Abstract
The effect of arsenic bioaccumulation in liver, kidney, skin, muscle, and intestinal tissues of mirror carp (Cyprinus carpio carpio) was investigated on lipid peroxidation and certain antioxidant enzyme activities. In this study, three aquarium groups were formed from mirror carp: control group, 0.5-, and 1-mg/L arsenic concentrations. The fish were dissected after 1 month. Arsenic bioaccumulation, malondialdehyde (MDA) levels, catalase (CAT), and superoxide dismutase (SOD) enzyme activities were determined in the tissues. Results showed that arsenic was accumulated in liver, kidney, muscle, skin, and intestinal tissues. As the final product of lipid peroxidation, MDA levels were determined to have increased in all tissues with the exception of muscle. On the other hand, CAT and SOD enzyme activities in the fish tissues were decreased as compared to the control group. In the muscle tissue, differences were observed in the enzyme activities depending on arsenic concentration. Considering the increases in enzyme inhibition and MDA levels, liver was observed to be the main tissue affected in response to the arsenic toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to natural and industrial causes, water resources are contaminated by arsenic compounds. Arsenic is a highly toxic element and its carcinogenic effect on living organisms is well known (Wang et al. 2006). Therefore, water contamination by arsenic has become a serious health and environmental problem with increasing severity (Allen and Rana 2004a). Organisms inhabiting aqueous media are directly exposed to arsenic intoxication. At the same time, this situation also threatens human health by using arsenic-contaminated water as drinking water and through food chain as a result of arsenic bioaccumulation in living organisms exposed to arsenic.
Accumulation of heavy metals in the fish tissue leads to both formation of hydroxyl radicals that cause oxidation of fatty acids and increase in hydrogen peroxides concentration (Atli et al. 2006). Arsenic emission to aqueous media as a result of anthropogenic activities leads to arsenic concentration in high concentrations in the sediment, water, benthic invertebrates, and fish (Culioli et al. 2009). Arsenic might accumulate in aqueous media tissues at levels of 1–200 mg/kg if the amount of arsenic in water is above tolerable levels (Entwisle and Hearn 2006). The avoidance threshold for golden shiner (Notemigonus crysoleucas) was 28 μg/L arsenic in flow-through tests (Hartwell et al. 1989). Accumulation of arsenic in living tissues above tolerable levels causes numerous problems such as oxidative damage and deterioration of numerous structural proteins (Akter et al. 2005). Furthermore, arsenic causes oxidative stress directly since it damages pro-oxidative and anti-oxidative balance by stimulating the formation of free radicals like superoxide, hydroxyl radical, and peroxyl radicals (Ventura-Lima et al. 2010).
One of the biggest factors in the formation of lipid peroxidation is the increase of reactive oxygen species (ROS) (Sare et al. 2002). Arsenic compounds that produce ROS and reactive nitrogen species (RNS) might cause oxidation of cellular components, especially lipid, DNA, and proteins (Volodymyr 2011). Biological indicators and modifications play an important role in determining oxidative stress (Wasowicz et al. 1993). Malondialdehyde (MDA) is the most important one of these indicators, which is the final product of lipid peroxidation (Papadimitriou and Loumbourdis 2002).
As the first defense line against free radicals, antioxidant defense system involves antioxidant enzymes like catalase (CAT), superoxide dismutase (SOD), GR, and GP-x and nonenzymatic antioxidants like glutathione (GSH), protein-SH, and other antioxidants (Kelly et al. 1998). Therefore, antioxidant enzymes both preserve cellular stability and play an important role in keeping free radicals away (Halliwel and Gutteridge 1989).
In animals, toxicity of arsenic compounds depends on species, gender, age, dosage, and exposure time (Allen et al. 2004b). Previous studies show that arsenic exposure of fishes at levels of 0.1–8.4 mg/mL resulted in arsenic accumulation and oxidative stress in fish tissues (Allen and Rana 2004a; Allen et al. 2004b; Bagnyukova et al. 2007; Bhattacharya and Bhattacharya 2007). Another study on Cyprinus carpio showed that there was no arsenic accumulation while antioxidant system changes were still detected if C. carpios were exposes to arsenic (AsIII and AsV) at levels of 0.1 and 1 mg/mL for 2 days (Ventura-Lima et al. 2009). In this study, bioaccumulation levels in the tissues of mirror carp exposed to arsenic (long time at high concentrations) and lipid peroxidation and at the same time changes in antioxidants system were researched together. As a result, it was aimed to determine the target organs where arsenic accumulated and the difference between the antioxidant reactions of target tissues against oxidative stress caused by arsenic intoxication.
Material and method
Material
In this study, the Cyprinus carpio carpio 15 ± 1 cm long and 50 ± 5 g were obtained from Mediterranean Aquaculture Research, Production, and Education Institute (Kepez/Antalya). Totally, 36 fishes were used in the experiment. The fishes were acclimatized in an aquarium for 15 days. Later, three aquariums were kept as control while three were filled with water with 0.5-mg/L concentration of arsenic and the other three with 1-mg/L concentration of arsenic. Four fishes were put into each aquarium. Sodium arsenite (NaAsO2) was used as arsenic source. The experiments were carried out in duplicate with three groups (control, As-0.5 mg/L, and As-1 mg/L) of mirror carps each in 120-L aquaria, under controlled temperature (21.3–23.6 ◦C), pH (7.0–7.5), photoperiod (12-h light/12-h dark), and dissolved oxygen 100 % saturation. Throughout the experiment, in order to keep the water quality of the aquariums at such a level not to affect fish health, one third water was changed every week and water quality parameters were checked frequently. The fish were kept in the aquariums for a month. At the end of the experiment, the fishes were anesthetized by immersion in 50 mg/L tricaine methane sulphonate (MS-222) solution for 5–10 min before they were killed by transection of the spinal cord. Liver, kidney, skin, muscle, and intestinal tissues of fishes were removed in ice. After the tissues were washed with physiologic water, dried, and weighed, they were stored at −80 ºC until analysis.
Bioaccumulation analysis
After drying the tissue samples at 105 ± 5 ºC, they were ground in mortar to prepare a homogenous mixture. Dry and homogenous samples were stirred after concentrated nitric acid and concentrated perchloric acid were added. Later, they were exposed to saturation treatment in a microwave unit (CEM Mars Xpress). After completing organic breakdown, the samples were cooled, centrifuged, and filtered through filter paper. Afterwards, they were analyzed using atomic absorption spectroscopy (AAS). All the operations were repeated on blind samples in the same way (APHA 1992).
Preparation of the samples
Before starting biochemical analyses, the tissues were homogenized for 5 min at 10,000 rpm in 50 μM, pH 7.4, cooled sodium-phosphate tampon containing 0.25 M sucrose at 1/10 weight/volume (w/v) rate. In order to prevent enzyme activity loss due to heat, the samples were homogenized in ice. Homogenates were centrifuged for 30 min at +4 ºC and 9,500g. The resultant supernatant was used while determining protein amounts and CAT and SOD enzyme activities.
In order to perform MDA levels analyses, the tissues were homogenized for 5 min at 10,000 rpm in 50 μM, pH 7.4, cooled sodium-phosphate tampon at 1/10 weight/volume (w/v) rate. The samples were homogenized in ice. Homogenates was used while determining MDA levels.
Malondialdehyde assay
Malondialdehyde was determined by the double-heating method of Draper and Hadley (1990). The principle of the method was spectrophotometric measurement of the pink color produced during the reaction to thiobarbituric acid (TBA) with MDA at 532 nm. The concentration of MDA was calculated from the standard chart of MDA–TBA complex by using 1,1,3,3-tetraethoxypropane on behalf of MDA. Results were expressed as nanomoles per milligram protein.
Determination of CAT activity
Catalase activity was measured according to the method of Aebi (1984). The principle of the assay is based on the determination of the rate constant of hydrogen peroxide decomposition by CAT enzyme. One unit (1U) of CAT equals the enzyme activity that recognized 1 μmol of hydrogen peroxide in 60 s at 37°C. CAT activity was measured by observing the change on absorbance of sample and blank for a minute spectrophotometricly at 240 nm. Results were expressed as units per milligram protein.
Determination of SOD activity
The determination method of SOD activity depends on the spectrophotometric measurement of SOD’s inhibition effect on autoxidation of 6-hydroxyidopamine (6-OHDA) (Heikkila and Cabbat 1976; Crosti et al. 1987). 1U of SOD activity is accepted as the amount of enzyme that decreases the autoxidation of 6-OHDA in 50 % in 1 min at 37 °C. Since the curve of autoxidation speed is stable in the 1st minute, the spectrophotometric measurement was done at 490 nm until the 60th second of oxidation in this reaction. Results were expressed as units per milligram protein.
Determination of protein
Tissue homogenates protein concentration was calculated according to the method of Lowry et al. (1951) as milligrams per milliliter using bovine serum albumin.
Statistical analysis
The data are expressed as mean ± standard deviations. The Levene test was used to analyze the homogeneity of the variances. If the variances were not homogenous, the differences were analyzed using the Kruskal–Wallis variance analysis, and bilateral comparisons were performed using the Mann–Whitney U test. The t test for paired samples was used when the variances were normally distributed. Statistical analysis was performed using the Statistical Analysis System (SPSS) 14.0 for Windows.
Results and discussion
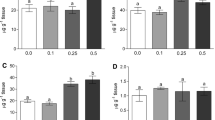
It was determined that arsenic accumulated in the kidney, muscle, and skin tissues of mirror carp raised in 00.5 mg/L arsenic concentration significantly compared with the control group (p < 0.05). It was also determined that as well as in kidney, muscle, and skin tissues, arsenic accumulation was significant in liver and intestinal tissues of mirror carp raised in 1-mg/L concentration compared with the control group (p < 0.05) (Fig. 1).
It was found that, in both concentrations, compared with the control group, MDA levels in most tissues of the mirror carp exposed to arsenic—liver, kidney, skin, and intestinal tissues—increased significantly (p < 0.05); the change in MDA levels in muscle tissue was not significant (p > 0.05) (Fig. 2).
Compared with the control group, while significant increases in SOD enzyme activities were seen in kidney and muscle tissues of the fish exposed to 0.5 mg/L arsenic (p < 0.05), in liver, skin, and intestinal tissues, SOD activity was significantly decreased (p < 0.05). In addition, In SOD enzyme activities, the decrease in liver, kidney, muscle, skin, and intestinal tissues of the fish exposed to 1 mg/L arsenic was determined to be significant (p < 0.05) (Fig. 3).
The decrease in CAT enzyme activities in all tissues of the fish exposed to 1 mg/L arsenic was found significant (p < 0.05). However, while an increase occurred in the muscle tissue of the fish exposed to 0.5 mg/L arsenic, a decrease was determined in the other tissues (Fig. 4).
Arsenic is a common environmental contaminant. Heavy metals might accumulate in tissues (Farombi et al. 2007). Therefore, tissue accumulation analysis of toxic materials in the aqueous organisms might be a reasonable assessment for animal and human health standards (Kumar and Banerjee 2012). In the literature, after exposure to arsenite, a significant amount of arsenic accumulation is reported in liver, gills, skin, muscle, brain, and blood tissues of the catfish (Kumar and Banerjee 2012). In this study, as a result of exposure to 0.5 mg/L arsenic for a month, the increase order in the arsenic bioaccumulation values in mirror carp tissues was skin > muscle > kidney > liver > intestine, whereas the arsenic accumulation change in the fish exposed to 1-mg/L concentration was determined to be skin > muscle > kidney > intestine > liver. Accordingly, it was observed that, in the mirror carp exposed to high concentrations of arsenic, the most arsenic accumulation occurred in skin and muscle tissues, whereas it was less in liver and intestinal tissues. Although arsenic accumulation showed discrepancies in freshwater fish tissues, the most accumulation was reported in muscle and gills (Allen et al. 2004b). In areas exposed to metal contamination, arsenic accumulation was determined in the liver, kidney, and heart tissues of the fish (Farombi et al. 2007). Arsenic accumulation was less in the liver and kidney of the rats exposed to chronic arsenic than in their skin and hair (Germolec et al. 1998). Differences might be observed in the arsenic accumulation among tissues depending on the duration and amount of inorganic arsenic (AsIII) exposure in different species. As a result of the study on different arsenic concentrations, arsenic accumulation ratio in liver, kidney, skin, and intestinal tissues significantly increased by increasing arsenic concentration in living media.
Similar to MDA, aldehydes occur during lipid peroxidation and are commonly used as an indicator of oxidative damage (Wang et al. 2012). In this study, an increase was observed in MDA levels—final product of lipid peroxidation—in the tissues of the fish due to prolonged arsenic exposure. An increase was reported in the lipid peroxidation products in liver tissue of goldfish (Bagnyukova et al. 2007), liver and gills tissues of C. carpio (Ventura-Lima et al. 2009), and liver tissue of Clarias batrachus (Bhattacharya and Bhattacharya 2007) depending short-term arsenic exposure. A minor increase in ROS formation in animals exposed to arsenite was enough to trigger a significant increase in lipid peroxidation (Zarazúa et al. 2006). A significant correlation (p < 0.05) was obtained between arsenic accumulation and the increase of MDA levels in our study (Table 1). Free radicals existing due to biotransformation of arsenic components and arsenic accumulation in tissues caused oxidation in cellular components—mainly lipids and proteins—and an increase was observed in the oxidative stress markers such as MDA in the cell.
Reactive oxygen species produced as a result of oxidative stress is deactivated by antioxidant defense system (Gumustekin et al. 2005). SOD represents the first defense line during ROS neutralization process against superoxide radicals (Guney et al. 2009). Antioxidant enzymes like SOD play an important role in suppressing ROS increase (Wang et al. 2012). In this study, while a decrease was determined in SOD activity in certain tissues of fish exposed to arsenite, an increase was determined in others. Differences might be observed among tissues in terms of antioxidant reaction against arsenic toxicity. It was observed that as a result of arsenic induction in rats, SOD activity did not change significantly or decrease in blood, liver, and brain tissues depending on age and exposure time (Jain et al. 2012). SOD enzyme activity in heart, brain, and liver tissues of female rats exposed to arsenite is reported to decrease (Bharti et al. 2012). Our study show that SOD enzyme activity in tissues that arsenic accumulated significantly decreased (p < 0.05) by likely depending on arsenic toxicity, when tissues of C. carpio—except muscle and kidney tissues—were exposed to 0.5 mg/L arsenic.
Catalase is a very crucial enzyme to protect the cell from toxic effects of H2O2 and radical oxygen species (Coban et al. 2007; Altikat et al. 2006). In this study, although changes occurred depending on arsenic concentration, a decrease was observed in CAT activity in all the tissues in high arsenic concentration. In similar studies, a decrease was reported in CAT activity in the tissues of the living exposed to arsenic (Wang et al. 2006; Ventura-Lima et al. 2009). Superoxide radical inhibits CAT enzyme activity (Kono and Fridovich 1982). At the same time, after arsenic exposure, a decrease in CAT enzyme activity could be due to depression of protein synthesis because of the damage caused by free radicals (Humtsoe et al. 2007; Palaniappan and Vijayasundaram 2008). A significant decrease of CAT enzyme activity in tissues that arsenic accumulated was observed, excepting the exposure of 0.5 mg/L as concentration in muscle tissue.
Conclusions
As a result of arsenic exposure, MDA—a product of lipid peroxidation—was reported to increase in mirror carp tissues, and at the same time, antioxidant enzyme activities were determined to be inhibited. Arsenic was reported to accumulate more in skin, kidney, and muscle, respectively. Considering the increase in MDA levels and decrease in CAT, SOD enzyme activities in the fish tissues, the most susceptible tissue to arsenic exposure was determined to be the liver. It was observed in all the tissues of mirror carp exposed to high concentrations of arsenic that antioxidant system was affected negatively. In the muscle tissue, on the other hand, the enzyme activities were found to be affected differently depending on arsenic concentration (increase in CAT and SOD activities in 0.5-mg/L concentration, decrease in CAT and SOD activities in 1-mg/L concentration). As a result, induction of oxidative stress and negative effect on antioxidant enzymes depending on arsenic accumulation in tissues of C. carpio were found after the exposure of high arsenic concentration.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Akter KF, Owens G, Davey DE, Naidu R (2005) Arsenic speciation and toxicity in biological systems. Rev Environ Contam Toxicol 184:97–149
Allen T, Rana S (2004) Effect of arsenic (AsIII) on glutathione-dependent enzymes in liver and kidney of the freshwater fish Channa punctatus. Biol Trace Elem Res 100(1):39–48
Allen T, Singhal R, Rana S (2004) Resistance to oxidative stress in a freshwater fish Channa punctatus after exposure to inorganic arsenic. Biol Trace Elem Res 98(1):63–72. doi:10.1385/BTER:98:1:63
Altikat S, Coban A, Ciftci M, Ozdemir H (2006) In vitro effects of some drugs on catalase purified from human skin. J Enzym Inhib Med Ch 21(2):231–234
American Public Health Association (APHA) (1992) Standard methods for the examination of water and wastewater. 18th ed., Washington, USA
Atli G, Alptekin O, Tukel S, Canli M (2006) Response of catalase activity to Ag+, Cd2+, Cr6+, Cu2+ and Zn2+ in five tissues of freshwater fish Oreochromis niloticus. Comp Biochem Physiol 143:218–224
Bagnyukova TV, Luzhna LI, Pogribny IP, Lushchak VI (2007) Oxidative stres and antioxidant defenses in goldfish liver in response to short-term exposure to arsenite. Environ Mol Mutagen 48:658–665
Bharti VK, Srivastava RS, Sharma B, Malik JK (2012) Buffalo (Bubalus bubalis) epiphyseal proteins counteract arsenic-induced oxidative stress in brain, heart, and liver of female rats. Biol Trace Elem Res 146(2):224–229. doi:10.1007/s12011-011-9245-0
Bhattacharya A, Bhattacharya S (2007) Induction of oxidative stress by As in Clarias batrachus: involvement of peroxissomes. Ecotox Environ Saf 66:178–187
Coban A, Ciftci M, Ozdemir H, Altikat S (2007) Purification and characterization of catalase enzymes from chicken liver and sheep erythrocytes. Asian J Chem 19(5):3941–3953
Crosti N, Servidei T, Bajer J, Serra A (1987) Modification of 6-hydroxydopamine technique for the correct determination of superoxide dismutase. J Clin Chem Clin Biochem 25:265–266
Culioli JL, Calendini S, Mori C, Orsini A (2009) Arsenic accumulation in a freshwater fish living in a contaminated river of Corsica, France. Ecotox Environ Safe 72:1440–1445
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Entwisle J, Hearn R (2006) Development of an accurate procedure for the determination of arsenic in fish tissues of marine origin by inductively coupled plasma mass spectrometry. Spectrochim Acta B 61:438–443
Farombi EO, Adelowo OA, Ajimoko YR (2007) Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African Cat Fish (Clarias gariepinus) from Nigeria Ogun River. Int J Environ Res Public Health 4(2):158–165
Germolec DR, Humble MC, Bruccoleri A, Spalding J, Boorman GA, Foley JF, Yu HS, Chen GS, Simeonova PP, Luster MI, Yoshida T (1998) Arsenic enhancement of skin neoplasia by chronic stimulation of growth factors''. Am J Pathol 153(6):1775–1785
Gumustekin K, Ciftci M, Coban A, Altikat S, Aktas O, Gul M, Timur H, Dane S (2005) Effects of nicotine and vitamin E on glucose 6-phosphate dehydrogenase activity in some rat tissues in vivo and in vitro. J Enzym Inhib Med Ch 20(5):497–502
Guney T, Yıldız B, Altikat S, Kural N, Alatas O (2009) Decreased antioxidant capacity and increased oxidative stress in patients with juvenile idiopathic arthritis. J Pediatric Sci 1:1–6
Halliwel B, Gutteridge JMC (1989) Free radicals in biology and medicine, 2nd edn. Clarendon Pres, Oxford
Hartwell SI, Jin JH, Cherry DS, Cairns J (1989) "Toxicity versus avoidance response of golden shiner, Notemigonus crysoleucas, to five metals. J Fish Biol 35(3):447–456
Heikkila RE, Cabbat F (1976) A sensitive assay for superoxide dismutase based on the autoxidation of 6-hydroxydopamine. Anal Biochem 75:356–362
Humtsoe N, Davoodi R, Kulkarni BG, Cavan B (2007) Effect of arsenic on the enzyms of the rohu carp, Labeo rohita (Hamilton, 1822). Raffles B Zool 14:17–19
Jain A, Flora G, Bhargava R, Flora S (2012) Influence of Age on Arsenic-Induced Oxidative Stress in Rat. Biol Trace Elem Res 149(3):382–390. doi:10.1007/s12011-012-9432-7
Kelly SA, Havrilla CHM, Brady TC, Abramo KH, Levin ED (1998) Oxidative stress in toxicology: Established mammalian and emerging piscine model systems. Environ Health Persp 106:375–384
Kono YY, Fridovich II (1982) Superoxide radical inhibits catalase. J Biol Chem 257(10):5751–5754
Kumar RR, Banerjee TK (2012) Analysis of arsenic bioaccumulation in different organs of the nutritionally important catfish, Clarias batrachus (L.) exposed to the trivalent arsenic salt, sodium arsenite. Bull Environ Contam Toxicol 89(3):445–449. doi:10.1007/s00128-012-0714-8
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265
Palaniappan PR, Vijayasundaram V (2008) FTIR study of arsenic induced biochemical changes on the liver tissues of fresh water fingerlings Labeo Rohita. Rom J Biophys 18:135–144
Papadimitriou EE, Loumbourdis NS (2002) Exposure of the frog Rana ridibunda to copper: impact on two biomarkers, lipid peroxidation, and glutathione. Bull Environ Contam Toxicol 69(6):0885–0891. doi:10.1007/s00128-002-0142-2
Sare M, Hamamci D, Yilmaz I, Birincioglu M, Mentes BB, Ozmen M, Yesildag O (2002) Effects of carbon dioxide pneumoperitoneum on free radical formation in lung and liver tissues. Surg Endosc 16:88–192
Ventura-Lima J, Fattorini D, Regoli F, Monserrat JM (2009) Effects of different inorganic arsenic species in Cyprinus carpio (Cyprinidae) tissues after short-time exposure: Bioaccumulation, biotransformation and biological responses. Environ Pollut 157(12):3479–3484
Ventura-Lima J, Bogo MR, Monserrat JM (2010) Arsenic toxicity in mammals and aquatic animals: a comparative biochemical approach. Ecotox Environ Safe 74:211–218
Volodymyr IL (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Wang L, Xu ZR, Jia XY, Jiang JF, Han XY (2006) Effects of arsenic (AsIII) on lipid peroxidation, glutathione content and antioxidant enzymes in growing pigs. Asian-Aust J Anim Sci 19(5):727–733
Wang J, Zhang W, Sun D, Song L, Li Y, Xu C (2012) Analysis of neuroglobin mRNA expression in rat brain due to arsenite‐induced oxidative stress. Environ Toxicol 27(9):503–509
Wasowicz W, Neve J, Peretz A (1993) Optimized steps in fluorometric determination of thiobarbuturic acid-reactive substance in serum: Importance of extraction pH and influence of sample preservation and storage. Clin Chem 39:2522–2526
Zarazúa S, Delgado J, Martínez L, Ortiz-Pérez D, Jiménez-Capdeville M, Pérez-Severiano F (2006) Decreased nitric oxide production in the rat brain after chronic arsenic exposure. Neurochem Res 31(8):1069–1077. doi:10.1007/s11064-006-9118-7
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Proceedings of the Second International Conference on Water, Energy and the Environment Kusadası, Turkey, September 21–24, 2013, paper no. 338
Rights and permissions
About this article
Cite this article
Altikat, S., Uysal, K., Kuru, H.I. et al. The effect of arsenic on some antioxidant enzyme activities and lipid peroxidation in various tissues of mirror carp (Cyprinus carpio carpio). Environ Sci Pollut Res 22, 3212–3218 (2015). https://doi.org/10.1007/s11356-014-2896-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2896-6