Abstract
The present study evaluates the relation between chronic arsenic (As) exposure in the natural distribution area of wild brown trout (Salmo trutta), oxidative stress and antioxidant enzymatic defenses. Two rivers of the same watershed were evaluated to highlight the correlation between As accumulation and the resulting stress: (i) the Presa River, which has high chronic As levels (2281.66 µg/L) due to past mining activity, and (ii) the Bravona River (control river). This metalloid was measured in main fish tissues (gills, kidney, liver, muscle, gonads and fins) and water. As organotropism in S. trutta was kidney > liver > gill > fin > gonad > muscle. The HepatoSomatic Index (HSI) and somatic condition (CF) were used to compare fish population conditions from both sites. Arsenic can be absorbed by the gills and can induce oxidative stress and disturb antioxidant defenses. The aim of this study was to evaluate oxidative stress response by measuring malondialdehyde (MDA) content, as a marker of lipid peroxidation, and antioxidant enzymatic defenses (Superoxide dismutase (SOD), catalase CAT, glutathione peroxidase (GPx) and glutathione S-transferase (GST)), in the main tissues of control and exposed trout. The highest MDA content was found in the kidney and liver of exposed trout. SOD and CAT activities in exposed livers and kidneys were considerably increased while a significant rise of GPx activity was observed only in the liver. GST activity was found to be significantly induced in the liver of exposed trout. The results demonstrate that arsenic bioaccumulation can induce lipid peroxidation and substantial modifications in antioxidant enzymatic defenses in main wild trout tissues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Our environment is subject to several perturbations such as climatic changes and environmental pollution (heavy metals, metalloids…). Aquatic ecosystems may be particularly vulnerable to these disturbances (Livingston et al. 1994). Freshwater systems are major vectors for pollutant dispersion, acting as “sinks for environmental contaminants” (Amundsen et al. 2011). Pollutants that enter the water cause undesirable changes that directly or indirectly affect the ecological environmental balance (Biswas et al. 2011). Heavy metals and metalloids are an important source of pollution in water streams. Heavy metal or metalloid pollution can contaminate fish via their body surface, gills or digestive tract. Thereby, fish are good bio-indicators of metal pollution in water streams. Some of these metallic trace elements (MTE) are potentially toxic and can be very harmful with chronic exposure (Munoz-Olivas and Camara 2001).
Arsenic (As) is a ubiquitous element. Natural sources of As in the aquatic environment include the erosion of As rocks and soils, volcanic activity and degradation of fauna and flora that have accumulated As. Main anthropogenic sources of As are mining activities, combustion of fossil fuels, agriculture, and industrial applications (Cockell and Hilton 1988; Pedlar and Klaverkamp 2002). For example, concentrations up to 3500 µg/g have been found in sediment of the Kam Lake in Canada due to long-term As contamination from gold mining activity (Pedlar and Klaverkamp 2002).
In Corsica (a Mediterranean island), the past exploitation of an Arsenic mine near a tributary of the Bravona River caused environmental pollution. The deposits in Matra (name of the surrounding village) located in Alpine Corsica come from a fold that has a serpentine core surmounted by prasinites which overlaps lustrous shale. The vein is 500 m long and almost 100 m deep. The most abundant mineral is realgar (AsS). Stibnite (Sb2S3) and orpiment (As2S3) are present in lower levels. At the end of 1880, following flooding of the Presa River, arsenic sulfide outcrop was found in the river bed. In 1913, the ore production reached about 4000 tons containing 30–50% As (Mori et al. 1999). The last period of mining was from 1935 to 1945, after a total production of 30,000 tons of ore (Foata et al. 2009). Now, As (V) represents 99.6% of the total As dissolved in the Presa river, whereas As (III) is essentially present in the mine bank (Calendini 2000). Arsenic toxicity is highly dependent on the oxidation state, its chemical form and the species of animal studied (Jain and Ali 2000). Generally, As (III) is more toxic than As (V) (Spehar et al. 1980, Ercal et al. 2001).The rapid of conversion of As (V) to As (III) in cells and the faster rate of cellular uptake of As (III) could be one reasons why As (III) is more toxic (Dopp et al. 2004).
Direct contaminant analysis of such substances does not provide information about their effect on the ecosystem, so the use of biomonitors is recommended to understand the bioavailable pollutant loads (Lamas et al. 2007). Trout are considered excellent bio-indicators due to their long life, their capacity to assimilate pollutants for a long time and their high position in food chain. In Corsica, above 500 m altitude, trout are predominant in the rivers as the temperature is low and the current speed and slope are high (Roche and Mattei 1997). This is the case for the two stations (exposed and control sites) considered in this study.
Exposure of organisms to xenobiotics can induce ROS formation leading to oxidative stress. Oxidative stress is involved in DNA damage, protein oxidation, lipid peroxidation (Winston and Digiulio 1991) and it disrupts signal transduction (Druwe and Vaillancourt 2010). In order to offset molecular and cellular damage, aerobic organisms have developed sophisticated antioxidant mechanisms. These mechanisms include enzymatic systems. Superoxide dismutase (SOD) catalyzes dismutation of the superoxide anion (O2 ●−) in hydrogen peroxide (H2O2). H2O2 can then be reduced by catalase (CAT) and glutathione peroxidase (GPx). Glutathione S-transferase (GST) uses reduced glutathione to protect cells against pollutant and toxic xenobiotics by conjugating them to endogenous and exogenous electrophilic compounds (Townsend and Tew 2003).
Among the major environmental pollutants, heavy metals and metalloids are a true ecological problem. For example, Almeida et al. (2002) showed that higher SOD and GPx activities in the liver of Nile tilapia (Oreochromis niloticus) were correlated with cadmium (Cd) exposure. It has been shown that As can also generate ROS (Allen et al. 2004b; Bhattacharya and Bhattacharya 2007; Dautremepuits et al. 2009; Sarkar et al. 2014), although the mechanisms of As toxicity are not clear. Monitoring of antioxidant defense and oxidative damage in the natural distribution area of fish may provide a more complete understanding of the effects of xenobiotics on exposed fish. Characterization of xenobiotic toxicity can provide important data for environmental studies.
The present study analyzes the level and the location of As bioaccumulation in wild brown trout. We investigated the effects of chronic As exposure under environmental conditions on oxidative stress by analyzing malondialdehyde content, a biomarker of lipid peroxidation, and antioxidant enzymatic defenses such as SOD, CAT, GPx and the xenobiotic-detoxifying enzyme, GST, in trout tissues. To exclude other variables that may cause stress but are not linked to the presence of As in water, we used a non-contaminated sector of the Bravona river as a control site.
Materials and methods
Studied area
The Bravona River (30 km length) begins at 1700 m altitude and meets the Tyrrhenian Sea on the Eastern plain of Corsica, north of Aleria (42°17’ N, 9°23’ E). Its watershed spans 140 km2 (Mori et al. 1999). The Presa River is a tributary of the Bravona. The distance from the mine to the Bravona River is approximately 10 km. The Presa station (P), where samples were collected, is downstream from the abandoned mine. This site was selected to provide As levels close to the source of pollution. Antimony (Sb) is also known to be present at this site, however the Sb levels in fish tissues were below the detection level (data not shown); there is no other known contamination at this site. The Bravona station, upstream from the confluence with the Presa (approximately 6 km), was the control site (B) (Fig.1).
Map of Corsican freshwater streams, showing the Presa River (exposed site) and the Bravona River (control site). P: Presa exposed site (2281.66 ± 52.03 µg As/L); B: Bravona control site (As <5 µg/L); Bc: Bravona upstream confluence (As <5 µg/L); Pc: Presa upstream confluence (1480 ± 32.26 µg As/L); Bf: Bravona after confluence with Presa (117.8 ± 11.04 µg As/L). In red, studied streams; in blue, other major Corsican streams
Sample collection
Thirty fish—15 from site (B) and 15 from site (P)—were captured in October 2015 (14.2–18.4 cm in length). The fish were immediately placed in separate bags for transport that contained river water. These bags were placed in a cooler to reduce thermal shock during transport (<18 °C) and to keep the fish in the dark (to avoid any stress). This method allowed us to keep them alive until dissection, thereby removing the impact of cell breakdown from our analysis.
During the dissection, the size (in centimeters), individual weight (in grams), and liver weight (in grams) of each fish were recorded. The analyzed tissues (fins, liver, muscle, gonads, kidney, and gills) were collected using stainless steel instruments. The organs were cut in half in order to measure two parameters: oxidative stress and As concentration. After rinsing the internal organs (liver, muscle, gonads and kidney) with physiological saline and the external organs (fins and gills) with Milli-Q® pure water, the first batch was immediately immersed in liquid nitrogen and stored at −80 °C for the oxidative stress analysis. The second batch was stored at −20 °C for the contamination analysis.
To evaluate arsenic contamination in the water, 50 mL water samples were taken from five sites (indicated in Fig.1): P, Presa exposed site; B, Bravona control site; Bc, Bravona upstream confluence with Presa; Pc, Presa upstream confluence with Bravona; Bf, Bravona after confluence with Presa. For this sampling, 50 mL sterile tubes were immersed at the center of the river and opened 10 cm below water surface. They were transported in a cooler at 5 °C. Upon arrival at the laboratory, we proceeded to acidify the samples with nitric acid (2%). The water samples were stored at 5 °C until analysis.
Metal concentration analyses
Analytical procedure
The collected samples were analyzed to determine their As concentration. These samples were placed in a refrigerator for a few hours before the analysis. Sample ranging from 30 to 500 mg were weighed and placed in pressure-resistant PTFE vessels (100 mL); 5 mL of HNO3 (>69%, TraceSELECT) and 2 mL of H2O2 (30%, TraceSELECT) was added. Mineralization was carried out by digestion in a microwave reaction system (Anton Paar Multiwave 3000) and involved three stages: 200 W (1 min), 500 W (5 min), 1100 W (15 mins), then 0 W (15 min). The stages were graduated to optimize mineralization. After mineralization, solutions were diluted with 25 mL of Milli-Q® water. The solutions were then analyzed by ICP-OES (Perkin Elmer, Optima 7000DV). The analytical procedure for As was checked using certified fish protein reference material (DORM-4) for trace metal analyses (National Research Council, Canada) as quality assurance/quality control (QA/QC). The water samples were analyzed after acidification (3% HNO3); they were not filtered as they do not contain impurities.
Inductively coupled plasma optical emission spectroscopy
The arsenic calibration solution (5, 50, 100 µg/L of As) was prepared by diluting an Instrument Calibration Standard 4 (PerkinElmer Pure). The limit of quantification (LOQ) for As (5 µg/L) was determined using relevant concentrations (1, 2, 3, 4, 5, 10, 20, 50, 100) of multi-element solution certified standards (PerkinElmer). Analytical blanks were run the same way as the samples and analyzed every eight samples. Analyses were carried out with three replicates to refine the measurement. Only data showing an RSD percentage lower than 10% were considered as reproducible and were included in our results.
The measurement accuracy was certified by a quality assurance program carried out for metal analyses with an ICP-OES (using DORM-4). The recovery percentage between certified values and measured values was always higher than 90% (6.87 mg/kg As ±0.44). Element concentrations are expressed as micrograms per kilogram of wet weight (µg/kg) for tissues and as micrograms per liter (µg/L) for water analyses.
Indexes and factors
Several biometric indexes were calculated. The index of somatic condition (CF) and the HepatoSomatic Index (HSI) were calculated according to the formulas used by Dupuy et al. (2012) and Galland et al. (2012), respectively. Multiplication factor is the ratio of contaminant concentrations between the concentrations in an exposed and a control organ. When trout from Bravona River (control site) had an average concentration below the detection limit, we used the detection limit for the contaminant as the average concentration to calculate the multiplication factor. Bioconcentration factor (BCF) is the ratio between environmental concentration and an organ concentration for the same contaminant (Alexander 1999).
Biochemical analyses
Preparation of homogenates
Organs were homogenized using a Potter-Elvehjem homogenizer in chilled phosphate buffer (100 mM, pH 7.4) (25 mg w/w per mL of buffer) containing 20% glycerol and 0.2 mM phenylmethylsulfonyl fluoride as a serine protease inhibitor. The homogenates were centrifuged at 15,000 × g for 30 min at 4 °C and the supernatant used for biochemical assays. Antioxidant enzyme activity and malondialdehyde (MDA) levels were determined using a Specord 205 spectrophotometer (Analytic jena, Wembley, UK).
Enzyme activity assays
Superoxide dismutase activity
Superoxide dismutase (SOD) (E.C 1.15.1.1) activity was measured using xanthine/xanthine oxidase as a source of superoxide radical (Oberley and Spitz, 1984); 100 µL of extract was added to a reaction mixture containing 1 mM DETAPAC buffer (pH 7.8), 1.25 units of catalase, 70 µM nitro-blue tetrazolium (NBT), 0.2 mM xanthine and 0.010 units of xanthine oxidase in a total volume of 1 mL. One unit of SOD was defined as the amount of enzyme that inhibits 50% of NBT reduction at 560 nm at 25 °C.
Catalase activity
Catalase (CAT) (E.C 1.11.1.6) activity was measured using the method described by Aebi (1984). The reaction mixture contained 30 mM sodium phosphate buffer (pH 7), 10 mM H2O2 and 100 µL of extract in a total volume of 1 mL. The decrease in absorbance was measured at 240 nm (ɛ = 39.4 mM−1 cm−1). One unit of CAT was defined as 1 µmol H2O2 degraded per minute at 25 °C.
Glutathione peroxidase activity
Glutathione peroxidase (GPx) (E.C. 1.11.1.7) activity was measured using the method described by Flohe et al. (1973). The reaction mixture contained 0.1 mM EDTA phosphate buffer (pH 7.4), 1 unit of glutathione reductase, 0.25 mM reduced glutathione, 0.12 mM NADPH, 10 mM NaCN and 1 mM cumene hydroperoxide. The mixture was pre-incubated for 15 min at 37 °C. The reaction was initiated by adding 125 µL of extract in a total volume of 1 mL. The decrease in absorbance was measured at 340 nm (ɛ = 6. 22.103 M−1.cm−1). One unit of GPx was defined as 1 µmol NADPH oxidized per minute at 37 °C.
Glutathione S-transferase activity
Glutathione S-transferase (GST) (E.C. 2.5.18) activity was determined using the method described by Habig et al. (1974). The reaction mixture contained 80 mM sodium phosphate buffer (pH 6.9), 0.25 mM GSH and 0.12 mM 1-chloro-2,4-dinitrobenzene (CDNB). The reaction started by adding 100 µL of extract. The change in absorbance was measured at 340 nm (ɛ = 9.6 × 103 mM−1 cm−1). One unit was defined as 1 mmol CDNB conjugate formed per minute at 25 °C.
Protein concentration was measured by spectrophotometry at 595 nm using the Bradford 1976) with serum albumin (Sigma-Aldrich Chemicals, France) as the standard. All enzyme activities were expressed in units.mg−1 protein (specific enzymatic activities).
Determination of malondialdehyde content
The MDA concentration of trout tissues was determined using the thiobarbituric acid (TBA) reaction described by Ohkawa et al. (1979). For extraction, 80 mg of frozen organ tissue was homogenized with inert sand in 2 mL of extraction buffer (v/v), followed by centrifugation at 3000 × g for 10 min at 4 °C.
Statistical analyses
Data were expressed as mean ± SD and analyzed using R statistical software (http://www.R-project.org). Two-way ANOVA and multiple mean comparisons were performed with the Least Significant Difference (LSD) test at P < 0.05. Data were compared between the two trout groups (control and assay) for each parameter and for the same organ. In addition, for each trout group, the data obtained between organs were compared.
Results
Water contamination
Results of metal content testing of water from the five analyzed sites (Fig. 1) show that the Presa River (P), just downstream of the Matra mine, contains a high arsenic concentration (2281.66 ± 52.03 µg/L). The concentration at a point upstream of the confluence with the Bravona was lower (Pc: 1480 ± 32.26 µg/L). In the Bravona, the samples taken at the control site (B) and at a point upstream of the confluence with the Presa (Bc) had a concentration under the detection limit (<5 µg/L). In the samples taken after the confluence between the Bravona and the Presa (Bf), the concentration was 117.8 ± 11.04 µg/L, evidence of the contaminant having been diluted.
Bioaccumulation of arsenic
Table 1 summarizes mean As concentrations in each tissue of trout from the Presa and Bravona rivers and their relative standard deviations, multiplication factors and bioconcentration factors. Concentrations are very heterogeneous between different tissues and between both sites. Average As concentration was higher in each trout tissue from the Presa River.
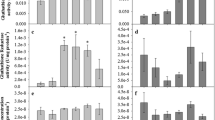
In the Presa River fish (Fig. 2), their kidneys had accumulated the most As (22,797.40 µg/kg). Liver is the second target-organ for As (3522.42 µg/kg). The gills and fins had an average As concentration higher than 1 mg/kg w/w (2004.91 and 1444.46 µg/kg respectively). Arsenic is less present in gonads (795.77 µg/kg) and muscle (613.87 µg/kg). The As concentrations found in control tissues were very low. The arsenic concentrations in tissues of trout from the Presa River were significantly different from those taken from Bravona River. Arsenic was detected in only four organs in the fish from the Bravona river: livers and kidneys had a value near to 130 µg/kg, gills and gonads were under 50 µg/kg, while fins and muscle had an As concentration below the detection limit. Arsenic is 175 times more concentrated in kidneys from trout of polluted sites than those from trout taken at the control site. For other tissues (gills, liver, and gonad), the multiplication factor is near 30 (Table 1). Bioconcentration factors indicate that As concentration in the kidney of exposed trout is nearly 10 fold higher than in water. Only two organs had a higher As concentration than water: kidney and liver. In other organs, the water concentration of As was higher than the tissue concentration of As.
Arsenic distribution by organ in control trout (white bars) and trout exposed to arsenic (black bars). All data are presented as the mean (± SD) of fifteen fish samples (n = 15). Data were analyzed using ANOVA and Fisher’s LSD tests (P < 0.05). Different uppercase letters indicate significant differences between the two trout groups for the same organ
Biometric indexes
Just like energy metabolism, the study of condition indices allows us to assess the general physiological status of an individual. The HSI highlights the importance of the liver relative to the carcass weight of an individual. In the Bravona River, more female trout were caught: 10 compared to 8 in the Presa River. CF and HSI indexes were relatively similar for both sites (Table 2). The HSI was somewhat more important in the Presa River.
Biochemical analyses
Changes in MDA content
Significant differences between exposed trout and their controls were observed in all organs except gonads (Fig. 3). The kidneys had the highest MDA level. An increase of 53 and 30% was observed in liver and kidney of exposed trout, respectively, compared to the controls. A slight increase was also observed in muscles and fins of exposed trout compared to their controls, while MDA level in control gills were higher than in exposed trout.
Changes in malondialdehyde (MDA) content in different organs of control trout (white bars) and trout exposed to arsenic (black bars). All data are presented as the mean (±SD) of fifteen fish samples (n = 15). Data were analyzed using ANOVA and Fisher’s LSD tests (P < 0.05). Different uppercase letters indicate significant differences between the two trout groups for the same organ. Different lowercase letters indicate significant differences between organs within one trout group
Changes in antioxidant enzymatic activities
Chronic As exposure significantly affected the SOD, CAT, GPx and GST activities in exposed trout compared to control trout.
SOD activity in all organs, except muscle and fins, was significantly greater in exposed trout compared to their controls (Fig. 4a). The liver and kidney of exposed trout had the highest SOD activities. A significant increase of 53 and 26% was observed in the kidney and liver of trout contaminated by As, respectively.
Changes in a superoxide dismutase (SOD) and b catalase (CAT) specific activity in organs of control trout (white bars) and trout exposed to arsenic (black bars). All data are presented as the mean (±SD) of fifteen fish samples (n = 15). CAT activity was not detected in fins. Data were analyzed using ANOVA and Fisher’s LSD tests (P < 0.05). Different uppercase letters indicated significant differences between the two trout groups for the same organ. Different lowercase letters indicate significant differences between organs within one trout group
CAT activity differed between control and As exposed trout. Significant differences were observed in liver, kidney and gonads (Fig. 4b). Highest CAT activities were found in the liver and kidney of exposed trout. CAT activity was 4.5-fold higher in liver when compared to the control. In the kidney of exposed trout, an increase of 28% was observed compared to the control trout. No CAT activity was detected in the fins.
GST activity was significantly higher in the liver and kidney of exposed trout compared to their controls (Fig. 5a). A significant increase was also observed in muscle and gills of exposed trout; however, the values were lower than those of the liver and kidney. No significant change was observed in the fins and gonads. The liver of exposed trout had the highest GST activity. Liver and kidney exhibited the largest increases (3.6-fold and 53%, respectively).
Changes in a glutathione S-transferase (GST) and b glutathione peroxidase (GPx) activity in different organs of control trout (white bars) and trout exposed to arsenic (black bars). All data are represented as the mean (±SD) of fifteen fish samples (n = 15). GPx activity was not detected in fins. Data were analyzed using ANOVA and Fisher’s LSD tests (P < 0.05). Different uppercase letters indicate significant differences between the two trout groups for the same organ. Different lowercase letters indicate significant differences between organs within one trout group
GPx activity was significantly increased in the liver and gonads of exposed trout compared to their controls (Fig. 5b). The highest GPx activities were found in the kidney and liver; however no significant difference was observed between exposed trout and the controls in the kidney tissues. The values were 2.2-fold higher in the liver of exposed trout than in control trout. No significant increase was found in the other organs of exposed trout compared to their controls. No GPx activity was detected in the fins.
Discussion
Biometric indexes
Biometric indexes reflect the condition of captured populations. In fact, a higher HSI gives a good interpretation of water contamination. For the same length, thinner is often considered as having a worse physiological condition than bigger (Froese 2006). The relative index homogeneity of both exposed and control sites can be translated as having the same physiological conditions between studied groups. The HSI was somewhat higher in the Presa River, evidence of slightly higher detoxification activity in the liver of exposed trout. This index can vary throughout a year due to temperature, diet and other abiotic factors. Consequently, biometric indexes alone are unable to translate a degree of As pollution in water streams; they have to be cumulated with more extensive analyses to determine tissue concentrations. Allen et al. (2004a) suggest that chromatophores could be also used as a quick biomarker of As exposure. Sex seems to have no influence on As accumulation in tissue, excepted for gonads, as females have a higher concentration (1030 µg/kg on average) than males (326 µg/kg on average).
Arsenic concentration
Water from the Bravona River is in the range of accepted values (<5 µg/L) for the control sites for analyzed elements, according to the World Health Organization (WHO) and European Directive (98/83/CE). Water from the Presa River is considered as highly polluted (2281.66 µg/L) and greatly exceeds the maximal acceptable As concentrations for drinking water supply (10 µg/L). In fish tissues, variations in metalloid content may have different causes. In addition to metal pollution, there are endogenous parameters that influence metal content (sex, length, age, etc.) (Linde et al. 1999). Experimental As exposure has been studied with several fish species, e.g. Clarias batrachus (Bhattacharya and Bhattacharya 2007), Ictalurus punctatus (Schlenk et al. 1997) and Gambusia holbrooki (Tuulaikhuu et al. 2016). In all studied species, As has accumulated in tissues. Nevertheless, some fish accumulate more As than others: Mora et al. (2001) compared As concentration between species and found it to be higher in Oreochromis aurea than in Ictioobus bubalus or in Cichlasoma cyanoguttatum (Mora et al. 2001). Many in vivo studies show bioaccumulation of As in freshwater fish (Maeda et al. 1990; Spehar et al. 1980; Tariq et al. 1993; Winger et al. 1990) but only a few studies describe the As distribution in different tissues (Calendini 2000; Culioli et al. 2009). As organotropism in S. trutta was kidney > liver > gill > gonad > muscle > fin. The kidney and liver seem to be central organs for bioaccumulation of heavy metals and metalloids (De Jonge et al. 2014; Ghosh et al. 2007), especially for S. trutta (Lamas et al. 2007; Linde et al. 1999). Bioaccumulation improves the accuracy of trace pollutant analysis close to the detection limits compared to direct water analysis (Rainbow and Phillips 1993).
It has been observed that As accumulates primarily in the retina, liver and kidney of fish species (Takatsu et al. 1999). In lake whitefish, reduction of As content in the liver and kidney was found after 30 days of As diet (Pedlar and Klaverkamp 2002), evidence of a time-dependent alteration. Consumption of fish exposed to As could result in As exposure to humans and lead to adverse health effects. Kar et al. (2011) have suggested that inhabitants exposed by means of tilapia and shrimp consumption growing in aquaculture using As groundwater are being subject to a moderately high As exposure. Many in vitro studies provide proof of the harmful effect of As compounds on fish tissues (Ananth et al. 2014; Guardiola et al. 2013; Moeller et al. 2003; Schlenk et al. 1997). Dabrowski (1976) has shown that sodium arsenate or As trioxide solution have a negative effect on the survival rate of rainbow trout embryos. Lastly, As is recognized as a source of disturbance during spermatogenesis of some fish (Shukla and Pandey 1984). This metalloid also induces cellular alteration, which are clearly obvious in a histological study of the kidney and spleen of Clarias batrachus after As exposure (Ghosh et al. 2006). Besides cellular alteration, As exposure can induce degenerative changes and necrosis in tissues, and affects embryonal development (Salmo gairdneri) (Dabrowski 1976). Muscle, gonads and fins have lower As concentrations. Nevertheless, these tissues are also hazardous because these parts of the fish are consumed by humans. Due to the high hepatotoxicity of As, such concentrations in blood clearing organs can lead to the development of cancer. Kar et al. (2011) calculated a hazard quotient (HQ) that reflects the increase or decrease of cancer risk, relative to human intake of aquaculture fish exposed to As. The mean cancer risk associated with the ingestion of Oreochromis mossambicus exposed to lower As concentrations than those we found in the Presa River is 200 times greater than the “normal” risk.
Despite the numerous studies on pollutant exposure in several fish species, only a few studies have been carried out to investigate the mechanism of oxidative stress induced by chronic As exposure on the natural distribution area of fish.
Arsenic contamination and lipid peroxidation induction
Lipid peroxidation is one of the first mechanisms of cell damage by xenobiotics (Recknagel and Glende 2011). Lipid peroxidation is involved in altering membrane fluidity and compromising membrane integrity (Milinkovitch et al. 2013). Malondialdehyde (MDA), one of the breakdown product of lipid peroxidation, is considered as a useful indicator of oxidative damage because of the susceptibility of membranes to be targeted by ROS (Ohkawa et al. 1979). Arsenic is known to induce lipid peroxidation in animals and cellular models (Bhattacharya and Bhattacharya 2007). In our study, a significant increase in MDA between exposed and control trout was observed in all organs except gonads. The highest MDA content was found in the kidney and liver, indicating oxidative damage by As, such as lipid peroxidation of biological membranes. When compared to As content, the highest values were also found in the liver and kidney. Enhanced MDA levels were observed in muscles and fins, which also accumulate high As concentrations. Dautremepuits et al. (2009) suggested that oxidative damage is related to exposure duration and specific bioaccumulation in tissues. Indeed, different behaviors have been observed in different organs, especially between liver and kidney and other organs. Arsenic accumulates more in the liver, because it is actively biomethylated in this tissue (Roy and Bhattacharya 2006). It has been showed that the liver is the main bioconversion tissue for organic xenobiotics in aquatic organisms (Livingston et al. 1994). For example, hepatomegaly and hepatocyte membrane damage have been found in C. batrachus after 14 days of arsenic exposure (0.50 µM) (Datta et al. 2007). Furthermore, the significant accumulation of As in kidney is the result of its ability to detoxify xenobiotics like liver tissue (Vinodhini and Narayanan 2008). Dopp et al. (2004) showed the kidneys play a major role in biomethylation of arsenic and excretion of arsenic compounds, which can explain the greater accumulation of arsenic in this organ during chronic exposure. Our study’s findings are in agreement with Bhattacharya and Bhattacharya (2007) who reported elevated lipid peroxidation in Indian catfish exposed to 8.4 mg/L arsenic after 7 days of treatment. Sarkar et al. (2014) reported a significant MDA increase in zebrafish with up to 15 days of As trioxide exposure (50 µg/L). Elevated MDA levels in the present study may indicate oxidative stress induced by ROS production, such as hydroxyl radical mainly involved in lipid peroxidation (Schaich 1992).
Antioxidant enzymatic defenses and arsenic contamination
The accumulation of metalloids such as As may lead to the overproduction of reactive oxygen species (ROS) causing oxidative stress (Farombi et al. 2007). Antioxidant enzymatic defenses like SOD, CAT, GPx and GST play a major role in protecting tissues from oxidative stress (Valavanidis et al. 2006). SOD is the first defense mechanism against oxygen toxicity; it catalyzes dismutation of the superoxide anion (Winston and Digiulio 1991). In this study, SOD activity increased significantly in all organs of trout contaminated by As, except muscle and fins. The highest SOD activity was found in the kidney and liver, although the greatest increment was observed only in kidney. SOD activity seems to be consistent with As accumulation in liver and kidney suggesting that As induces production of the superoxide radical. Kim and Kang (2015) found a significant increase of SOD activity in the liver and gill of rockfish (Sebastes schlegelii) exposed to 200 µg/L of sodium arsenite for 20 days, like Bhattacharya and Bhattacharya (2007). Thus, under environmental conditions, trout antioxidant responses are clearly related to chronic arsenic exposure.
CAT and GPx are both involved in H2O2 removal in the peroxisome and cytosol, respectively. H2O2 can be used as precursor of radical hydroxyl production, mainly involved in lipid peroxidation (Digiulio et al. 1989). Our results showed a significant increase in CAT and GPx activities in the liver of exposed trout while in the kidney, only CAT activity was increased. Tuulaikhuu et al. (2016) suggest that CAT activity was related to As toxicity, specifically in the liver. The high CAT and GPx activity are consistent with SOD activity, which highlights the needs to rapidly eliminate H2O2 produced by SOD in order to prevent an accumulation in trout tissues. In addition, this finding suggests that the antioxidant response to As exposure depends on metal bioaccumulation in tissues. Ventura-Lima et al. (2009) reported no significant changes in CAT and GPx activities in gills of zebrafish exposed to 100 µg As/L for 48 h, whereas Sarkar et al. (2014) found a significant increase in GPx and CAT activities in zebrafish exposed to As trioxide (As2O3). In this study, a significant increase in liver GPx activity was observed concomitantly with an elevation of MDA. The situation was different in kidney where high MDA levels were observed although no significant changes in GPx activity were found. This suggests that the kidney accumulates As and its detoxification mechanism is not effective at limiting oxidative damage in this tissue. Because it functions as a barrier between the external and internal environment, gills constitute the primary target of As toxicity (Pelgrom et al. 1995), which can explain the high MDA content, while low antioxidant enzymatic activities were found in this tissue. The low CAT and GPx activities observed in muscle and gonads can increase the level of hydrogen peroxide and other hydroperoxides, which could cause enhanced oxidative damage.
Glutathione S-transferase (GST) is one of the most important enzymes that protects organisms from oxidative stress (Ercal et al. 2001); it is used as an environmental biomarker (Capkin and Altinok 2013). The present study showed that maximum induction of GST activity was observed in the liver of exposed trout, which shows the protective response of this enzyme to prevent further arsenic-induced oxidative damage (Mardirosian et al. 2015). A slight augmentation was also found in muscle, gills and kidney of exposed trout, while no significant changes were observed in fins and gonads. Kim and Kang (2015) and Tuulaikhuu et al. (2016) reported a considerable increase in GST activity in liver exposed to As from Korean rockfish (Sebastes schlegelii) and Eastern mosquitofish (Gambusia holbrooki), respectively. Some authors reported that As and heavy metals can inhibit GST activity in fish tissues (Atli and Canli 2010; Sarkar et al. 2014) due to their great affinity for protein thiol groups and cellular non-protein thiols such as glutathione. This antioxidant molecule plays an important role in ROS scavenging, in addition to its role as a GPx and GST substrate. Consequently, a decrease in GSH content led to a reduction of GPx and GST activities, which can explain the lower level of GST activity found in exposed muscles, gills, fins and gonads.
Conclusion
The oxidative stress and antioxidant defense response to As present in the natural distribution area of Salmo trutta were studied here. The Presa River contains a high concentration of arsenic (2281.66 µg/L) just downstream the Matra mine. Arsenic (V) is the predominant form in the Presa River. Arsenic organotropism in S. trutta was kidney > liver > gill > fin > gonad > muscle. Biometric indexes of S. trutta caught at both sites reveal a homogeneous population. These indexes coupled with tissue concentrations are evidence of an environmental hazard in the Presa ecosystem. Arsenic bioaccumulation is a major aspect of environment safety because it can affect fish health in the river by altering biochemical functions and inducing tissue damage. Our results suggest that arsenic exposure can induce oxidative damage, reflected by MDA content, and can disrupt the antioxidant enzymatic responses in Salmo trutta organs. These data clearly indicate that the liver and kidney play a key role in arsenic bioaccumulation and oxidative stress response. High levels of antioxidant enzymatic activity were found in these organs, evidence of their detoxification function. However, the kidney seems to be more sensitive than the liver to arsenic exposure. These findings demonstrate the importance of selecting appropriate tissues and sensitive biomarkers for biomonitoring of metal toxicity in an aquatic environment.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Alexander DE (1999) Bioaccumulation, bioconcentration, biomagnification. Springer Netherlands, Dordrecht, pp 43–44
Allen T, Awasthi A, Rana SVS (2004a) Fish chromatophores as biomarkers of arsenic exposure. Env Biol Fish 71:7–11
Allen T, Singhal R, Rana SVS (2004b) Resistance to oxidative stress in a freshwater fish Channa punctatus after exposure to inorganic arsenic. Biol Trace Elem Res 98:63–72
Almeida JA, Diniz YS, Marques SFG, Faine LA, Ribas BO, Burneiko RC, Novelli ELB (2002) The use of the oxidative stress responses as biomarkers in Nile tilapia (Oreochromis niloticus) exposed to in vivo cadmium contamination. Environ Int 27:673–679
Amundsen P-A, Kashulin NA, Terentjev P, Gjelland KO, Koroleva IM, Dauvalter VA, Sandimirov S, Kashulin A, Knudsen R (2011) Heavy metal contents in whitefish (Coregonus lavaretus) along a pollution gradient in a subarctic watercourse. Environ Monit Assess 182:301–316
Ananth S, Mathivanan V, Ganesh Prabu P, Selvisabhanayakam (2014) Studies on the impact of heavy metal Arsenic trioxide on freshwater fish grass carp, Ctenopharyngodon idella in relation to enzyme activity of succinate dehydrogenase. Int J Toxicol Appl Pharmacol 4:1–5
Atli G, Canli M (2010) Response of antioxidant system of freshwater fish Oreochromis niloticus to acute and chronic metal (Cd, Cu, Cr, Zn, Fe) exposures. Ecotoxicol Environ Saf 73:1884–1889
Bhattacharya A, Bhattacharya S (2007) Induction of oxidative stress by arsenic in Clarias batrachus: involvement of peroxisomes. Ecotoxicol Environ Saf 66:178–187
Biswas S, Prabhu RK, Hussain KJ, Selvanayagam M, Satpathy KK (2011) Heavy metals concentration in edible fishes from coastal region of Kalpakkam, southeastern part of India. Environ Monit Assess 184:5097–5104
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Calendini S (2000) Impact of an old arsenic mine on chemical and biological components of a Corsican freshwater system. Thèse de 3ème cycle. Université de Corse, Corte, p 188
Capkin E, Altinok I (2013) Effects of chronic carbosulfan exposure on liver antioxidant enzyme activities in rainbow trout. Environ Toxicol Pharmacol 36:80–87
Cockell KA, Hilton JW (1988) Preliminary investigations on the comparative chronic toxicity of 4 dietary arsenicals to juvenile rainbow-trout (Salmo gairdneri R). Aquat Toxicol 12:73–82
Culioli J-L, Calendini S, Mori C, Orsini A (2009) Arsenic accumulation in a freshwater fish living in a contaminated river of Corsica, France. Ecotoxicol Environ Saf 72:1440–1445
Dabrowski KR (1976) The effect of arsenic on embrional development of rainbow trout (Salmo gairdneri, RICH.). Water Res 10:793–796
Datta S, Saha DR, Ghosh D, Majumdar T, Bhattacharya S, Mazumder S (2007) Sub-lethal concentration of arsenic interferes with the proliferation of hepatocytes and induces in vivo apoptosis in Clarias batrachus L. Comp Biochem Physiol C Toxicol Pharmacol 145:339–349
Dautremepuits C, Marcogliese DJ, Gendron AD, Fournier M (2009) Gill and head kidney antioxidant processes and innate immune system responses of yellow perch (Perca flavescens) exposed to different contaminants in the St. Lawrence River, Canada. Sci Total Environ 407:1055–1064
De Jonge M, Belpaire C, Van Thuyne G, Breine J, Bervoets L (2014) Temporal distribution of accumulated metal mixtures in two feral fish species and the relation with condition metrics and community structure. Environ Pollut 197:43–54
Digiulio RT, Washburn PC, Wenning RJ, Winston GW, Jewell CS (1989) Biochemical responses in aquatic animals - a review of determinants of oxidative stress. Environ Toxicol Chem 8:1103–1123
Dopp E, Hartmann LM, Florea AM, von Recklinghausen U, Pieper R, Shokouhi B, Rettenmeier AW, Hirner AV, Obe G (2004) Uptake of inorganic derivatives of arsenic associated with induced cytotoxic and genotoxic effects in Chinese hamster ovary (CHO) cells. Toxicol Appl Pharmacol 201:156–165
Druwe IL, Vaillancourt RR (2010) Influence of arsenate and arsenite on signal transduction pathways: an update. Arch Toxicol 84:585–596
Dupuy C, Calves I, Pedron N, Franca S, Vasconcelos RP, Auffret M, Quiniou L, Laroche J (2012) Cellular and molecular responses of the European flounder (Platichthys flesus) to the effects of climate change, hypoxia and chemical stress, in several estuaries from France to Portugal. Comp Biochem Physiol A Mol Integr Physiol 163:S5
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 6:529–39
Farombi EO, Adelowo OA, Ajimoko YR (2007) Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun River. Int J Environ Res Publ Health 4:158–65
Flohe L, Gunzler WA, Schock HH (1973) Glutathione Peroxidase - Selenoenzyme. Febs Lett 105:132–134
Foata J, Quilichini Y, Torres J, Pereira E, Spella MM, Mattei J, Marchand B (2009) Comparison of arsenic and antimony contents in tissues and organs of brown trout caught from the river Presa Polluted by ancient mining practices and from the river Bravona in Corsica (France): a survey study. Arch Environ Contam Toxicol 57:581–589
Froese R (2006) Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J Appl Ichthyol 22:241–253
Galland C, Dupuy C, Quiniou L, Auffret M, Laroche J, Pichereau V (2012) Response of the European flounder Platichthys flesus to experimental and in situ contaminations: a proteomic approach. Comp Biochem Physiol A Mol Integr Physiol 163:S49
Ghosh D, Bhattacharya S, Mazumder S (2006) Perturbations in the catfish immune responses by arsenic: organ and cell specific effects. Comp Biochem Physiol C Toxicol Pharmacol 71:455–463
Ghosh D, Datta S, Bhattacharya S, Mazumder S (2007) Long-term exposure to arsenic affects head kidney and impairs humoral immune responses of Clarias batrachus. Aquat Toxicol 81:79–89
Guardiola FA, Gonzalez-Parraga MP, Cuesta A, Meseguer J, Martinez S, Martinez-Sanchez MJ, Perez-Sirvent C, Esteban MA (2013) Immunotoxicological effects of inorganic arsenic on gilthead seabream (Sparus aurata L.). Aquat Toxicol 134:112–119
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Jain CK, Ali I (2000) Arsenic: occurrence, toxicity and speciation techniques. Water Res 34:4304–4312
Kar S, Maity JP, Jean J-S, Liu C-C, Liu C-W, Bundschuh J, Lu H-Y (2011) Health risks for human intake of aquacultural fish: arsenic bioaccumulation and contamination. J Environ Sci Health A Tox Hazard Subst Environ Eng 46:1266–1273
Kim J-H, Kang J-C (2015) The arsenic accumulation and its effect on oxidative stress responses in juvenile rockfish, Sebastes schlegelii, exposed to waterborne arsenic (As3+). Environ Toxicol Pharmacol 39:668–676
Lamas S, Fernandez JA, Aboal JR, Carballeira A (2007) Testing the use of juvenile Salmo trutta L. as biomonitors of heavy metal pollution in freshwater. Chemosphere 67:221–228
Linde AR, Sanchez-Galan S, Klein D, Garcia-Vazquez E, Summer KH (1999) Metallothionein and heavy metals in brown trout (Salmo trutta) and European eel (Anguilla anguilla): a comparative study. Ecotoxicol Environ Saf 44:168–173
Livingston DR, Förlin L, George SG (1994) Molecular biomarkers and toxic consequences of impact by organic pollution in aquatic organisms. In: Sutcliffe, DW (ed) Water quality & stress indicators in marine and freshwater systems: linking levels of organisation. Ambleside, UK, Freshwater Biological Association, p 154–171
Maeda S, Inoue R, Kozono T, Tokuda T, Ohki A, Takeshita T (1990) Arsenic metabolism in a freshwater food chain. Chemosphere 20:101–108
Mardirosian MN, Lascano CI, Ferrari A, Bongiovanni GA, Venturino A (2015) Acute toxicity of arsenic and oxidative stress responses in the embryonic development of the common South American toad Rhinella arenarum. Environ Toxicol Chem 34:1009–1014
Milinkovitch T, Imbert N, Sanchez W, Le Floch S, Thomas-Guyon H (2013) Toxicological effects of crude oil and oil dispersant: biomarkers in the heart of the juvenile golden grey mullet (Liza aurata). Ecotoxicol Environ Saf 88:1–8
Moeller A, MacNeil SD, Ambrose RF, Hee SSQ (2003) Elements in fish of Malibu Creek and Malibu Lagoon near Los Angeles, California. Mar Pollut Bull 46:424–429
Mora MA, Papoulias D, Nava I, Buckler DR (2001) A comparative assessment of contaminants in fish from four resacas of the Texas, USA-Tamaulipas, Mexico border region. Environ Int 27:15–20
Mori C, Orsini A, Migon C (1999) Impact of arsenic and antimony contamination on benthic invertebrates in a minor Corsican river. Hydrobiologia 392:73–80
Munoz-Olivas R, Camara C (2001) Speciation related to human health. In: Ebdon, L (ed) Trace element speciation for environment, food and health. Royal Society of chemistry, Great Britain, pp 331–353
Oberley LW, Spitz DR (1984) Assay of superoxide dismutase activity in tumor-tissue. Methods Enzymol 105:457–464
Ohkawa H, Ohishi N, Yagi K (1979) Assay for Lipid Peroxides in animal-tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pedlar RM, Klaverkamp JF (2002) Accumulation and distribution of dietary arsenic in lake whitefish (Coregonus clupeaformis). Aquat Toxicol 57:153–166
Pelgrom S, Lamers LPM, Lock RAC, Balm PHM, Bonga SEW (1995) Interactions between Copper and Cadmium modify metal organ distribution in mature tilapia, Oreochromis mossambicus. Environ Pollut 90:415–423
Rainbow PS, Phillips DJH (1993) Cosmopolitan biomonitors of trace metals. Mar Pollut Bull 26:593–601
Recknagel RO, Glende EA (2011) Lipid Peroxidation: A Specific form of Cellular Injury. Comprehensive Physiology. pp 591–601. http://www.comprehensivephysiology.com/WileyCDA/CompPhysArticle/refId-cp090138.html
Roche B, Mattei J (1997) The animal species introduced into the Corsican freshwaters. Bull Fr Peche Piscicult 344-45:233–239
Roy S, Bhattacharya S (2006) Arsenic-induced histopathology and synthesis of stress proteins in liver and kidney of Channa punctatus. Ecotoxicol Environ Saf 65:218–229
Sarkar S, Mukherjee S, Chattopadhyay A, Bhattacharya S (2014) Low dose of arsenic trioxide triggers oxidative stress in zebrafish brain: expression of antioxidant genes. Ecotoxicol Environ Saf 107:1–8
Schaich KM (1992) Metals and lipid oxidation - contemporary-issues. Lipids 27:209–218
Schlenk D, Wolford L, Chelius M, Steevens J, Chan KM (1997) Effect of arsenite, arsenate, and the herbicide monosodium methyl arsonate (MSMA) on hepatic metallothionein expression and lipid peroxidation in channel catfish. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 118:177–183
Shukla JP, Pandey K (1984) Impaired spermatogenesis in arsenic treated fresh-water fish, Colisa fasciatus (Bl and Sch). Toxicol Lett 21:191–195
Spehar RL, Fiandt JT, Anderson RL, Defoe DL (1980) Comparative toxicity of arsenic compounds and their accumulation in invertebrates and fish. Arch Environ Contam Toxicol 9:53–63
Takatsu A, Kuroiwa T, Uchiumi A (1999) Arsenic accumulation in organs of the fresh water fish Tribolodon hakonensis. J Trace Elem Med Biol 13:176–179
Tariq J, Ashraf M, Jaffar M (1993) Metal pollution status of the river chennab, pakistan through fish, water and sediment analysis. Toxicol Environ Chem 38:175–181
Townsend DM, Tew KD (2003) The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 22:7369–7375
Tuulaikhuu B-A, Bonet B, Guasch H (2016) Effects of low arsenic concentration exposure on freshwater fish in the presence of fluvial biofilms. Sci Total Environ 544:467–475
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64:178–189
Ventura-Lima J, de Castro MR, Acosta D, Fattorini D, Regoli F, de Carvalho LM, Bohrer D, Geracitano LA, Barros DM, Marins LFF, Souza da Silva R, Bonan CD, Bogo MR, Monserrat JM (2009) Effects of arsenic (As) exposure on the antioxidant status of gills of the zebrafish Danio rerio (Cyprinidae). Comp Biochem Physiol C Toxicol Pharmacol 149:538–543
Vinodhini R, Narayanan M (2008) Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (Common carp). Int J Environ Sci Technol 5:179–182
Winger PV, Schultz DP, Johnson WW (1990) Environmental contaminant concentrations in biota from the Lower Savannah river, Georgia and South-Carolina. Arch Environ Contam Toxicol 19:101–117
Winston GW, Digiulio RT (1991) Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol 19:137–161
Acknowledgements
We thank the Collectivité Territoriale de Corse (CTC) for the financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Samuel Greani and Radia Lourkisti contributed equally to this work.
Jérémie Santini and Yann Quilichini contributed equally to this work.
Rights and permissions
About this article
Cite this article
Greani, S., Lourkisti, R., Berti, L. et al. Effect of chronic arsenic exposure under environmental conditions on bioaccumulation, oxidative stress, and antioxidant enzymatic defenses in wild trout Salmo trutta (Pisces, Teleostei). Ecotoxicology 26, 930–941 (2017). https://doi.org/10.1007/s10646-017-1822-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-017-1822-3