in Table 2.
Significantly positive correlation was found between the operation time and all the enzyme activities (p < 0.001), which can be attributed to the IVCW system operation condition. For the relationship between operation time and pollutant removal, only significant negative correlation were found with TP removal (p = 0.003, F = −0.545). Meanwhile, significant positive correlations between nitrate reductase activities and TN and NH4 +-N removal efficiencies were found. The result was not similar to Li et al. (2011). The negative relationship between phosphatase activity and TP removal was also found, which can be explained by the fact that TP removal was mainly absorbed by the substrate in the constructed wetland, and phosphatase activity had inverse relationship with substrate P concentration (Wright and Reddy 2001; Allison et al. 2007).
Relationships between microbial community structures and function
Enzyme activity has been regarded as an important indicator of microbial community function (Mentzer et al. 2006; Zhang et al. 2011), and microbial community structure has been demonstrated to be closely related to specific enzyme activities (Zhang et al. 2006).
In the present study, the correlations between microbial community structure and function were analyzed by RDA, a significant association (p = 0.002) was observed (Fig. 9).
The biplot of the first two RDA axes of enzyme activity and microbial communities. Enzyme activity parameters (expressed as “response variables”) were presented as black solid arrow, whereas microbial communities (expressed as explanatory variables) were presented as empty dashed arrow. The microbial communities were labeled with their p values to show the significance of their relationship to RDA axes. In the diagram, Gram + for Gram-positive bacteria abundance, Gram − for Gram-negative bacteria abundance, Anaerobic for anaerobic bacteria abundance; DHA for dehydrogenase, Ure for urease, Nitr for nitrate reductase, Pho for phosphatase
The most significant diagnostic FA groups associated with the enzyme data included Gram-positive bacteria (p = 0.002), anaerobic bacteria (p = 0.024), Gram-negative bacteria (p = 0.078) and Saturated FA groups (p = 0.096). The results showed that Gram-negative bacteria were mainly responsible for urease and phosphatase activities, also important contributors for dehydrogenase and nitrate reductase activities. This finding was consistent with Zhang et al. (2011). Meanwhile, urease and phosphatase activities were also greatly impacted by the abundance of anaerobic bacteria (Fig. 9).
There was significant negative correlation between Gram-positive bacteria and urease and phosphatase activities, while no significant correlation between enzyme activity and Gram-negative bacteria was found (Table 3). The results were similar to the findings reported by Mentzer et al. (2006).
Relationship among microbial community structure, environmental parameters, and pollutant removal
Previous studies have demonstrated that microorganisms play different roles in the removal or transformation of pollutants in the constructed wetlands (Ahn et al. 2007; Krasnits et al. 2009). Microbial community structure has been proposed to be an important determinant of water quality improvement in the wetland systems (Calheiros et al. 2009; Faulwetter et al. 2009), and temperature (Smith et al. 2010), hydrologic regime and pollutant treatments (Mentzer et al. 2006; Steenwerth et al. 2006), plant diversity and function group richness (Zhang et al. 2010, 2011) and biotic succession (Kent et al. 2007), could strongly influence the microbial community structure.
In the present study, the effects of microbial community structure (four diagnostic fatty acid (FA) groups) and environmental parameters on pollutant removal efficiencies were also determined by RDA (Fig. 10).
The biplot of the first two RDA axes of microbial communities, environmental parameters and pollutant removal efficiencies. The pollutant removal efficiencies (expressed as “response variables” in the RDA analysis) were presented as black solid arrow, whereas the specific diagnostic FA groups and environmental parameters (expressed as explanatory variables) were presented as empty dashed arrow. The explanatory variables were labeled with their p values to show the significance of their relationship to RDA axes. The following abbreviations were used in the diagram: Gram + for Gram-positive bacteria abundance, Gram − for Gram-negative bacteria abundance, Anaerobic for anaerobic bacteria abundance; Time for operation time, T for average temperature of influent and effluent
The most significant explanatory variables associated with removal efficiencies of pollutants included temperature (p = 0.016), operation time (p = 0.044), and Gram-positive bacteria (p = 0.074). The removal of TN and NH4 +-N were positively correlated with temperature, pH, abundance of saturated FA groups, and Gram-negative bacteria. TP removal were significantly positively correlated with the abundance of Gram-positive bacteria indicators (branched fatty acids) (Table 3), but negatively correlated with sampling time. Abundance of anaerobic bacteria was contributed to the removal of COD.
The results indicated that operation time and temperature were the key environment factor to influence removal of pollutants. Gram-positive bacteria were mainly responsible for TP removal, and anaerobic bacteria might be important contributors for COD removal, while the removal of N was primarily dependent on the abundance of saturated FA groups and Gram-negative bacteria.
Conclusions
-
(1)
Mean removal efficiencies of 81.03 % for COD, 51.66 % for TN, 42.50 % for NH4 +-N and 68.01 % for TP were achieved by the IVCWs to treat domestic wastewater at a loading rate of 125 mm/day under the subtropical monsoon climate, respectively. HRT and temperature was the main limited factors for nitrogen removal.
-
(2)
Nitrate reductase activities significant positive correlated with TN and NH4 +-N removal efficiencies, the nitrate reductase activities could be used to indicate the efficiency of nitrogen removal in IVCWs.
-
(3)
Anaerobic bacteria, saturated FA groups, and Gram-positive bacteria were mainly responsible for COD (p = 0.388), N (p = 0.236), and TP (p = 0.074) removal, respectively.
References
Abdelmagid HM, Tabatabai MA (1987) Nitrate reductase activity of soils. Soil Biol Biochem 19:421–427
Ahn C, Gillevet PM, Sikaroodi M (2007) Molecular characterization of microbial communities in treatment microcosm wetlands as influenced by macrophytes and phosphorus loading. Ecol. Indicat 7:852–863
Akratos CS, Tsihrintzis VA (2007) Effect of temperature, HRT, vegetation and porous media on removal efficiency of pilot-scale horizontal subsurface flow constructed wetlands. Ecol Eng 29(2):173–191
Allison VJ, Condron LM, Peltzerc DA, Richardsonc SJ, Turner BL (2007) Changes in enzyme activities and soil microbial community composition along carbon and nutrient gradients at the Franz Josef chronosequence. New ZealandSoil Biol. Biochem 39:1770–1781
Billore SK, Singh N, Sharma JK, Dass P, Nelson RM (1999) Horizontal subsurface flow gravel bed constructed wetland with Phragmites Karka in central India. Water Sci Technol 40:163–171
Calheiros CSC, Duque AF, Moura A, Henriques IS, Correia A, Rangel AOSS, Castro PML (2009) Changes in the bacterial community structure in two-stage constructed wetlands with different plants for industrial wastewater treatment. Bioresour Technol 100:3228–3235
Chang J, Wu S, Dai Y, Liang W, Wu Z (2012) Treatment performance of integrated vertical-flow constructed wetland plots for domestic wastewater. Ecol Eng 44:152–159
Chung AKC, Wu Y, Tam NFY, Wong MH (2008) Nitrogen and phosphate mass balance in a sub-surface flow constructed wetland for treating municipal wastewater. Ecol Eng 32:81–89
D’Angelo EM, Karathanasis AD, Sparks EJ, Ritchey SA, Wehr-McChesney SA (2005) Soil carbon and microbial communities at mitigated and late successional bottomland forest wetlands. Wetlands 25:162–175
Dong Y, Wiliński PR, Dzakpasu M, Scholz M (2011) Impact of hydraulic loading rate and season on water contaminant reductions within integrated constructed wetlands. Wetlands 31:499–509
Duarte B, Reboreda R, Cacdor I (2008) Seasonal variation of extracellular enzymatic activity (EEA) and its influence on metal speciation in a polluted salt marsh. Chemosphere 73:1056–1063
Faulwetter JL, Gagnon V, Sundberg C, Chazarenc F, Burr MD, Brisson J, Camper AK, Stein OR (2009) Microbial processes influencing performance of treatment wetlands: a review. Ecol Eng 35:987–1004
Fountoulakis MS, Terzakis S, Chatzinotas A, Brix H, Kalogerakis N, Manios N (2009) Pilot-scale comparison of constructed wetlands operated under high hydraulic loading rates and attached biofilm reactors for domestic wastewater treatment. Sci Total Environ 407:2996–3003
Gikas GD, Tsihrintzis VA, Akratos CS (2011) Performance and modeling of a vertical flow constructed wetland—maturation pond system. J. Environ. Sci. Health A 46(7):692–708
Gikas GD, Tsihrintzis VA (2012) A small-size vertical flow constructed wetland for on-site treatment of household wastewater. Ecol Eng 44:337–343
Huang J, Reneau JRRB, Hagedorn C (2000) Nitrogen removal in constructed wetlands employed to treat domestic wastewater. Water Res 34:2582–2588
Kent AD, Yannarell AC, Rusak JA, Triplett EW, McMahon KD (2007) Synchrony in aquatic microbial community dynamics. ISME J 1:38–47
Klose S, Tabatabai MA (2000) Urease activity of microbial biomass in soils as affected by cropping systems. Biol. Fert. Soils 31:191–199
Kotti EP, Gikas GD, Tsihrintzis VA (2010) Effect of temperature, HRT, vegetation and porous media on removal efficiency of pilot-scale free water surface flow constructed wetlands. Ecol Eng 36(7):862–875
Krasnits E, Friedler E, Sabbah I, Beliavski M, Tarre S, Green M (2009) Spatial distribution of major microbial groups in a well established constructed wetland treating municipal wastewater. Ecol Eng 35:1085–1089
Kuschk P, Wiebner A, Kappelmeyer U, Weibbrodt E , Kastner M, Stottmeister U (2003) Annual cycle of nitrogen removal by a pilot-scale subsurface horizontal flow in a constructed wetland under moderate climate. Water Res 37:4236–4242
Leps J, Smilauer P (2003) Multivariate analysis of ecological data using CanocoTM. Cambridge University Press, Cambridge, p 62
Liang W, Wu Z, Cheng S, Zhou Q, Hu H (2003) Roles of substrate microorganisms and urease activities in wastewater purification in a constructed wetland system. Ecol Eng 21:191–195
Li S, Li H, Liang XQ, Chen YX, Cao ZH, Xu ZH (2009) Rural wastewater irrigation and nitrogen removal by the paddy wetland system in the Tai Lake region of China. J. Soils. Sediments 9:433–442
Li YH, Li HB, Wang X, Sun TH (2011) Changes in microbial populations and enzyme activities during nitrogen biodegradation of domestic sewage treatment in the subsurface wastewater infiltration system (SWIS). Bull Environ Contam Toxicol 87:431–435
Martens DA, Johanson JB, Frankenberger WT (1992) Production and persistence of soil enzymes with repeated addition of organic residues. Soil Sci 153:53–61
Mentzer JL, Goodman RM, Balser TC (2006) Microbial response over time to hydrologic and fertilization treatments in a simulated wet prairie. Plant Soil 284:85–100
Mina IPO, Costa M, Matos A, Calheiros CSC, Castro PML (2011) Polishing domestic wastewater on a subsurface flow constructed wetland: organic matter removal and microbial monitoring. Int. J. Phytorem 13:947–958
van de Moortel AMK, Rousseau DPL, Tack FMG, Pauw ND (2009) A comparative study of surface and subsurface flow constructed wetlands for treatment of combined sewer overflows: a greenhouse experiment. Ecological Engineering 35:175–183
Pankhurst CE, Yu S, Hawke BG et al (2001) Capacity of fatty acid profiles and substrate utilization patterns to describe differences in soil microbial communities associated with increased salinity or alkalinity at three locations in South Australia. Biol. Fertil. Soils 33:204–217
Perfler R, Laber J, Langergraber G, Haberl R (1999) Constructed wetlands for rehabilitation and reuse of surface waters in tropical and subtropical areas—first results from small-scale plots using vertical flow beds. Water Sci Technol 40(3):155–162
Saeed T, Sun GZ (2011) Enhanced denitrification and organics removal in hybrid wetland columns: comparative experiments. Bioresour Technol 102:967–974
Schinner F, von Mersi W (1990) Xylanase-, CM-cellulase- and invertase activity in soil: an improved method. Soil. Biol. Biochem 22:511–515
Schutter ME, Dick RP (2000) Comparison of fatty acid methyl ester (FAME) methods for characterizing microbial communities. Soil Sci Soc Am J 64:1659–1668
Smith J, Wagner-Riddle C, Dunfield K (2010) Season and management related changes in the diversity of nitrifying and denitrifying bacteria over winter and spring. Appl Soil Ecol 44:138–146
State Environmental Protection Administration of China (2002) Standard methods for testing water and wastewater, 4th edn. China Environment Science Press, Beijing
Steenwerth KL, Jackson LE, Carlisle EA, Scow KM (2006) Microbial communities of a native perennial bunchgrass do not respond consistently across a gradient of land-use intensification. Soil Biol Biochem 38:1797–1811
Stefanakis AI, Tsihrintzis VA (2012) Effect of loading, resting period, temperature, porous media, vegetation and aeration on performance of pilot-scale vertical flow constructed wetlands. Chem Eng J 181–182:416–430
Tabatabai MA (1994) Soil enzymes. In: Weaver RW, Angel JS, Bottomley PS (eds) Methods of soil analysis, part 2-microbiological and biochemical properties. SSSA Book Series No. 5. Soil Science Society of America, Madison, pp 775–833
ter Braak CJF (1994) Canonical community ordination: part I. Basic theory and linear methods. Ecoscience 1:127–140
Tsihrintzis VA, Gikas GD (2010) Constructed wetlands for wastewater and activated sludge treatment in north Greece: a review. Water Sci Technol 61(10):2653–2672
Vymazal J (2002) The use of sub-surface constructed wetlands for wastewater treatment in the Czech Republic: 10 years experience. Ecol Eng 18:633–646
Wang LM, Guo FH, Zheng Z, Luo XZ, Zhang JB (2011) Enhancement of rural domestic sewage treatment performance, and assessment of microbial community diversity and structure using tower vermifiltration. Bioresour Technol 102:9462–9470
Weaver MA, Zablotowicz RM, Krutz LJ, Bryson CT, Locke MA (2012) Microbial and vegetative changes associated with development of a constructed wetland. Ecol Indic 13:37–45
Wilkinson SG (1988) Gram negative bacteria. In: Ratledge C and Wilkinson SG (eds) Microbial Lipids. Academic Press, London, pp. 299–488
Wright AL, Reddy KR (2001) Phosphorus loading effects on extracellular enzyme activity in everglades wetland soils. Soil Sci Soc Am J 65:588–595
Wu HM, Zhang J, Li PZ et al (2011) Nutrient removal in constructed microcosm wetlands for treating polluted river water in northern China. Ecol. Eng 37:560–568
Xiong JB, Qin Y, Mahmood Q, Liu HH, Yang DJ (2011) Phosphorus removal from secondary effluents through integrated constructed treatment system. Mar Pollut Bull 63:98–101
Zelles I, Bai QY, Beck T, Beese F (1992) Signature fatty acids in phospholipids and lipopolysaccharides as indicators of microbial biomass and community structure in agricultural soils. Soil Biol Biochem 24:317–323
Zelles L (1999) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterization of microbial communities in soil: a review. Biol Fertil Soils 29:111–129
Zhang CB, Huang LN, Luan TG, Jin J, Lan CY (2006) Structure and function of microbial community during the early stages of revegetation near Shaoguan Pb/Zn Smelter, Guangdong, P.R. China. Geoderma 136:555–565
Zhang CB, Liu WL, Wang J, Chen T, Yuan QQ, Huang CC, Ge Y, Chang SX, CHANG J (2011) Plant functional group richness-affected microbial community structure and function in a full-scale constructed wetland. Ecol. Eng 37:1360–1368
Zhang CB, Wang J, Liu WL, Zhu SX, Ge HL, Chang SX, Ge Y, Chang J (2010) Effects of plant diversity on microbial biomass and community metabolic profiles in a full-scale constructed wetland. Ecol Eng 36:62–68
Zhang D, Gersberg RM, Keat TS (2009) Constructed wetlands in China. Ecol Eng 35(10):1367–1378
Zhao YJ, Hui Z, Chao X, Nie E, Li HJ, He J, Zheng Z (2011) Efficiency of two-stage combinations of subsurface vertical down-flow and up-flow constructed wetland systems for treating variation in influent C/N ratios of domestic wastewater. Ecol Eng 37:1546–1554
Zhou QH, He F, Zhang LP, Wang YF, Wu ZB (2009) Characteristics of the microbial communities in the integrated vertical-flow constructed wetlands. J. Environ. Sci. China 21:1261–1267
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (51179184, 41272272), Key Project of the National Twelfth-Five Year Research Program of China (2012BAD25B05-02), Major Science and Technology Program for Water Pollution Control and Treatment (2011ZX07303-001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Wu, Sq., Chang, Jj., Dai, Y. et al. Treatment performance and microorganism community structure of integrated vertical-flow constructed wetland plots for domestic wastewater. Environ Sci Pollut Res 20, 3789–3798 (2013). https://doi.org/10.1007/s11356-012-1307-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1307-0