Abstract
The feeding activity and subsequent assimilation of the products resulting from food digestion allow organisms to obtain energy for growth, maintenance and reproduction. Among these biological parameters, we studied digestive enzymes (amylase, cellulase and trypsin) in Gammarus fossarum to assess the impact of contaminants on their access to energy resources. However, to enable objective assessment of a toxic effect of decreased water quality on an organisms’ digestive capacity, it is necessary to establish reference values based on its natural variability as a function of changing biotic and abiotic factors. To limit the confounding influence of biotic factors, a caging approach with calibrated male organisms from the same population was used. This study applied an in situ deployment at 23 sites of the Rhone basin rivers, complemented by a laboratory experiment assessing the influence of two abiotic factors (temperature and conductivity). The results showed a small effect of conductivity on cellulase activity and a significant effect of temperature on digestive enzyme activity but only at the lowest temperature (7 °C). The experimental conditions allowed us to define an environmental reference value for digestive enzyme activities to select sites where the quality of the water impacted the digestive capacity of the organisms. In addition to the feeding rate, this study showed the relevance of digestive enzymes as biomarkers to be used as an early warning tool to reflect organisms’ health and the chemical quality of aquatic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A great diversity of chemical compounds has been introduced into aquatic ecosystems. These contaminants can have toxic effects on the biological components of ecosystems (Chapman 2007; Chapman et al. 2003). One of the challenges of aquatic ecotoxicology is to develop tools and indicators to assess the chemical pollution of aquatic systems as well as to determine or predict the effects or suspected effects on living organisms (Hagger et al. 2006). In this context, multi-level studies are needed to link toxic effects obtained at biochemical and cellular levels with impacts observed on populations and communities. Many biological parameters called biomarkers have been developed to monitor the quality of aquatic systems. However, the responses of these biomarkers can be influenced by a number of confounding factors, and consequently, their variation may not directly reflect an impact caused by contamination. This requires that the natural variability of these biomarkers to be known and quantified in relation to biotic and abiotic factors so that their levels are reliably interpreted in terms of contamination or toxicity level of aquatic systems (Geffard et al. 2007; Xuereb et al. 2009).
Among freshwater species, crustacean amphipods are often used as sentinel species in aquatic ecotoxicological studies (Coulaud et al. 2011; Dedourge-Geffard et al. 2009; Lacaze et al. 2011) because their sensitivity towards many contaminants is relatively well known (Bundschuh et al. 2011). The genus Gammarus has a key role in the food web as common shredders, which play a major role in leaf litter breakdown, and reductions in its feeding activity were directly correlated with reductions in the processing of leaf litter (Forrow and Maltby 2000). Furthermore, in Gammarus sp., the ingestion of leaves leads to the assimilation of energy and the production of faecal pellets. Gammarids and their faeces represent a food resource for many aquatic species. In Central and Eastern Europe, Gammarus fossarum is widespread and common, and this species has been defined as a very good candidate to study the impact of chemical stressors on processes used to obtain energy and on overall metabolism.
The acquisition of energy for growth, maintenance and reproduction depends on food availability, ingestion and assimilation (De Coen and Janssen 1998). The study of biological responses, including the feeding rate, digestive enzyme activities and energy storage, constitutes a relevant approach for identifying stressed organisms, especially those exposed to contaminants (Coulaud et al. 2011; Dedourge-Geffard et al. 2009; Gismondi et al. 2012; Sroda and Cossu-Leguille 2011). In the same way, energy metabolism impairments appear to be good predictive markers of disturbances observed at higher biological levels, including reproduction and growth (Hyne and Maher 2003). Consequently, under chemical stress, organisms’ energy metabolism could be modified by (1) initiating defence mechanisms and/or (2) toxic effects on the feeding rate and food digestion. Digestive enzyme activities are therefore an early warning tool that can help assessing changes in energy metabolism. Various studies have shown direct effects of contaminants on digestive enzyme activities (Chen and Mayer 1998; Chen et al. 2002). However, some investigators suggested that polluted waters had decreased the quality of added food, inhibiting food intake, ingestion and assimilation of nutrients (Allen et al. 1995; De Schamphelaere et al. 2007; Meyer et al. 2005). In contrast, Douhry and Sayah (2009) demonstrated that the cells responsible for the synthesis of digestive enzymes suffered a cytotoxic effect from pollution. In addition to a toxic insult, various biotic and abiotic factors can also influence the level of these enzymatic responses (Dedourge-Geffard et al. 2012). Therefore, it is essential to characterise the natural variability of the biological responses studied to enable distinguishing the impact of contaminants from that of environmental factors. Concerning the influence of biotic factors on digestive capacity, some studies have highlighted differences in activities according to the species, gender and life stage (Ibarrola et al. 1998; Kuz’mina 1996; Palais et al. 2012). In crustaceans, Johnston et al. (2005) and Johnston and Freeman (2005) showed differences of digestive enzyme activities (cellulase, laminarinase, β-glucosidase, proteases, α-glucosidase, α-amylase) in six species of crabs and also in three species of amphipods (laminarinase, cellobiase, carboxymethyl-cellulase, xylanase, α and β-glucosidase and lipase). Similarly, Perera et al. (2008) found that ontogenic parameters can influence digestive enzyme activities. Thus, it appears important to understand and take into account how the physiological state (age, sex, reproductive stage) of organisms influences the activity levels of these biomarkers. The influence of biotic factors can be limited by using organisms that are as homogenous as possible (Coulaud et al. 2011; Dedourge-Geffard et al. 2009; Lacaze et al. 2011). Among environmental abiotic factors, previous studies emphasised temperature and conductivity as major confounding parameters (Pöckl 1992; Pöckl et al. 2003; Sornom et al. 2010). Maltby et al. (2002, 1990) and Coulaud et al. (2011) observed that temperature strongly influences the feeding rate in G. fossarum, with higher activity in summer than in winter. Consequently, it could be suggested that this parameter modulates digestive enzyme activities as well (Trellu and Ceccaldi 1980b).
The aim of our study was to assess the feasibility and the relevance of various molecular responses related to digestive capacity as potential biomarkers to monitor the quality of aquatic system. The present study focused on three digestive enzymes: (1) amylase, which is responsible for starch hydrolysis, an essential step for shredder feeding; (2) endocellulase, which breaks the crystallin structure of cellulose into polysaccharide fragments; and (3) trypsin, a protease found in the digestive system of many vertebrate and invertebrate organisms in which it cleaves peptide chains primarily at the carboxyl side of certain amino acids. We therefore studied the variability of digestive enzyme activities in G. fossarum transplanted to several sites with different contamination histories. Active biomonitoring based on the use of selected organisms collected from a control population allowed us to limit the impact of biotic factors (age, gender, size and reproductive state). The potential impact of temperature and conductivity as confounding abiotic factors on the above-described enzyme activities was investigated during laboratory experiments.
Materials and methods
Sampling and maintenance of transplanted G. fossarum
Gammarids were collected by kick sampling at La Tour du Pin, upstream of the Bourbre River (France). This station displayed good water quality according to the data records of the RNB (French Watershed Biomonitoring Network), and a high density of gammarids was found. Sexually mature G. fossarum were collected using a handheld net and were sieved (2–2.5 mm). The organisms were kept during a 15-day acclimatisation period in 30-L tanks under constant aeration at 12 ± 0.5 °C using a 10/14 h light/dark photoperiod (Besse et al. 2013). They were continuously supplied with drilled groundwater mixed with soft water (obtained by reverse osmosis) at constant conductivity, 200 or 600 μS cm−1, depending on the conductivity level of the subsequent experimental environment. The organisms were fed ad libitum with alder leaves (Alnus glutinosa) previously conditioned for at least 6 ± 1 days in drilled groundwater. Twice a week, freeze-dried Tubifex sp. worms were added as a dietary supplement (Coulaud et al. 2011).
In situ exposure
Description of sites
For this study, 23 stations were selected on rivers in the Rhône–Alpes region, seeking to cover a wide range of physicochemical characteristics and geographical locations (Fig. 1). All deployments were implemented during one campaign in June 2010. The physicochemical parameters of the water (pH, temperature and conductivity) were analysed by a French accredited chemical analysis laboratory (Laboratoire d’analyses physico-chimiques des milieu aquatiques, IRSTEA, UR Milieux Aquatiques, Ecologie et Pollutions). Measured water temperature ranged mainly between 12 and 18 °C (with one exception of 20.8 °C on site S17), and conductivity fluctuated between 55 and 650 μS cm−1 (Table 1). Temperature was continuously measured using the Tinytag temperature logger Aquatic 2 from Gemini Data Loggers (Chichester, UK).
Location of study sites in the Rhône-Alpes region (France). Non-impacted sites are indicated with white squares while impacted sites are indicated in grey (modified from Coulaud et al. 2011)
During a previous study that included the same 23 sites that were investigated in the present study, Besse et al. (2013) proposed threshold values of bioaccumulation for five metallic compounds and 30 organic compounds including seven pesticides + DDTs in G. fossarum above which the concentration reveals a bioaccumulation of compounds significantly higher than the natural background contamination in this species, tracing thus an anthropogenic source of pollution. Based on the results obtained by Besse et al. (2013), the sites investigated in the present study were organised into two groups according to the number of compounds measured in the caged gammarids with concentrations higher than the threshold values (Table 1). Five sites (R1–R5) were considered as references with concentrations of compounds in caged gammarids less than the threshold values or only slightly higher for only one compound (Pb for R2 and R4; perylene for R5). For the other sites (18 stations, S1–S18), caged gammarid bioaccumulation (Besse et al. 2013) was higher than the threshold values for at least two compounds and maximal for 28 compounds (Table 1). These sites (S1–S18) were considered as impacted by contamination.
Caging procedure
The in situ assay was performed according to the protocol described previously (Lacaze et al. 2011). Briefly, 24 h before initiating the experiment, four replicates of 20 adult male gammarids were caged in polypropylene cylinders (length, 10 cm; diameter, 5.5 cm) capped at the ends with pieces of net (mesh, 1 mm) to guarantee the free circulation of water. The cylinders were protected by a rigid plastic container. Rocks were placed on the top of protective containers in order to weigh them in the river bed. Adult gammarids with an average body length of 10–11 mm were selected so that mature animals of the same age would be exposed. During the tests, gammarids were fed with the same alder leaves as in the laboratory (A. glutinosa). After 15 days of exposure, gammarids and alder leaf discs were collected and carried to the laboratory. Gammarids from the same site were pooled, counted (for survival rate assessment), dried, organised into six pools of three organisms, weighed, flash frozen in liquid nitrogen and stored at −80 °C until the digestive enzyme activity was analysed. The remaining pool of gammarids was used in different studies.
Laboratory exposure
The influence of water temperature and conductivity on digestive enzyme activities was studied by exposing adult male gammarids (10.6 ± 0.7 mm) to three temperatures (7, 12 and 16 °C) and three conductivity levels (200, 500 and 800 μS cm−1). These levels corresponded to the range of physicochemical characteristics usually encountered in streams in the Rhône–Alpes region. Twenty-four hours before initiating the experiment, the gammarids were placed in each water temperature and conductivity treatment (Coulaud et al. 2011). Three replicates of three organisms each were analysed for each of the nine conditions.
During this experiment, conductivity, temperature, pH and dissolved oxygen were monitored daily. We used a flow-through system which consisted of 0.5-L glass beakers filled with continuously renewed water (four renewals per day), a continuous pumping system and a 10/14 h light/dark photoperiod. In each beaker, gammarids were fed with 20 alder leaves. After 20 days of exposure, gammarids were counted (for survival rate assessment), dried on paper, weighed, frozen in liquid nitrogen and stored at −80 °C until biochemical analyses.
Analysis of digestive enzyme activities
Samples were homogenised in Tris–HCl buffer (0.01 M, pH 7) for 2 min using a mixer mill (Retsch) with a frequency of 30 Hz. The homogenate was centrifuged at 10,000×g for 10 min at 4 °C. The supernatant was stored at −80 °C until enzyme activity analysis.
The cellulase and amylase activities were measured according to the protocol adapted from Palais et al. (2010) with the artificial substrates carboxymethyl-cellulose (2 %) and starch (1 %), respectively. Trypsin activity was determined according to the protocol described by Garcia-Carreño and Haard (1993), using N-benzoyl-dl-arginine 4-nitroanilide hydrochloride (BAPNA, 3 mM) as the substrate. Enzyme activities were expressed in micrograms of the final product released per minute and per milligram of protein. The protein content in the supernatant was determined according to Bradford (1976) using bovine serum albumin (BSA) as the protein standard.
Statistics
Statistical analyses were performed with R software (R Development Core Team 2008). For in situ experiments, the normality and homogeneity of data variance for digestive activities in the reference stations were first checked using the Shapiro-Wilk test and the Levene test, respectively. Then, following the methodology reported in Coulaud et al. (2011), a reference distribution of in situ activity levels based on the measurements in selected reference stations was built with the Mass R-package to compute p values associated with the mean activities measured at the different stations of the study and test their conformity to this reference distribution. Homoscedasticity of measurements between sites occurred for the three enzymes even both under reference and contaminated conditions. Thus, conformity to the reference distribution was checked by comparing the mean value of the measurements obtained at a site against the confidence threshold defined for a mean of the same number of replicates.
For laboratory experiments, the normality and homogeneity of data variance for digestive activities were evaluated using the Shapiro-Wilk test and the Levene test, respectively. To determine the temperature and conductivity effects on digestive enzyme activities, an analysis of variance (ANOVA) was chosen. Then, Tukey’s HSD post hoc test was used to identify differences between groups of exposed G. fossarum. A value of p < 0.05 was considered statistically significant.
Results
Large-scale in situ caging study
During the 2 weeks of caging, water temperature and conductivity ranged between 11.6 °C and 20.8 °C and between 55 and 650 μS cm−1, respectively (Table 1).
The survival percentages of caged gammarids (Table 1) ranged between 86.2 % and 93.7 % for the reference sites (R1–R5) and between 60 % and 94.1 % for suspected contaminated stations (S1–S18).
For the three enzymes, ANOVA tests showed that no significant differences existed in the levels of enzymatic activities among the five reference stations (Fig. 2). Therefore, all replicate measurements were pooled to fit a normal distribution, defining by this means the variability of values expected under reference conditions and allowing for testing of deviations from this range in the measurements obtained for a given station.
Digestive enzyme activities of amylase (a), cellulase (b) and trypsin (c) for all sites studied (in grey areas: reference stations; in white areas: compared stations). Each boxplot represents a deployment (diamond: site mean, squares: interquartile, black dot: outlier, segments: range). The solid and dotted lines represent the mean and the first species risk at 1 % evaluated within reference stations. Deployments with a significant deviation of enzyme activity values from the reference distribution are marked with stars
The mean amylase activity (Fig. 2a) in gammarids in the reference group (R1–R5 sites) was 827 (±138) μg maltose/mg BSA/min. With a p value set at 0.01, only stations with a mean value below 696 μg maltose/mg BSA/min were considered to have significantly lower activity levels than the reference group. The threshold of p < 0.01 was selected because the variability within reference measurements, combined with the number of replicates, led to a p value lower than 0.05 for the reference station R5. We therefore decided to be more conservative with the risk of decreasing our ability to detect significant inhibitions in contaminated stations. Nevertheless, we calculate that for six replicates, an inhibition beyond only 16 % will be detected as significant. With this constraint, at seven sites (S1, S2, S3, S4, S5, S6 and S9), the gammarids had an average amylase activity that was not significantly different from gammarids exposed to the reference sites. On the contrary, gammarids exposed to the 11 other sites (S7, S8, S10, S11, S12, S13, S14, S15, S16, S17 and S18) presented a significant inhibition of amylase activity when compared with the reference group.
Cellulase activity (Fig. 2b) of gammarids at the reference stations showed a mean value of 58.6 (±4.72) μg maltose/mg BSA/min. With a p value set at 0.01, cellulase activity was considered as inhibited for values below 54.1 μg maltose/mg BSA/min. Under this condition, only station S7 showed a significant inhibition (8 %).
Finally, for trypsin activity (Fig. 2c), the reference mean value was 7 (±1.88) μg p-Na/mg BSA/min (p-Na corresponding to p-nitroaniline, the product of the reaction). With a p value set at 0.01, trypsin activity could be considered significantly inhibited for values below 5.23 μg p-Na/mg BSA/min (corresponding to ≥25 % inhibition). Under these conditions, gammarids exposed at nine sites (S4, S7, S10, S11, S12, S14, S15, S16 and S18) showed a significant decrease in the activity of this enzyme. An inhibition of trypsin activity of 25 % or more compared with the reference group was significant; this high value, compared with those of the other enzyme activities, was related to the high variability of this enzyme activity in control gammarids.
The amylase/trypsin (A/T) ratio reflects a potential change in the relative proportion of protein or carbohydrate in the animal’s diet. Under the conditions tested here, except for S8, the A/T ratio was constant for all sites considered (not shown). This is in accord with the standardisation of food given to the gammarids during the different experiments.
For an easier comparison of the data (digestive enzyme inhibition) with the results of feeding rate inhibition (FI) obtained by Coulaud et al. (2011) during the same campaign (June 2010), an inhibition rate of digestive enzyme activities (IDEA) was calculated for each deployment and for each digestive enzyme using the following equation (Table 2):
where DEAref is the mean digestive enzyme activity in gammarids exposed to reference sites (R1 to R5) and DEAsite is the mean digestive enzyme activity in gammarids exposed to each suspected contaminated stations (S1 to S18). Inhibition of digestive enzyme activities was observed for amylase and trypsin but not for cellulase (except for site S7; Fig. 2). Concerning amylase and trypsin, in organisms exposed to the suspected contaminated stations (S1 to S18), the lowest enzyme activity inhibition was approximately 20 %. However, these activity inhibitions reached 54 % and 62 % for amylase and trypsin, respectively, at site S14. Of the 12 sites presenting an inhibition of activity for at least one enzyme, eight sites were similar for amylase and trypsin.
Laboratory experiment: influence of water conductivity and temperature on enzyme activities
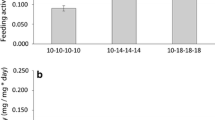
Amylase activity was not significantly influenced by water conductivity (p > 0.05, data not shown). So to define the effect of temperature on enzyme activities, gammarid samples from all water conductivity conditions tested (200, 500 and 800 μS cm−1) were pooled. No effect of conductivity was observed on enzymes activities, consequently, in order to test the effect of temperature on enzymes activities, for each temperature, we used the mean of values obtained for all conductivity conditions. Significantly lower amylase activity was recorded in gammarids maintained at 7 °C compared with those maintained at 12 °C or 16 °C, (p = 0.013 for 12 °C and p = 0.029 for 16 °C) (Fig. 3). However, no significant variation of amylase activity was detected between gammarids exposed at 12 °C or 16 °C. At 7 °C, the level of enzyme activities was reduced by 21 % compared with the level observed for 12 °C and 16 °C.
For cellulase activity, ANOVA analysis indicated a strong temperature effect (p < 0.001) and a weak conductivity effect (p < 0.05) with no significant interactions (Fig. 4). For each conductivity tested, significantly lower cellulase activities were observed at 7 °C than at 12 and 16 °C (Tukey post hoc test; p < 0.0001) except in the case of 500 μS cm−1 at 12 °C. In addition, cellulase activity significantly increased (p < 0.05) with conductivity but only for gammarids exposed at 7 °C (Fig. 4).
Cellulase activity measured in male gammarids exposed to three temperatures (7, 12 and 16 °C) and conductivity (200, 500 and 800 μS cm−1). Diamond: mean for each condition, n = 3; squares: interquartile, segments: range. Boxplots with same letter are not statistically different at 95 % level (samples for 200–16 were lost)
For trypsin activity, no significant differences were observed regardless of the temperature or conductivity tested (data not shown).
Discussion
Digestive enzymes in gammarids
According to MacNeil et al. (1997), Gammarus spp. may be mainly shredders in one habitat in one season, collector–gatherers in the same habitat in a different season, mainly predators in a third ecosystem and generalist–detritivores in other habitats. Consequently, gammarids could have a large digestive enzymatic package composed of carbohydrases and proteases to digest these different food sources. However, to our knowledge, few studies have focused on the digestive enzyme activities in Gammarus sp. Amylase activity has been found in Gammarus palustris (Borowsky and Guarna 1989; Guarna and Borowsky 1995). Similarly, endoglucanases and β-glucosidases have been detected in Gammarus lacustris (McGrath and Matthews 2000) and in Gammarus pulex (Chamier and Willoughby 1986). Bärlocher and Porter (1986) have also demonstrated the presence of other cellulase activities such as β-galactosidase and β-glucosidase in Gammarus tigrinus. Dedourge-Geffard et al. (2009) have shown the presence of five digestive enzyme activities, namely amylase, endoglucanase, β-glucosidase, esterase and β-galactosidase in G. fossarum. In accordance with previous studies (Borowsky and Guarna 1989; Dedourge-Geffard et al. 2009), the highest activities observed were for amylase. At the reference stations, amylase activity was 14 and 118 times higher than those measured for cellulase and trypsin, respectively. The activities of digestive enzymes observed during the present study were similar to those obtained in previous in situ studies with G. fossarum (Dedourge-Geffard et al. 2009). Amylase and cellulase activities in reference gammarids were 685 (±153) and 64 (±12) μg maltose/mg BSA/min, respectively, and 6 (±1) μg p-Na/mg BSA/min for trypsin.
Many studies have supported the assumption that enzyme activities are indicative of the most common substrates in the diet. According to Johnston and Freeman (2005), digestive enzyme activities are effective tools for identifying particular components of an animal’s diet. Thus, a high proteolytic activity reflects a diet rich in protein, and a high carbohydrase activity reflects a diet rich in starch or cellulose. Our results are in accord with shredder behaviour of gammarids but are also explained mainly by the feeding conditions applied in these experiments, in which only alder leaves were supplied to the organisms as food.
Digestive enzyme activities in G. fossarum as field biomarkers
Influence of abiotic factors on digestive enzyme activities and definition of in situ reference values
A toxic effect on digestive enzyme activities could suggest a decrease in the animal’s energy access. As a result, digestive enzymes could be interesting biomarkers in animal organisms (De Coen and Janssen 1997a, b). However, to use digestive enzyme activities for assessing water quality, it is necessary to distinguish the impact of environmental factors other than chemical stressors on these responses or to avoid these factors, depending on the methodology applied. In fact, several biotic (reproduction status, phase of life cycle, food quantity) and abiotic factors are known to modulate digestive enzyme activities (Albentosa and Moyano 2008; Ibarrola et al. 2000; Palais et al. 2012; Sanchez-Paz et al. 2006).
The variability of digestive enzyme activities observed for the reference sites was low. These results could be related to our experimental design (transplantation of standard organisms and food). They also showed a low impact of abiotic factors, in particular, conductivity, on these digestive enzyme activities. So the mean digestive enzyme activities measured in organisms exposed to these five control sites could be proposed as reference values for our field study (827 ± 138 μg maltose/mg BSA/min for amylase, 58.6 ± 4.72 μg maltose/mg BSA/min for cellulase and 7 ± 1.9 μg p-Na/mg BSA/min for trypsin; see Fig. 2). In our experimental conditions, threshold values below which the digestive capacity of an organism is significantly impacted could be proposed (696 μg maltose/mg BSA/min for amylase, 54.1 μg maltose/mg BSA/min for cellulase and 5.23 μg p-Na/mg.BSA/min for trypsin; see Fig. 2).
Responses of digestive enzyme activities to water quality
Among the organisms exposed to the 18 suspected contaminated sites, several gammarid groups showed significant changes in amylase and trypsin activities, particularly at sites S7–S18. These changes corresponded to a decrease in enzyme activities that could represent, in comparison to the reference values defined above, a 10–60 % inhibition of activity (IDEA) (Table 2). These inhibitions could potentially have severe consequences for the digestive capacity and energy access of organisms, particularly in the case of amylase for shredder behaviour organisms such as gammarids. Similar patterns were observed for amylase and trypsin activities for all sites studied, whereas no effect on cellulase activity was shown. Many studies have reported an effect on digestive enzyme activities in vertebrate and invertebrate species related to a contamination source (Barfield et al. 2001; Chen et al. 2002; De Coen and Janssen 1997b; Essedaoui et al. 1998; Le Bihan et al. 2004; Li et al. 2008; Mukherjee and Bhattacharya 1977; Reddy and Fingerman 1994; Yan et al. 1996). Under lab condition experiments, in crustaceans, digestive enzyme activities were usually downregulated under contaminated conditions (De Coen and Janssen 1998; De Coen et al. 1998; Lebrun et al. 2012).Under in situ studies, similar results were observed in gammarids. Dedourge-Geffard et al. (2009) have observed inhibitions of digestive enzyme activities linked with very high levels of heavy metal contamination related to mine activities and severe toxic effect (mortality) after 2 weeks of exposure. In the present study, the range of contaminants was greater, including pesticides and organic contaminants. In fact, bioaccumulation of organic compounds (i.e., S7 for pesticides + DDs and S14, S15 for HAPs + PCBs; Besse et al. (2013) and Table 1) was observed at several sites. Complementary to the previous in situ studies investigating the effects of metals, the present study suggested that two types of enzymes (carbohydrases and proteases) were promise tools for identifying contaminated sites.However, further calibration studies are needed in support of using digestive enzymes in biomonitoring programs.
Variability of digestive enzyme activities in G. fossarum under laboratory conditions
During field experiments, differences in abiotic parameter ranges were observed between the reference (temperature, 14.5–15.8 °C; conductivity, 130–345 μS cm−1) and impacted sites (temperature, 11.6–20.8 °C; conductivity, 55–650 μS cm−1). We therefore conducted a laboratory experiment to test a wider range of temperature and conductivity on the digestive enzyme activities under controlled conditions in support of field observations and the discussion of potentially impacted sites. The results showed a small effect of conductivity on digestive enzyme activities (only on cellulase at a low temperature) and a significant effect of temperature (only at the lowest temperature; 7 °C) for both enzymes. These results indicate that conductivity is not a key parameter influencing digestive enzyme activities recorded during the short-term bioassays with G. fossarum described here. In our experiments, the temperatures measured were all above 11.5 °C. Nevertheless, for further experiments in other seasons (autumn or winter), temperature should be taken into account for a reliable interpretation of field data, especially for cold systems with temperatures lower than 12 °C. For these two abiotic parameters, similar patterns were observed for the feeding rate in G. fossarum (Coulaud et al. 2011). These results confirmed the low variability of enzyme activities in organisms from the five reference sites, which had similar temperatures (14.5–15.8 °C). In the same way, given that there was no temperature effect on the enzyme activities at the highest temperatures tested in the laboratory experiment (12 and 16 °C), these additional results supported the hypothesis that the IDEA observed on several impacted sites was principally related to the water quality.
Differences in digestive enzyme activities were observed between organisms from reference sites during the in situ experiments and organisms maintained in the laboratory with a similar temperature (reference sites, 14.5–15.8 °C; laboratory, 12–16 °C). In fact, amylase, trypsin and cellulase activities, respectively, averaged 25 %, 34 % and 15.5 % lower in laboratory experiments than in the in situ study. Several hypotheses could explain these results. The symbiotic microorganisms present in the digestive apparatus of gammarids could differ between the in situ and laboratory conditions. In fact, many studies have considered these interactions in the gammarids’ digestive process (Chamier and Willoughby 1986; Monk 1977). Among natural factors, seasonal variability could influence energy metabolism. Many studies have already shown a seasonal impact on biomarkers including digestive enzymes (Geffard et al. 2007; Palais et al. 2012; Sroda and Cossu-Leguille 2011). In this case, the field study was conducted in June, whereas the laboratory assay was carried out in July, in the same season of the year. Concerning seasonal effects, important parameters were generally linked to temperature or reproductive process (Palais et al. 2012; Geffard et al. 2007). In our conditions, temperatures between experiments were similar (reference sites 14.5–15.8 °C; lab 12–16 °C). Besides, all organisms were male, avoiding effect of reproduction, important in female. In fact, in the field experiments, the gammarids were caged in polypropylene cylinders away from sunlight, whereas organisms exposed in the laboratory experiments were exposed to a day/night cycle (10 h light and 14 h dark). Thus, in our case, the variations observed between laboratory and field measurements were most likely dependent on light intensity. In this way, some studies have focused on variations of digestive enzyme activity in crustacean species (Van Wormhoudt 1977), and some have mentioned the influence of a circadian rhythm (Galgani et al. 1983; Trellu and Ceccaldi 1980a; Van Wormhoudt and Malcoste 1976). Van Wormouth (1977) noted a peak of amylase activity at approximately 21 h (during the dark exposure) for organisms exposed to a day/night cycle. So we can hypothesize that, in our in situ condition, an activation of digestive enzyme activities occurs such as it could happened during the night in organisms exposed to light/dark cycle.
The results of the laboratory experiment suggested that further studies are needed to detail the potential effect of several environmental factors such as light on the digestive capacity of G. fossarum. These results also showed the need to have reference values for the biological responses under environmental conditions (the in situ experiment), to allow comparisons with organisms transplanted to different sites and to assess whether the exposure to a contamination environment induced a toxic effect. In view of using digestive enzyme activities as biomarkers on a very large geographical scale or for several seasons, it seems necessary to assess the range of reference values for these digestive enzymes at more reference sites covering a wider range of temperatures.
Digestive enzyme activities versus individual parameters: feeding rate and mortality
The acquisition of energy is dependent on food availability and two intrinsic organism parameters: feeding rate and digestive capacity. The sensitivity of the feeding rate to chemical stress is well established in gammarids (Coulaud et al. 2011; Maltby et al. 2002). For digestive capacities, fewer data are available, but the present study underlines the influence of contaminant bioaccumulation on this parameter in G. fossarum, in an in situ experiment, and indicates the relevance of these responses. During this in situ experiment, Coulaud et al. (2011) measured the feeding rate of the same organisms so we could legitimately consider the comparison between these two biomarkers and relate the feeding behaviour to the digestive process expressed as IDEA (Table 2). At the nine studied sites where the feeding rate was inhibited, seven also showed an inhibition of at least one digestive enzyme (amylase and/or trypsin). The inhibition of the feeding rate was more pronounced than the inhibition of the digestive enzyme activities, with the percentage inhibition being greater than 50 % for five of the nine sites impacted. In some cases, the feeding rate was the only impacted response (i.e., S2, S3), which could suggest an effect of water quality on the palatability of leaves but not on the organism’s physiology. These results underlined the importance of a multi-marker approach including feeding rate and digestive enzyme activity.
In previous study on digestive enzymes in gammarids (Dedourge-Geffard et al. 2009), we investigated four sites in a river basin known to be highly contaminated by heavy metals originating primarily from acid mine drainage. A strong inhibition of digestive enzyme activities was observed at the most impacted sites in association with high mortality after 7 or 14 days of caging (Dedourge-Geffard et al. 2009). In the present study, based on a larger geographical scale and considering several contamination sources, the highest sensitivity of digestive enzyme responses compared with mortality confirmed the relevance of these measurements to assessing the effect of water quality on the digestive capacity of G. fossarum.
Conclusions
The transplantation of gammarids for 15 days on 23 multi-contaminated stations with a wide range of contamination sources showed the usefulness of the inhibition of digestive enzyme activities (amylase and trypsin) as a sensitive tool to suggest an organism exposure to contaminant. The accuracy and suitability of enzyme activities is related to the active biomonitoring approach. The caging process avoids the potential influence of biotic factors (population, size, sex, etc.) and helps to distinguish the variability in biological responses related to the water quality. However, abiotic environmental factors might influence the data and bias the interpretations. The laboratory bioassay highlighted the small effect of abiotic confounding factors (temperature and conductivity) on digestive enzyme activities. However, some environmental parameters such as rainfall, water flow, suspended matter and light conditions need to be investigated to specify their effects on digestive enzyme activities. The investigation of the inhibition of digestive enzyme activities was complementary to the study of feeding rate responses for monitoring the impact of xenobiotics on energy acquisition in organisms. These results suggest a possible disturbance of the energy purchase for gammarids. It will be advantageous to define whether this disturbance impacts energy reserves and/or different fitness parameters, including growth and reproduction.
Abbreviations
- IDEA:
-
Inhibition rate of digestive enzyme activity
References
Albentosa M, Moyano FJ (2008) Influence of nutritional stress on digestive enzyme activities in juveniles of two marine clam species, Ruditapes decussatus and Venerupis pullastra. J Sea Res 59:249–258
Allen Y, Calow P, Baird DJ (1995) A mechanistic model of contaminant induced feeding inhibition in Daphnia magna. Environ Toxicol Chem 14:1625–1630
Barfield ML, Farris JL, Black MC (2001) Biomarker and bioaccumulation responses of Asian clams exposed to aqueous cadmium. J Toxicol Environ Health Part A 63:495–510
Bärlocher F, Porter CW (1986) Digestive enzymes and feeding strategies of three stream invertebrates. J North Am Benthol Soc 5:58–66
Besse JP, Coquery M, Lopes C, Chaumot A, Budzinski H, Labadie P, Geffard O (2013) Caged Gammarus fossarum (crustacea) as a robust tool for the characterization of bioavailable contamination levels in continental waters. Toward the determination of threshold values. Water Res 47:650–660
Borowsky R, Guarna MM (1989) Excess amylase in Gammarus palustris (Crustacea: Amphipoda); its release into and possible roles in the environment. Mar Biol (Berl) 101:529–534
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bundschuh M, Zubrod JP, Schulz R (2011) The functional and physiological status of Gammarus fossarum (Crustacea; Amphipoda) exposed to secondary treated wastewater. Environ Pollut 159:244–249
Chamier A-C, Willoughby LG (1986) The role of fungi in the diet of the amphipod Gammarus pulex (L.): an enzymatic study. Freshw Biol 16:197–208
Chapman PM (2007) Determining when contamination is pollution—weight of evidence determinations for sediments and effluents. Environ Int 33:492–501
Chapman PM, Wang F, Janssen CR, Goulet RR, Kamunde CN (2003) Conducting ecological risk assessments of inorganic metals and metalloids: current status. Hum Ecol Risk Assess 9:641–697
Chen Z, Mayer LM (1998) Digestive proteases of the lugworm (Arenicola marina) inhibited by Cu from contaminated sediments. Environ Toxicol Chem 17:433–438
Chen Z, Mayer LM, Weston DP, Bock MJ, Jumars PA (2002) Inhibition of digestive enzyme activities by copper in the guts of various marine benthic invertebrates. Environ Toxicol Chem 21:1243–1248
Coulaud R, Geffard O, Xuereb B, Lacaze E, Quéaau H, Garric J, Charles S, Chaumot A (2011) In situ feeding assay with Gammarus fossarum (Crustacea): modelling the influence of confounding factors to improve water quality biomonitoring. Water Res 45:6417–6429
De Coen WM, Janssen CR (1997a) The use of biomarkers in Daphnia magna toxicity testing. II. Digestive enzyme activity in Daphnia magna exposed to sublethal concentrations of cadmium, chromium and mercury. Chemosphere 35:1053–1067
De Coen WM, Janssen CR (1997b) The use of biomarkers in Daphnia magna toxicity testing. IV. Cellular energy allocation: a new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J Aquat Ecosyst Stress Recov 6:43–55
De Coen WM, Janssen CR (1998) The use of biomarkers in Daphnia magna toxicity testing. I. The digestive physiology of daphnids exposed to toxic stress. Hydrobiologia 367:199–209
De Coen WM, Vangheluwe ML, Janssen CR (1998) The use of biomarkers in Daphnia magna toxicity testing. III. Rapid toxicity testing of pure chemicals and sediment pore waters using ingestion and digestive enzyme activity. Chemosphere 37:2677–2694
De Schamphelaere KAC, Forrez I, Dierckens K, Sorgeloos P, Janssen CR (2007) Chronic toxicity of dietary copper to Daphnia magna. Aquat Toxicol 81:409–418
Dedourge-Geffard O, Palais F, Biagianti-Risbourg S, Geffard O, Geffard A (2009) Effects of metals on feeding rate and digestive enzymes in Gammarus fossarum: an in situ experiment. Chemosphere 77:1569–1576
Dedourge-Geffard O, Palais F, Geffard A, Amiard-Triquet C (2012) Origin of energy metabolism impairments. In: Amiard-Triquet C, Amiard JC, Rainbow P (eds) Ecological biomarkers—indicators of ecotoxicological effects. CRC Press, Taylor & Francis Group, Boca Raton, pp 279–306
R Development Core Team (2008) R: a language and environment for statistical computing, R Foundation for Statistical Computing
Douhri H, Sayah F (2009) The use of enzymatic biomarkers in two marine invertebrates Nereis diversicolor and Patella vulgata for the biomonitoring of Tangier's bay (Morocco). Ecotoxicol Environ Saf 72:394–399
Essedaoui A, Sif J, Kerambrun P (1998) Effet du cadmium sur l’activité de l’α-amylase chez Mytilus galloprovincialis: effect of cadmium on α-amylase activity in Mytilus galloprovincialis. Marine life 8:51–61
Forrow DM, Maltby L (2000) Toward a mechanistic understanding of contaminant-induced changes in detritus processing in streams: direct and indirect effects on detritivore feeding. Environ Toxicol Chem 19:2100–2106
Galgani F, Benyamin Y, Van Worhmoudt A, Cecaldi J (1983) Variations des activites digestives en fonction des facteurs du milieu chez les crustaces. Bases biologiques de l’aquaculture 1:277–292
Garcia-Carreño FL, Haard NF (1993) Characterization of proteinase classes in langostilla (Pleuroncodes planipes) and crayfish (Pacifastacus Planipes) extracts. J Food Biochem 17:97–113
Geffard A, Quéau H, Dedourge O, Biagianti-Risboug S, Geffard O (2007) Influence of biotic and abiotic factors on metallothionein level in Gammarus pulex. Comp Biochem Physiol Part C Toxicol Pharmacol 145:632–640
Gismondi E, Beisel J-N, Cossu-Leguille C (2012) Influence of gender and season on reduced glutathione concentration and energy reserves of Gammarus roeseli. Environ Res. doi:10.1016/j.envres.2012.06.004
Guarna MM, Borowsky RL (1995) Biochemical properties of amylase isozymes from Gammarus palustris. A comparative study. Comp Biochem Physiol Part B Biochem Mol Biol 112:619–628
Hagger JA, Jones MB, Leonard DRP, Owen R, Galloway TS (2006) Biomarkers and integrated environmental risk assessment: are there more questions than answers? Integr Environ Assess Manag 2:312–329
Hyne RV, Maher WA (2003) Invertebrate biomarkers: links to toxicosis that predict population decline. Ecotoxicol Environ Saf 54:366–374
Ibarrola I, Larretxea X, Iglesias JIP, Urrutia MB, Navarro E (1998) Seasonal variation of digestive enzyme activities in the digestive gland and the crystalline style of the common cockle Cerastoderma edule. Comp Biochem Physiol Part A Mol Integr Physiol 121:25–34
Ibarrola I, Etxeberria M, Iglesias JIP, Urrutia MB, Angulo E (2000) Acute and acclimated digestive responses of the cockle Cerastoderma edule (L.) to changes in the food quality and quantity: II. Enzymatic, cellular and tissular responses of the digestive gland. J Exp Mar Biol Ecol 252:199–219
Johnston D, Freeman J (2005) Dietary preference and digestive enzyme activities as indicators of trophic resource utilization by six species of crab. Biol Bull 208:36–46
Johnston M, Johnston D, Richardson A (2005) Digestive capabilities reflect the major food sources in three species of talitrid amphipods. Comp Biochem Physiol Part B Biochem Mol Biol 140:251–257
Kuz’mina VV (1996) Influence of age on digestive enzyme activity in some freshwater teleosts. Aquaculture 148:25–37
Lacaze E, Devaux A, Mons R, Bony S, Garric J, Geffard A, Geffard O (2011) DNA damage in caged Gammarus fossarum amphipods: a tool for freshwater genotoxicity assessment. Environ Pollut 159:1682–1691
Le Bihan E, Perrin A, Koueta N (2004) Development of a bioassay from isolated digestive gland cells of the cuttlefish Sepia officinalis L. (Mollusca Cephalopoda): effect of Cu, Zn and Ag on enzyme activities and cell viability. J Exp Mar Biol Ecol 309:47–66
Lebrun J, Perret M, Geffard A, Gourlay-Francé C (2012) Modelling copper bioaccumulation in Gammarus pulex and alterations of digestive metabolism. Ecotoxicology 21:2022–2030
Li N, Zhao Y, Yang J (2008) Effects of water-borne copper on digestive and metabolic enzymes of the giant freshwater prawn Macrobrachium rosenbergii. Arch Environ Contam Toxicol 55:86–93
Macneil C, Dick JTA, Elwood RW (1997) The Trophic ecology of freshwater Gammarus spp. (Crustacea: Amphipoda): problems and perspectives concerning the fonctional feeding group concept. Biol Rev 72:349–364
Maltby L, Naylor C, Calow P (1990) Field deployment of a scope for growth assay involving Gammarus pulex, a freshwater benthic invertebrate. Ecotoxicol Environ Saf 19:292–300
Maltby L, Clayton SA, Wood RM, McLoughlin N (2002) Evaluation of the Gammarus pulex in situ feeding assay as a biomonitor of water quality: robustness, responsiveness, and relevance. Environ Toxicol Chem 21:361–368
McGrath CC, Matthews RA (2000) Cellulase activity in the freshwater amphipod Gammarus lacustris. J North Am Benthol Soc 19:298–307
Meyer JS, Adams WJ, Brix KV, Luoma SN, Mount DR, Stubblefield WA, Wood CM (2005) Toxicity of dietborne metals to aquatic organisms. SETAC, Pensacola, FI, USA
Monk DC (1977) The digestion of cellulose and other dietary components, and pH of the gut in the amphipod Gammarus pulex (L.). Freshw Biol 7:431–440
Mukherjee S, Bhattacharya S (1977) Variations in the hepatopancreatic α-amylase activity in fishes exposed to some industrial pollutants. Water Res 11:71–74
Palais F, Jubeaux G, Dedourge-Geffard O, Giambérini L, Biagianti-Risbourg S, Geffard A (2010) Amylolytic and cellulolytic activities in the cristalline style and the digestive diverticulae of the freshwater bivalve Dreissena polymorpha (Pallas, 1771). Molluscan Res 30:29–36
Palais F, Dedourge-Geffard O, Beaudon A, Pain-Devin S, Trapp J, Geffard O, Noury P, Gourlay-Francé C, Uher E, Mouneyrac C, Biagianti-Risbourg S, Geffard A (2012) One-year monitoring of core biomarker and digestive enzyme responses in transplanted zebra mussels (Dreissena polymorpha). Ecotoxicology 21:888–905
Perera E, Moyano FJ, Diaz M, Perdomo-Morales R, Montero-Alejo V, Rodriguez-Viera L, Alonso E, Carrillo O, Galich GS (2008) Changes in digestive enzymes through developmental and molt stages in the spiny lobster, Panulirus argus. Comp Biochem Physiol Part B Biochem Mol Biol 151:250–256
Pöckl M (1992) Effects of temperature, age and body size on moulting and growth in the freshwater amphipods Gammarus fossarum and G. roeseli. Freshw Biol 27:211–225
Pöckl M, Webb BW, Sutcliffe DW (2003) Life history and reproductive capacity of Gammarus fossarum and G. roeseli (Crustacea: Amphipoda) under naturally fluctuating water temperatures: a simulation study. Freshw Biol 48:53–66
Reddy PS, Fingerman M (1994) Effect of cadmium chloride on amylase activity in the red swamp crayfish, Procambarus clarkii. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 109:309–314
Sanchez-Paz A, Garcìa-Carreño F, Muhlia-Almazan A, Peregrino-Uriarte AB, Hernandez-Lopez J, Yepiz-Plascencia G (2006) Usage of energy reserves in crustaceans during starvation: status and future directions. Insect Biochem Mol Biol 36:241–249
Sornom P, Felten V, Médoc V, Sroda S, Rousselle P, Beisel J-N (2010) Effect of gender on physiological and behavioural responses of Gammarus roeseli (Crustacea Amphipoda) to salinity and temperature. Environ Pollut 158:1288–1295
Sroda S, Cossu-Leguille C (2011) Seasonal variability of antioxidant biomarkers and energy reserves in the freshwater gammarid Gammarus roeseli. Chemosphere 83:538–544
Trellu J, Ceccaldi HJ (1980a) Influence de l’intensité lumineuse sur quelques activités enzymatiques chez Palaemon serratus. Biochem Syst Ecol 8:181–191
Trellu J, Ceccaldi HJ (1980b) Influence de la température sur quelques activités enzymatiques chez Palaemon serratus. Biochem Syst Ecol 8:171–179
Van Wormhoudt A (1977) Activités enzymatiques digestives chez Palaemon serratus: variations annuelles de l’acrophase des rythmes circadiens. Biochem Syst Ecol 5:301–307
Van Wormhoudt A, Malcoste R (1976) Influence d’éclairements brefs, à différentes longeurs d’onde, sur les variations circadiennes des activitées enzymatiques digestives chez Palaemon serratus (Crustacea, Natantia). J Interdiscip Cycle Res 7:101–111
Xuereb B, Chaumot A, Mons R, Garric J, Geffard O (2009) Acetylcholinesterase activity in Gammarus fossarum (Crustacea Amphipoda): intrinsic variability, reference levels, and a reliable tool for field surveys. Aquat Toxicol 93:225–233
Yan T, Teo LH, Sin YM (1996) Effects of metals on α-amylase activity in the digestive gland of the green mussel, Perna viridis. Bull Environ Contam Toxicol 56:677–682
Acknowledgements
This research program was financially supported by the ANR CESA program GAMMA 021 02 “Variability–adaptation–diversity and ecotoxicology in gammarids” (2012–2015), and the PIREN-Seine Program. The authors sincerely thank the Elsevier site for the English revision.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Markus Hecker
Highlights
Digestive enzyme activities (amylase and trypsin) in Gammarus fossarum are sensitive biomonitoring tools that reflect the quality of aquatic ecosystems.
Abiotic factors (temperature and conductivity) have a small influence on the digestive enzyme activities of gammarids.
The caging procedure avoids the potential influence of biotic factors.
Rights and permissions
About this article
Cite this article
Charron, L., Geffard, O., Chaumot, A. et al. Effect of water quality and confounding factors on digestive enzyme activities in Gammarus fossarum . Environ Sci Pollut Res 20, 9044–9056 (2013). https://doi.org/10.1007/s11356-013-1921-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1921-5