Abstract
Typically, studies in digestive physiology in fish focus on a few enzymes and provide insight into the specific processes of the enzyme in a targeted species. Comparative studies assessing a wide number of digestive enzymes on fishes that compete for food resources are lacking, especially in the context of an introduced species. It is generally thought that the invasive silver carp (SVC; Hypophthalmichthys molitrix) directly compete for food resources with the native gizzard shad (GZS; Dorosoma cepedianum) in waters where they coexist. We compared 19 digestive enzymes between SVC and GZS throughout a year and in two rivers in the Midwestern United States: Illinois River and Wabash River. All digestive enzymes analyzed were detected in both SVC and GZS in both rivers. However, the profiles of the digestive enzymes varied by species. Alkaline phosphatase, valine arylamidase, acid phosphatase, naphthol-AS-BI-phosphohydrolase and N-acetyl-β-glucosaminidase were all much higher in SVC than in GZS. Differences between digestive enzyme profiles were also observed between rivers and months. This study demonstrates the utility of using an ecological approach to compare physiological features in fishes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of digestive enzymes is vital to understanding the nutritional needs of an animal and can provide insight beyond the presence and activities of an enzyme. The presence and activities of digestive enzymes are key components of an organism’s ability to break down food into the basic nutritional components required for maintenance, growth and health, and their presence is dependent on the presence of their substrate (Smith 1989). Typically, these studies in fish focus on a few enzymes (Dabrowski and Glogowski 1977; Bitterlich 1985; Das and Tripathi 1991; Infante and Cahu 1994; Cahu and Infante 1994; Hidalgo et al. 1999; Harpaz and Uni 1999; German et al. 2004) in a single species and provide insight into specific processes such as protein digestion. Comparative studies assessing a wide number of digestive enzymes on fishes that compete for food resources are lacking, especially in the context of an introduced species.
Silver carp (SVC; Hypophthalmichthys molitrix) and gizzard shad (GZS; Dorosoma cepedianum) provide an ideal comparison for two fishes that compete for food resources in systems where they coexist (Irons et al. 2007; Sampson et al. 2008). SVC were imported into North America in the 1970s and subsequently escaped into the nearby Mississippi River. Their populations expanded throughout much of the Mississippi River drainage. Well-established populations are found in both the Illinois and Wabash Rivers. Filter-feeding adult SVC primarily feed on zooplankton and phytoplankton, which is a niche few adult native fishes in the Illinois and Wabash Rivers occupy. However, GZS is one species of fish native to this region that does feed on zooplankton and phytoplankton and stable isotope studies have indicated that they directly compete with SVC for food (Irons et al. 2007; Sampson et al. 2008). Both SVC and GZS lack true stomachs, but GZS do have a gizzard and cecae, whereas SVC do not. SVC have a significant impact on native fishes by sequestering essential nutrients, like fatty acids (Gutreuter et al. 2011). However, GZS have been found to feed on detritus, while SVC have not (Yako et al. 1996; Ye et al. 2014). Contributions from nutritional resources such as detritus, bacteria, and cyanobacteria are not typically well identified (or well characterized) through stable isotope analysis, but differences among these food resources may be observed though changes in gut enzyme composition and activities.
A better understanding of digestion and nutritional physiology in SVC is expected to improve our ability to predict their ecological effects. We compared 19 digestive enzymes between SVC and GZS on a temporal and spatial scale using a commercially available kit previously found to be useful in assessing digestive enzymes in mussels and other invertebrates (Sauey et al. 2015; Sauey et al. 2016). We hypothesize that the profile of digestive enzymes will differ among months and that some enzymes will be more active in SVC than in GZS. The objectives of this study were: (1) to determine if a commercially available kit can be used to assess the activities of 19 different digestive enzymes; (2) to characterize the digestive enzyme profiles of SVC and GZS; (3) to determine seasonal changes in digestive enzymes for both of these species; and (4) to compare digestive enzyme profiles between these two species captured from two separate rivers.
Materials and methods
Animals and sample collection. To identify temporal changes in digestive enzyme activity, we captured six SVC and six GZS by electroshocking from the Illinois River near Havana, Illinois (40°18’26.22” N and 90°04’10.84”W) during both August and September of 2010 and each of March, May, and June of 2011. These fish were collected concurrently with those captured and analyzed for intestinal microbiome reported in Ye et al. (2014). To compare digestive enzyme activity between rivers within a species, we captured five SVC and six GZS by electroshocking from the Wabash River near Lafayette, Indiana (40°25’48.99” N and 86°93’49.55”W), in June two days after fish were captured from the Illinois River. We only sampled the Wabash River in June due to a malfunctioning of the electroshocking boat at a time similar to those when fish were captured from the Illinois River. We chose these two rivers because of the high abundance of both species and the consistent seasonal changes in hydrological conditions (USGS National Water Information System; https://waterdata.usgs.gov/nwis). Upon capture, all fish were immediately euthanized with Tricaine-S (Western Chemical, Inc., Ferndale, Washington, USA). The gastrointestinal tract (GIT) was removed from each fish and individual anterior gut contents were collected, flash-frozen, and stored at -80 °C until further processing. In this manuscript, we define the anterior gut in SVC as the section posterior to the duct connecting the gall bladder and anterior to the first distal loop, while the section posterior to the gizzard with cecae was considered the anterior gut in GZS.
Digestive enzyme sample. All GIT content samples were diluted with de-ionized water. Samples were individually homogenized with a Tissue Tearor™ (Cole-Palmer, Vernon Hills, IL, USA) and centrifuged at 13,000 × g for 10 minutes at 4 °C. Supernatants were retained for subsequent analysis and pellets were discarded. Total protein concentration of each supernatant was determined by BCA assay (Thermo Scientific, Rockford, IL) according to the manufacturer’s instructions at a wavelength of 562 nm on a MultiSkan Spectrum plate reader (Thermo Scientific, Rockford, IL, USA). All supernatants were then diluted with de-ionized water to a final total protein concentration of 1.0 µg µL−1. A buffer was not used because the commercial kit contains salts to buffer the enzymatic reactions.
Validation of the screening assay. A commercially available colorimetric kit for digestive enzymes (api®ZYM test kit, bioMérieux, Inc. Durham, NC) was validated with serially diluted intestinal contents. The wells of a strip were loaded with 6.00, 3.00, 1.50, 0.75, 0.38, or 0 µg total protein of the supernatant. One test strip was used for each concentration of total protein. Each strip was loaded with the supernatant and processed according to the manufacturer’s instructions. Following incubation at 37 °C for 4 h, 60 uL of the contents from each well was individually transferred to a 96-well plate. Absorbance was measured by spectrophotometry at 400, 450, 500, 550, 600, and 650 nm using a MultiSkan Spectrum plate reader. Absorbance of each well for each protein concentration was adjusted for the blank at each respective wavelength. Standard curves were developed, and the wavelength that provided the best fit model (R2 ≥ 0.95) was used for further analysis of each enzyme. Those enzymes that did not provide an appropriate model were only confirmed as present or absent. This process was repeated using the supernatant of a second SVC and GZS to verify that the appropriate wavelengths were chosen for analysis.
Screening enzyme assay. We assessed 19 digestive enzymes from individual fish using the api®ZYM test kit. For each fish, equal amounts of total protein (3.0 µg) were loaded into each of the 20 wells of a test strip. One test strip was used for each fish. Absorbance at the appropriate wavelength (determined during the validation process described above) was used to quantify the concentration of each specific enzyme using the equation (Absx − B)/C = Quantity (a.u.), where Absx is the absorbance at wavelength x and B and C are values determined from the linear model at wavelength x (B is the y-intercept and C is the slope). This concentration is referred to as an arbitrary concentration (a.u.) and was used in all subsequent analysis.
Statistical analysis. Total length (TL), mass, and condition factor (K) of fish are expressed as mean values (± SD). K was the ratio between the mass of the fish and its length. Differences in K within a species among months were determined using ANOVA and Tukey’s pairwise comparisons. A t-test was used to compare differences in condition factor between rivers. Only fish captured during June were used to compare between the Illinois and Wabash Rivers. These analyses were performed using R (Legendre and Anderson 1999) with a significance level of α < 0.05.
To compare enzyme profile, we used multivariate analysis to examine if enzyme activities differed by species, month, and river. Specifically, a distance-based redundancy analysis (dbRDA) was used (Kuczynski et al. 2010). A type of multivariable regression, dbRDA, generalizes redundancy analysis (RDA) to allow for non-Euclidean dissimilarities using Bray–Curtis dissimilarly (Oksanen et al. 2015). Enzyme activities were the response variables, and species, year, and site were the predictor variables. The regression included interaction terms among all of the predictor variables. The capscale function from the vegan package (Oksanen et al. 2015) in R (R Core Team 2016) was used. Data was visualized using the ggplot2 package (Wickham 2009) in R.
Results
Validation of enzyme assay. All 19 enzymes included in the api®ZYM test kit were present in both GZS and SVC from the Wabash River and Illinois River. We were able to generate positive response curves of each of the nine enzymes that was useful for semi-quantification. Enzymes that had responses which could be semi-quantified included according to the api®ZYM label were alkaline phosphatase (ALP), esterase (EST), valine arylamidase (VAL), cysteine arylamidase (CYS), trypsin (TRY), acid phosphatase (ACP), naphthol-AS-BI-phosphohydrolase (NAP), α-glucosidase (αGS), and N-acetyl-β-glucosaminidase (NAG). All other enzymes could not be semi-quantified; therefore, we classified them as present or absent.
Morphometric comparisons of fish. Temporal. Differences in condition factor were observed in GZS among months (P = 0.001, F = 6.276, df = 4). GZS captured in March had a lower condition factor than other months (Table 1). The condition factor increased from March (1.30 ± 0.14) to August and September (1.58 ± 0.05 and 1.57 ± 0.10, respectively). Differences were also observed in SVC condition factor among months (P = 0.021, F = 3.517, df = 4). Pairwise comparisons only indicated that the condition factor in SVC captured in March was different from those captured in September.
Spatial. No significant difference in condition factor was found between GZS captured from either the Illinois River or the Wabash River (P = 0.086, t = 1.903, df = 10). Mean TL of GZS was 246 ± 45 mm and 229 ± 45 mm from the Wabash River and Illinois River, respectively. The mass of GZS was also similar between the Wabash River and Illinois River, 217 ± 126 g and 192 ± 94 g, respectively. There was no significant difference in the condition factor between SVC captured contemporaneously from both of these rivers (P = 0.573, F = -0.585, df = 9). SVC from the Wabash River were 474 ± 125 mm in TL and weighed 1702 ± 1153 g, whereas SVC from the Illinois were 454 ± 125 mm long and weighed 1374 ± 841 g.
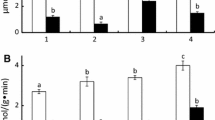
Digestive enzyme richness and diversity. Enzyme activities varied by species, month, and river and an interaction occurred among all three parameters (P = 0.001, F = 5.176, df = 11,). A clear separation between the species was observed (Fig. 1). Enzymes in both species varied by month (Figs. 2 and 3). Enzymes in SVC were similar to those in GZS during August (Fig. 2). Enzymes in SVC from the Illinois River differed from those of SVC captured from the Wabash River, whereas enzymes were similar in GZS for both rivers in June (Fig. 2). Overall, SVC had higher enzyme activities than GZS, except for CYS (Figs. 3 and 4). The activity of 4 enzymes (NAP, ACP, NAG, VAL) was notably greater in SVC than in GZS (Fig. 4). NAP was prominent in SVC when compared to levels in GZS with NAG as the second most prominent enzyme (Fig. 4). The two phosphatases, ACP and ALP, were also greater in SVC when compared with levels observed in GZS (Fig. 4).
Box plot of scores from the first axis of the distance-based redundancy analysis for digestive enzymes in SVC and GZS captured from the Illinois River in March, May, June, August, and September. The horizontal line in the box represents the median. Boxes represent 25 to 75% of the data and the whiskers indicate 0 to 25% and 75 to 100% of the data. Dots indicate outliers
Discussion
Our study was designed to determine if differences in digestive enzymes exist between two planktivorous fishes with dietary overlap, GZS and SVC. We were able to demonstrate that a commercial enzyme kit was useful for the determination of the presence or absence of 19 digestive enzymes in both species.
We determined that the activities of the digestive enzymes of SVC were different from those of GZS. These differences in enzyme activities could be related to the specific resources that the fish are consuming. SVC are known to directly compete with GZS for food resources (Irons et al. 2007; Sampson et al. 2008). SVC have significantly adapted to food resources in the Illinois River since their invasion in the 1990s (Irons et al. 2007; Sass et al. 2014) and it is plausible that SVC are now consuming cyanobacteria. In fish collected concurrently with these fish, Ye et al. (2014) found that SVC had high amounts of cyanobacteria within their anterior guts and few in their hindgut. GZS may have switched to detritus. Ye et al. (2014) found no cyanobacteria and high numbers of, and a high diversity of, microbes within their anterior guts. It has been reported that GZS will consume detritus when plankton is limited (Yako et al. 1996). Shifts such as these in diet may explain the differences in digestive enzymes between these two species. Our data, along with Ye et al. (2014), suggest SVC may be taking advantage of cyanobacteria as a food resource that GZS are not accessing.
The difference in primary productivity, and hence secondary productivity, between the two rivers studied likely has a role in the differences observed in enzyme activity/presence between SVC and GZS in these rivers. Headwaters of the Illinois River drain a highly urban area and receive a significant amount of treated wastewater from the City of Chicago, IL. Further downstream, the Illinois River watershed is dominated by crop agriculture and a series of navigation pools or impoundments. Thus, it is a very productive system that supports the greatest density of SVC in the world (Sass et al. 2009). The Wabash River is a free-flowing system without impoundments and drains agricultural fields. Similar to the Illinois River, the Wabash River is highly productive, but lacks impoundments for nutrient accumulation and cycling. Primary productivity in the Wabash River is likely more closely aligned with pulsed addition of key nutrients such as nitrogen and phosphorus associated with agriculture land runoff than with the nutrient cycling which likely occurs in the impounded sections of the Illinois River.
Phosphatases and proteases appear to be important groups of enzymes for SVC. The phosphatases NAP, ACP, and ALP were greater in SVC than in GZS, which may suggest that SVC have a higher requirement for phosphorus than GZS. Similar to the phosphatases, the proteases VAL and TRY were also greater in SVC. These elevated levels may be required by SVC to support their rapid growth rate (Williamson and Garvey 2005). Rapid growth would require greater amounts of phosphorus for the production of bone, and protein used in muscle. Unfortunately, the dietary requirements for SVC and GZS are unknown and should be further studied.
The differences in the activities of digestive enzymes (i.e. NAP, NAG, ACP, and VAL) suggest that the efficiency of digestion between SVC and GZS is dissimilar. The increased activity observed in SVC could be due to their rapid gut transit time (Opuszynski and Shireman 1991). The rapid evacuation rate of SVC would require quick digestion and rapid nutrient uptake of foodstuffs to maintain high growth rates (Williamson and Garvey 2005). Enteric microbes could also be responsible for the higher levels of certain digestive enzymes in SVC. Numerous microbes that may play a role in digestion have been found in the anterior gut of SVC (Ye et al. 2014).
Conclusion
In summary, we found that the api®ZYM test kit was a valuable tool for uncovering trends in activities of 19 digestive enzymes in SVC and GZS. Enzyme activities differed temporally and spatially within each species. Our results suggest that SVC may feed on different food sources at different times relative to native planktivorous species, even if they occupy similar trophic positions. Our research suggests that SVC digestive enzymes such as trypsin and alkaline phosphatase, which vary significantly over time and in comparison to native GZS, represent valuable biochemical and physiological differences between two species known to have dietary overlap. It is important to continue gathering information about seasonal digestive enzyme profiles of invasive SVC relative to those in native species to provide insight into the magnitude of overlap between their diets. This is important if management actions to reduce SVC populations allow GZS to shift back to other resources.
References
Bitterlich G (1985) Digestive enzyme pattern of two stomachless filter feeders, silver carp, Hypophthalmichthys molitrix Val., and bighead carp, Aristichthys nobilis Rich. J Fish Biol27:103–112
Cahu CL, Infante JLZ (1994) Early weaning of sea bass (Dicentrarchus labrax) larvae with a compound diet: Effect on digestive enzymes. Comp Biochem Physiol A Physiol 109:213–222
Dabrowski K, Glogowski J (1977) Studies on the role of exogenous proteolytic enzymes in digestion processes in fish. Hydrobiologia 54:129–134
Das KM, Tripathi SD (1991) Studies on the digestive enzymes of grass carp, Ctenopharyngodon idella (Val.). Aquaculture 92:21–32
German DP, Horn MH, Gawlicka A (2004) Digestive enzyme activities in herbivorous and carnivorous prickleback fishes (Teleostei: Stichaeidae): ontogenetic, dietary, and phylogenetic effects. Physiol Biochem Zool 77:789–804
Gutreuter S, Vallazza J, Knights B, Bartsch M, Bartsch L, Richardson W (2011) Early evidence of effects of invasive Asian carps on selected fishes of the Upper Mississippi River system. Project completion report. US Fish and Wildlife Service Project, Washington D.C.
Harpaz S, Uni Z (1999) Activity of intestinal mucosal brush border membrane enzymes in relation to the feeding habits of three aquaculture fish species. Comp Biochem Physiol A Mol Integr Physiol 124:155–160
Hidalgo M., Urea E, Sanz A (1999) Comparative study of digestive enzymes in fish with different nutritional habits. Proteolytic and amylase activities. Aquaculture 170:267–283
Infante JLZ, Cahu C (1994) Development and response to a diet change of some digestive enzymes in sea bass (Dicentrarchus labrax) larvae. Fish Physiol Biochem 12:399–408
Irons KS, Sass GG, McClelland MA, Stafford JD (2007) Reduced condition factor of two native fish species coincident with invasion of non-native Asian carps in the Illinois River, U.S.A. Is this evidence for competition and reduced fitness? J Fish Biol 71:258–273
Kuczynski J, Liu Z, Lozupone C, McDonald D, Fierer N, Knight R (2010) Microbial community resemblance methods differ in their ability to detect biologically relevant patterns. Nat Methods 7:813–819
Legendre P, Anderson MJ (1999) Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecol monogr 69:1–24
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara R, Simpson GL, Solymos P, Stevens M, Wagner H (2015) vegan: community ecology package. R package version 2.0-10. 2013
Opuszynski K, Shireman JV (1991) Food passage time and daily ration of bighead carp, Aristichthys nobilis, kept in cages. Environ Biol Fishes 30:387–393
R Development Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed 6 December 2016
Sampson SJ, Chick JH, Pegg MA (2008) Diet overlap among two Asian carp and three native fishes in backwater lakes on the Illinois and Mississippi rivers. Biol Invasions 11:483–496
Sass GG, Cook TR, Irons KS, McClelland MA, Michaels NN, O’Hara TM, Stroub MR (2009) A mark-recapture population estimate for invasive silver carp (Hypophthalmichthys molitrix) in the La Grange Reach, Illinois River. Biol Invasions 12:433–436
Sass GG, Hinz C, Erickson AC, McClelland NN, McClelland MA, Epifanio JM (2014) Invasive bighead and silver carp effects on zooplankton communities in the Illinois River, Illinois, USA. J Great Lakes Res 40:911–921
Sauey BW, Amberg JJ, Cooper ST, Grunwald SK, Newton TJ, Haro RJ (2015) Preliminary characterization of digestive enzymes in freshwater mussels. J Shellfish Res 34:415–422
Sauey BW, Amberg JJ, Cooper ST, Grunwald SK, Haro RJ, Gaikowski MP (2016) Digestive physiology comparisons of aquatic invertebrates in the Upper Mississippi River Basin. J Freshw Ecology 31:303–314
Smith LS (1989) Digestive functions in Teleost fish. In: Halver JE (ed) Fish Nutrition, 2nd edn. Academic Press, San Diego, California, pp 59–94
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York
Williamson CJ, Garvey JE (2005) Growth, Fecundity, and Diets of Newly Established Silver Carp in the Middle Mississippi River. Tran Am Fish Soc 134:1423–1430
Yako LA, Dettmers JM, Stein RA (1996) Feeding preferences of omnivorous gizzard shad as influenced by fish size and zooplankton density. Tran Am Fish Soc 125:753–759
Ye L, Amberg J, Chapman D, Gaikowski M, Liu W-T (2014) Fish gut microbiota analysis differentiates physiology and behavior of invasive Asian carp and indigenous American fish. ISME J 8:541–551
Acknowledgments
This project was funded through the Great Lakes Restoration Initiative as part of a larger project focused on the development of a species-specific control for bigheaded carps. The authors claim no conflicts of interests. The authors thank the staff at the Illinois History Survey in Havana, Illinois, and Dr. Reuben Goforth and students at Purdue University in West Lafayette, Indiana, for assisting in the capture of fishes. All vertebrate animals used in this study (AEH-10-ORAL-01) were handled according to the approved protocol procedures and the policies established by the USGS Upper Midwest Environmental Sciences Center Institutional Animal Care and Use Committee; the Committee reviewed and approved protocol-specific procedures. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Amberg, J.J., Jensen, N.R., Erickson, R.A. et al. Profiles of digestive enzymes of two competing planktivores, silver carp and gizzard shad, differ. Ichthyol Res 65, 245–251 (2018). https://doi.org/10.1007/s10228-018-0615-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-018-0615-x