Abstract
A 12-month active biomonitoring study was performed in 2008–2009 on the Vesle river basin (Champagne-Ardenne, France) using the freshwater mussel Dreissena polymorpha as a sentinel species; allochthonous mussels originating from a reference site (Commercy) were exposed at four sites (Bouy, Sept-Saulx, Fismes, Ardre) within the Vesle river basin. Selected core biomarkers (acetylcholinesterase (AChE) activity, glutathione-S transferase (GST) activity, metallothionein concentration), along with digestive enzyme activities (amylase, endocellulase) and energy reserve concentrations (glycogen, lipids), were monitored throughout the study in exposed mussels. At the Fismes and Ardre sites (downstream basin), metallic and organic contamination levels were low but still high enough to elicit AChE and GST activity induction in exposed mussels (chemical stress); besides, chemical pollutants had no apparent deleterious effects on mussel condition. At the Bouy and Sept-Saulx sites (upstream basin), mussels obviously suffered from adverse food conditions which seriously impaired individual physiological state and survival (nutritional stress); food scarcity had however no apparent effects on core biomarker responses. Digestive enzyme activities responded to both chemical and nutritional stresses, the increase in energy outputs (general adaptation syndrome—downstream sites) or the decrease in energy inputs (food scarcity—upstream sites) leading to mid- or long-term induction of digestive carbohydrase activities in exposed mussels (energy optimizing strategy). Complex regulation patterns of these activities require nevertheless the use of a multi-marker approach to allow data interpretation. Besides, their sensitivity to natural confounding environmental factors remains to be precised.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The zebra mussel Dreissena polymorpha (Pallas, 1771) is a freshwater bivalve frequently used for the monitoring of inland waters (Minier et al. 2006; Voets et al. 2006; Zorita et al. 2006; Bacchetta and Mantecca 2009; Contardo-Jara et al. 2009; Bourgeault et al. 2010; Faria et al. 2010). Bio-ecological traits of this bivalve (e.g. abundance, wide distribution, filtration activity) make it indeed an interesting sentinel species, especially as its tolerance to transplantation allows active monitoring of various types of water bodies. Numerous biochemical and physiological biomarkers of defense (e.g. metallothionein (MT) induction) and damage (e.g. DNA strand breaks) have been investigated in D. polymorpha for environmental assessment purposes (de Lafontaine et al. 2000). However, these ‘routine’ biomarkers often fail in predicting the effect of environmental stressors at individual or populational level. To overcome such deficiency, the use of generic markers related to energy allocation and metabolism could be of great interest as their disturbances under toxic stress may have adverse effects on individual growth and reproduction (metabolic cost hypothesis) and so, could be linked to effects at higher levels of organization (Calow and Sibly 1990; De Coen et al. 2000; Kalman et al. 2009).
In bivalves, dietary energy assimilation depends on a two-phase digestive process involving an extracellular phase followed by an intracellular one (reviewed in Morton 1983). The first phase occurs within the stomach under the mechanical and biochemical actions of the crystalline style (CS), a revolving gelatinous rod saturated with hydrolytic enzymes and secreted by the style sac epithelial cells. Activity of the CS promotes the grinding and mixing of food materials with digestive enzymes released in the stomach lumen by dissolution of the CS itself or by breakdown of ‘digestive spherules’ produced by disintegrating digestive cells from the neighboring digestive gland (DG). This extracellular digestion phase allows a preliminary breakdown of ingested food materials into particles small enough to enter the digestive tubules of the DG where the digestion is completed intracellularly within phagocytic digestive cells. Extra- and intracellular digestive processes are mediated by enzymes whose activity actually depends on their innate lytic properties, synthesis rate and secretion rate (extracellular enzymes only) (Yan et al. 1996). These enzyme activity regulators are sensitive to interactions with chemical toxicants which may lead to a disruption of enzyme activities under chemical stress, as previously reported for several aquatic invertebrate species (Yan et al. 1996; De Coen and Janssen 1997; Barfield et al. 2001; Chen et al. 2002; Le Bihan et al. 2004; Li et al. 2008; Dedourge-Geffard et al. 2009; Kalman et al. 2009; Boldina-Cosqueric et al. 2010; Bourgeault et al. 2010). Perturbation of digestive processes may affect individual energy balance and result in energy allocation disturbances with possible consequences on individual fitness. Nevertheless, digestive enzyme activities have been little studied in aquatic sentinel species and, in the case of D. polymorpha, had never been investigated until recently (Bourgeault et al. 2010).
Present paper reports the results of a 12-month monitoring study performed on the Vesle river system, a French basin whose surface waters and associated groundwater are potentially contaminated by pollutants originating from agricultural, industrial and urban activities (Rouxel-David et al. 2002). These sources of diffuse pollution represent a threat to water quality and raise several issues at regulatory (European Union Water Framework Directive 2000/60/EC), sanitary (drinking water source protection) and environmental (wetland protection) levels. In an attempt to address these issues, a biomonitoring study was performed in 2008–2009 with the objectives of (i) assessing the quality of surface waters through long-term exposure of transplanted zebra mussels and (ii) assessing the potential interest of digestive enzyme responses as complementary informative tools in multi-biomarker approaches. The study especially focused on CS and DG carbohydrase activities, consistently with the ‘herbivory’ regime of D. polymorpha. Along with digestive carbohydrase activities, core biomarkers were monitored throughout the study in exposed mussels: acetylcholinesterase (AChE) activity inhibition (indicative of neurotoxicity), glutathione S-transferase activity induction (indicative of phase II biotransformation of xenobiotics) and MT induction (indicative of metal exposure). A particular attention was also given to mussel condition and physiological state (mortality rate, body weight, energy reserve cycle, filtration activity).
Materials and methods
Site description
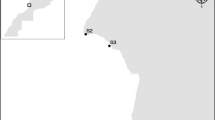
The biomonitoring study was performed on four sites within the Vesle river basin (Champagne-Ardenne, northern France): three along the Vesle river and one along the Ardre river, a tributary river (Fig. 1). The Bouy site (N 49°5′9.95″, E 4°21′12.73″) and the Sept-Saulx site (N 49°9′8.08″, E 4°14′56.07″) were located upstream of the Reims agglomeration, close to agricultural areas. According to data records from the Réseau de Contrôle de Surveillance (RCS), a French monitoring network, the upper Vesle river exhibited in 2008 a ‘good’ chemical state and a ‘medium’ ecological state (classes of water quality defined in the European Union Water Framework Directive 2000/60/EC) (DREAL Champagne-Ardenne 2010). The Fismes site (N 49°18′46.62″, E 3°40′51.00″) was located downstream of the Reims agglomeration, near urban areas. The lower Vesle river exhibited in 2008 a ‘bad’ chemical state (major degrading factor: concentration in Polycyclic Aromatic Hydrocarbons—PAHs) and a ‘good’ ecological state. Finally, the Ardre site (N 49°18′31.39″, E 3°39′41.68″) was located in the lower Ardre river, downstream of agricultural areas mainly dedicated to viticulture and vinification. The lower Ardre river exhibited in 2008 a ‘good’ chemical state and a ‘medium’ ecological state.
In situ experiment
In November 2008, zebra mussels with shell lengths of 18–24 mm were collected from a reference site located at Commercy (northeastern France), along the Meuse channel (N 48°45′26.13″, E 5°36′14.51″). In the laboratory, sampled mussels were sorted and then randomly distributed into 2 mm-mesh polyethylene experimental cages (60 cages × 70–80 mussels) were mussels were allowed to byssally reattach to each other; cages were kept in aerated water from the collection site until in situ deployment in the following 72 h. At each exposure site, 15 experimental cages were ballasted, immersed to a 0.2–1.0 m depth with a temperature data logger (TidbiT® v2 Temp), and secured to the riverbank with a rope. Cages were collected every month from December 2008 to October 2009; every time, water conductivity and O2 content were measured in the field while water samples were collected for further laboratory analysis. Besides, mussels from the reference site were sampled in November 2008 and in March, July and October 2009, for seasonal control measurements.
Chemical pollutant concentrations in the Vesle and Ardre rivers were monitored regularly over the study period, either by timely sampling of running waters (July, October—analysis of pesticides) or by short-term exposure of passive sampling systems (February, May, August, October—analysis of metallic and organic pollutants): Diffusive Gradient in Thin-films (DGTs—DGT Research, Lancaster, United Kingdom) (Davison and Zhang 1994) were deployed once a season for labile metal quantification (exposure time: 2 weeks), while Semi-Permeable Membrane Devices (SPMDs—ExposMeter AB, Trehörningen, Sweden) (Huckins et al. 1990) were deployed in April and October for labile PAHs quantification (exposure time: 2 weeks). Pre- and post-deployment treatments of DGTs and SPMDs were performed as described in Tusseau-Vuillemin et al. (2007).
Water analyses
Water pH, conductivity and O2 content were measured using a Mettler Toledo S30 pH meter, a WTW LF 318 meter (TetraCon 325 probe) and a WTW Oxi320 meter (CellOx 325 probe), respectively. Sodium (Na+), potassium (K+), calcium (Ca2+) and magnesium (Mg2+) concentrations were assayed by Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) (Varian Liberty Series II). Chlorophyll a concentrations were measured spectrophotometrically according to the SCOR-Unesco method (SCOR-Unesco 1966). Ammonium (NH4 +), nitrate (NO3 −), sulfate (SO4 2−), phosphate (PO4 3−) and chloride (Cl−) concentrations were measured according to the Standard Methods for the Examination of Water and Wastewater (www.standardmethods.org).
Pesticide concentrations were measured by liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS; MOP/MP/AN/10 internal method) or by high-performance liquid chromatography coupled to diode-array detection (HPLC/DAD; NFT 90-180-1, NF EN ISO 11369 standard methods) (Laboratoire Municipal et Régional de Reims, COFRAC accreditation 1-1205). Labile metals and PAHs were quantified by inductively coupled plasma mass spectrometry (ICP-MS, VG PlasmaQuad PQ2 Plus) and by gas chromatography–mass spectrometry (GC–MS, HP GCD 1800A), respectively.
Handling and dissection protocol
At every exposure site, one experimental cage was collected monthly, opened in the field and checked for dead mussels; in case of high mortality (>50%), a supplementary cage was collected. In the laboratory, sampled mussels were processed for either filtration rate measurement (n = 6–9, according to mortality rate) or for biometric analyses (shell length and soft body wet weight, ww) and tissue dissection (n = 40–50, according to mortality rate). In detail, 8–10 mussels were sacrificed for excision of the DG and remaining soft tissues, while 32–40 mussels were sacrificed for excision of the DG (n = 32–40) along (or not) with extraction of the CS (n = 24–30), gonad (n = 24–30) and gills (n = 16–20). Sampled tissues were weighted (ww), shock frozen in liquid nitrogen and stored at −80°C until assays. Similar procedures were applied to the native ‘control’ mussels sampled in November 2008, as well as in March, July and October 2009.
Filtration rate measurement
Mussel filtration rate was measured according to a procedure adapted from Faria et al. (2009) and based on the loss of neutral red dye particles from the water column as a result of mussel filtration activity (Coughlan 1969). For each study site, mussels were placed in 200 ml beakers (3 mussels per beaker) filled with 100 ml of a 5 mg ml−1 neutral red solution; a supplementary beaker, containing only 100 ml of the dye solution, was used as a control. Test and control beakers were placed for 2 h in the dark and at field temperature conditions. Dye particle concentration in the test beakers was then measured spectrophotometrically (340 nm), using the control beaker to generate a standard curve from which neutral red concentrations could be extrapolated. The filtration rate f, expressed in ml of water per individual and per hour (ml ind−1 h−1), was calculated using the following formula:
where V is the volume (ml) of the dye solution in the beaker, n the number of mussel in the beaker, t the duration of the filtration period (h), C 0 and C T the initial and final dye particle concentrations in the beaker (Coughlan 1969).
Biometric data processing
Condition index (CI) of sampled mussels was calculated using the following formula:
Besides, the soft body weight (ww) of a standard individual (21 mm in shell length, i.e. mean length of exposed mussels) was assessed for each site and each sampling date using the following length-weight regression equation:
where W is the soft body weight (mg, ww), L the shell length (mm), a and b some constants.
Body metal quantification
Copper (Cu), lead (Pb), nickel (Ni) and zinc (Zn) body concentrations were measured in mussel soft tissues after digestion in suprapur nitric acid for 24 h at 80°C. Resulting acid solutions, adjusted to 1.5 ml with deionized water, were processed for metal quantification by either flame (Zn) or flameless (Cu, Pb, Ni) atomic absorption spectrophotometry (flame AAS: Varian AA240FS; flameless AAS: Varian SpectrAA 220 Zeeman) as described in Dedourge-Geffard et al. (2009). Body metal concentrations were expressed in μg of metal per g of dry weight (μg g−1, dw). Tissue dry weights were estimated from tissue wet weights using a formula adapted from Schneider (1992):
and assuming a mean body water content of 85% in D. polymorpha (Voets et al. 2006; Baines et al. 2007).
Acetylcholinesterase activity assay
AChE (EC 3.1.1.7) activity was assayed in extracts prepared from pooled gills (minimal pool weight: 80 mg) homogenized in an ice cold phosphate buffer (100 mM, pH 7.8—0.1% TritonX-100) (buffer: 8 μl per mg of gill, ww) with an Ultra-Turrax® homogenizer. After centrifugation at 4°C and 9,000×g (15 min), the supernatants were collected and stored at −80°C until assays. AChE activity was measured by the 5,5′-dithiobis-2-nitrobenzoic (DTNB) acid method (Ellman et al. 1961) according to the microplate procedure described in Xuereb et al. (2009). The enzyme reaction was monitored spectrophotometrically (405 nm) during a 10-min incubation performed at pH 7.8 and 25°C in 76 mM acetylthiocholine iodide substrate (AChTh, Sigma A5751) mixed with 7.6 mM DTNB acid reagent (Sigma D8130). The supernatant protein content was measured by the method of Bradford (1976), using bovine serum albumin (Bio-Rad 500-0202) as a standard. AChE activity was expressed in nmol of hydrolyzed AChTh per minute and per mg protein (nmol min−1 mg−1 protein).
Glutathione S-transferase (GST) activity assay
GST (EC 2.5.1.18) activity was assayed in extracts prepared from individual DGs grounded by hand in a glass–glass potter and homogenized in an ice cold Tris buffer (10 mM, pH 7.5—1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride) (buffer: 10 μl per mg of DG, ww). After centrifugation at 4°C and 10,000×g (10 min), the supernatants were collected and stored at −80°C until assays. GST activity was determined spectrophotometrically (340 nm) by monitoring the conjugation of 1 mM 1-chloro-2,4-dinitrobenzene substrate (CDNB, Fluka 24440) (0.1 M phosphate buffer, pH 6.5) with 1 mM reduced glutathione (Sigma G4251), as described in Habig et al. (1974). The supernatant protein content was measured by the method of Bradford (1976). GST activity was expressed in nmol of conjugated CDNB per minute and per mg protein (nmol min−1 mg−1 protein).
Metallothionein quantification
MTs were quantified in extracts prepared from individual DGs grounded by hand in a glass–glass potter and homogenized in an ice cold Tris–HCl buffer (20 mM, pH 7.5—5 mM 2-mercapto-ethanol) (buffer: 12 μl per mg of DG, ww). The soluble (S1) and insoluble (P1) fractions of the homogenates were separated by centrifugation at 4°C and 25,000×g (55 min). S1 aliquots (200 μl) were heat-denatured (75°C, 15 min) and kept in ice for 60 min to precipitate the heat-denaturable compounds (C2) which were later separated from the heat-stable compounds (S2) by centrifugation at 4°C and 15,000×g (10 min). Isolated S2 compounds were stored at −80°C until MT quantification by Differential Pulse Polarography (DPP—Metrohm 797 VA Computrace). S2 compound sulfhydryl groups were measured at 4°C by the Brdička reagent method (Brdička 1933) (procedure described in Thompson and Cosson 1984), using MTs from rabbit liver (Sigma M7641) as a standard. MT levels were expressed in μg of metalloprotein per g of wet soft tissue (μg g−1, ww).
Energy reserve quantification
Glycogen and lipid reserves were quantified in extracts prepared from pooled DGs and gonads (minimal pool weight: 80 mg) grounded by hand in liquid nitrogen with a mortar and pestle. Resulting powder preparations were homogenized in 1 ml citrate buffer (0.1 M, pH 5.0), aliquoted and stored at −80°C until assays. Lipid and glycogen contents were determined according to the methods described in Frings et al. (1972) and Carr and Neff (1984), respectively. Olive oil (Sigma O1514) and glycogen from oyster (Sigma G8751) were used as standards for lipid and glycogen assays, respectively. Energy reserve levels were expressed in mg of glycogen or lipid per g of wet soft tissue (mg g−1, ww).
Digestive carbohydrase activity assay
Amylase (EC 3.2.1.1) and endocellulase (EC 3.2.1.4) activities were assayed in extracts prepared from pooled CSs or from individual DGs grounded by hand in a glass–glass potter and homogenized in an ice cold phosphate buffer (0.01 M, pH 6.5) (buffer: 500 μl per CS; 33 μl per mg of DG, ww). After centrifugation at 4°C and 15,000×g (30 min), the supernatants were collected and stored at −80°C until assays. Enzyme activities were measured by the 3,5-dinitrosalicylic (DNS) reagent method (Bernfeld 1955) according to the procedure described in Palais et al. (2010). Amylase activity was assayed after a 30-min incubation performed at pH 7.2 and 25°C in 1% soluble starch substrate (Sigma S9765) (0.2 M phosphate buffer, 6 mM NaCl); cellulase activity was assayed after a 60-min incubation performed at pH 5.2 and 50°C in 2% carboxymethylcellulose substrate (Sigma C5678) (0.2 M acetate buffer, 6 mM NaCl). Reaction products were quantified spectrophotometrically (540 nm), using maltose as a standard (Sigma M9171) (0.6–3.2 mg ml−1). The supernatant protein content was measured by the method of Bradford (1976). Carbohydrase activity was expressed in μg of maltose released per minute and per mg protein (μg min−1 mg−1 protein).
Statistical analysis
As collected data did not follow a normal distribution, intersite and intermonth variations were studied using a Kruskal–Wallis non-parametric ANOVA followed by a Dunn’s multiple comparison test (α = 5%). Correlations between parameters were studied using a Pearson’s correlation matrix (α = 5%), as well as a principal component analysis (PCA). Statistical tests were performed on the whole set of data except those collected within less than 6 weeks of exposure (i.e. December 2008 data) on account of the time period required for mussels to reach an equilibrium state with surrounding ambient waters (Bervoets et al. 2005). Statistical analyses were performed using R (2.13.1) and XLSTAT® (2010.3. 05).
Results
Water physicochemistry
Physicochemical features of water from the reference and exposure sites are reported in Table 1A, B, C and D. Chlorophyll levels recorded in the Vesle river system varied along an upstream–downstream gradient in spring and early summer, levels being the lowest at the Bouy and Sept-Saulx sites (2.4–5.9 μg l−1) and the highest at the Fismes and Ardre sites (6.9–31.3 μg l−1); July–August and September–October chlorophyll levels are not present in Table 1 as data records were unfortunately not available for these months. Water pH showed little seasonal variation and remained at slightly basic values throughout the study. Concentrations in mineral ions varied between upstream and downstream sites, the highest levels being generally measured at the Fismes and Ardre sites (e.g. Ca2+, NH4 +, Cl− or PO4 3− concentrations) (Table 1A).
Concentrations in metallic and organic pollutants varied along an upstream–downstream gradient, the highest concentrations in labile metals (Table 1B) and PAHs (Table 1C) being recorded at the Fismes site. Metal levels exhibited seasonal variations with [metal]max/[metal]min ratios of 1.8–2.0 for Cu, 1.5–2.1 for Ni, 1.9–4.0 for Pb and 1.6–5.7 for Zn; metal levels in waters were generally the lowest in summer (e.g. Ni, Pb, Zn) and the highest in spring (e.g. Cu, Pb). Seasonal variations of PAH concentrations could not have been assessed as October data records were not exploitable (accidental contamination of samples). Besides, much more pesticide substances were identified at the Fismes and Ardre sites (18 and 16 substances detected, respectively, over the 46 substances analyzed) than at the upstream sites (7 or less substances detected); detected compounds were mainly herbicides, fungicides or related metabolites (Table 1D).
Body metal concentrations
Average body metal concentrations measured in native and transplanted mussels are reported in Fig. 2. Collected data revealed significantly higher levels of Cu, Ni, Pb and Zn in mussels exposed at the Fismes and Ardre sites than in mussels exposed at the Bouy and Sept-Saulx sites (p < 0.05). Otherwise, metal concentrations recorded in February, May, August and October in exposed mussels (data not shown) were positively correlated with those recorded in exposure waters using the DGT sampling systems (Pearson’s correlation coefficient r = 0.63–0.80 according to the metal species; p < 0.05; n = 16).
Average body metal concentrations (μg g−1 dry weight, dw) measured in mussels sampled at the reference site (mean ± SD; n = 35–36) and transplantation sites (mean ± SD; n = 80–90) over the monitoring period. Groups with no common letters are statistically different from each other (p < 0.05). Dot-lines correspond to threshold values indicative of water contamination by metals (see text for explanation)
Filtration rates
Filtration rates of native and transplanted mussels varied significantly throughout the seasons, ranging from 1.5–1.8 ml ind−1 h−1 in winter to 4.7–7.6 ml ind−1 h−1 in summer (p < 0.05) (data not shown). Filtration rates were positively correlated with water temperature (r = 0.71), chlorophyll a concentrations (r = 0.72) and, to a lower extent, suspended matter concentrations (r = 0.34; p < 0.05; n = 30–48). No inter-site difference was ever noted as regard mussel filtration rates.
Body condition and mortality rates
CI of native and transplanted mussels exhibited a downward trend throughout the study, decreasing significantly from 0.44 in November 2008 to 0.36 (native mussels) or 0.28–0.30 (transplanted mussels) in October 2009 (p < 0.05) (data not shown). Still, at the end of the study, CI of native mussels remained significantly higher than those of transplanted mussels (p < 0.05).
Soft body weight (ww) variations of standard native and transplanted mussels are presented in Fig. 3. At the upstream sites (Bouy, Sept-Saulx), body weights exhibited a downward trend throughout the study while at the downstream sites (Fismes, Ardre), body weights increased in spring before decreasing in summer and autumn. Irrespective of the transplantation site, body weights recorded at the end of the study were always lower than those recorded at the beginning of the study. At the reference site, a rise in weight was recorded during spring months but occurred earlier and at higher amplitude than the rise exhibited by mussels exposed at the Fismes and Ardre sites. Still, at the end of the study, the body weight of mussels sampled at the reference site was close to the one recorded at the beginning of the study.
Mortality rates of transplanted mussels remained below 10% during the first months of exposure (data not shown). At the upstream sites, mortality rates started to increase in mid-spring (30.4 and 18.4% of dead mussels recorded in April at the Bouy and Sept-Saulx sites, respectively) and then underwent a steady upward trend until the end of the study (52.2 and 74.1% of dead mussels recorded in October, respectively). At the downstream sites, mortality rates started to increase later, in mid-summer (23.2 and 19.6% of dead mussels recorded in July at the Fismes and Ardre sites, respectively), and rose markedly in autumn, particularly at the Fismes site (59.8 and 39.8% of dead mussels recorded in October, respectively). On the whole, mortality rates were higher at the upstream sites and were generally twice those recorded at the downstream sites.
Core biomarker responses
AChE activities measured in the gills of native and transplanted mussels are reported in Fig. 4a (for statistical data, see Fig. 4A bis in Supplementary materials). Cholinesterase activities recorded in native mussels exhibited no clear seasonal trend throughout the study, ranging on the whole between 5.4 and 10.8 nmol min−1 mg−1 protein. AChE activities recorded in transplanted mussels increased in January-March (up to 25.7 nmol min−1 mg−1 protein) and in July–August (up to 60.4 nmol min−1 mg−1 protein) to levels significantly higher than those recorded in mussels from the reference site (p < 0.05).
Seasonal variation in acetylcholinesterase (AChE) activity (nmol hydrolyzed AThCh min−1 mg−1 protein), glutathione S-transferase (GST) activity (nmol conjugated CDNB min−1 mg−1 protein) and metallothionein (MT) concentration (mg g−1 ww tissue) recorded in the gills (AChE) or digestive gland (GST, MT) of mussels sampled at the reference site (Commercy) and transplantation sites (Bouy, Sept-Saulx, Fismes, Ardre) over the monitoring period: a AChE activity (mean ± SD, n = 4–6 pools of 2 organs); b GST activity (mean ± SD, n = 6–10 organs); c MT concentration (mean ± SD, n = 5–8 organs). For statistical data, see Fig. 4 bis in Supplementary materials
GST activities measured in the DG of native and transplanted mussels are reported in Fig. 4b (for statistical data, see Fig. 4B bis in Supplementary materials). GST activities recorded in native mussels exhibited no seasonal variations throughout the study, ranging on the whole between 336.2 and 395.5 nmol min−1 mg−1 protein. GST activities measured in transplanted mussels were overall higher than those recorded in native mussels, and increased markedly from July in mussels transplanted at the Sept-Saulx, Fismes and Ardre sites (up to 1003.9 nmol min−1 mg−1 protein). Besides, GST activities were positively correlated with water temperature over the monitoring period (r = 0.37; p < 0.05; n = 44—all sites considered).
MT concentrations measured in the DG of native and transplanted mussels are reported in Fig. 4c (for statistical data, see Fig. 4C bis in Supplementary materials). MT concentrations recorded in native mussels exhibited no seasonal variations throughout the study, ranging on the whole between 572.3 and 676.2 μg g−1 ww. MT concentrations measured in transplanted mussels were overall higher than those recorded in native mussels, and increased markedly from July in mussels transplanted at the Bouy and Sept-Saulx sites (up to 1174.5 nmol min−1 mg−1 protein).
Energy reserve responses
Glycogen and lipid concentrations measured in the DG of native and transplanted mussels are reported in Fig. 5a, b, respectively (for statistical data, see Fig. 5A bis and 5B bis, respectively, in Supplementary materials); concentrations measured in the gonad are not shown here as they were closely correlated to those measured in the DG (glycogen: r = 0.89–0.94, lipids: r = 0.76–0.89; p < 0.05; n = 20–53). Concurrently with the reproductive cycle of mussels, energy reserve concentrations varied considerably throughout the seasons: in every mussels, glycogen and lipid concentrations decreased significantly from January (glycogen: 26.6–36.1 mg g−1 ww; lipids: 10.1–16.1 mg g−1 ww) to July (glycogen: 7.5–11.9 mg g−1 ww; lipids: 4.5–7.1 mg g−1 ww) (p < 0.05). Energy reserve concentrations were the lowest in July but, whereas in the following months, a complete restoration of initial reserve concentrations was observed in mussels from the reference site, no or only partial recovery was observed in mussels from the exposure sites.
Seasonal variation in energy reserve concentration (mg g−1 wet weight, ww) recorded in the digestive gland of mussels sampled at the reference site (Commercy) and transplantation sites (Bouy, Sept-Saulx, Fismes, Ardre) over the monitoring period: a glycogen and b lipid concentrations (mean ± SD, n = 4–5 pools of 3–4 organs). For statistical data, see Fig. 5 bis in Supplementary materials
Digestive enzyme responses
Amylase (AmCS) and cellulase (CeCS) activities measured in the CS of native and transplanted mussels are reported in Fig. 6a, b, respectively (for statistical data, see Fig. 6A bis and 6B bis, respectively, in Supplementary materials). For both carbohydrases, activities recorded in native mussels exhibited no clear seasonal trend throughout the study, ranging between 287.1 and 537.9 μg min−1 mg−1 protein for amylase, and between 105.2 and 163.7 μg min−1 mg−1 protein for cellulase. As regard site-to-site variations, enzyme activities recorded in mussels transplanted on the Vesle river (Bouy, Sept-Saulx, Fismes) were significantly higher in spring and early summer than those recorded in mussels transplanted on the Ardre river (p < 0.05); over this period, CS enzyme activities measured in mussels from the Ardre site remained very close to those measured in mussels from the reference site. In late summer and autumn, no intersite difference was noted, except higher amylase activities in mussels exposed at the Fismes sites. Over the monitoring period, CS amylase activities were positively correlated with water temperature (r = 0.42; p < 0.05; n = 44—all sites considered), as did not cellulase activities. No significant correlation was observed between CS carbohydrase activities and chlorophyll levels (at least for the period during which chlorophyll data were available).
Seasonal variation in digestive enzyme activities (μg maltose min−1 mg−1 protein) recorded in the crystalline style (CS) and the digestive gland (DG) of mussels sampled at the reference site (Commercy) and transplantation sites (Bouy, Sept-Saulx, Fismes, Ardre) over the monitoring period: a CS amylase and b CS cellulase activities (mean ± SD, n = 4–5 pools of 3–4 organs); c DG amylase and d DG cellulase activities (mean ± SD, n = 7–8 organs). For statistical data, see Fig. 6 bis in Supplementary materials
Amylase (AmDG) and cellulase (CeDG) activities recorded in the DG of native and transplanted mussels are reported in Fig. 6c, d, respectively (for statistical data, see Fig. 6C bis and 6D bis, respectively, in Supplementary materials). For both carbohydrases, activities measured in winter (AmDG = 12.5–26.5 μg min−1 mg−1 protein, CeDG = 7.8–12.2 μg min−1 mg−1 protein) were, at all sites, significantly lower than those measured in summer (AmDG = 44.5–111.0 μg min−1 mg−1, CeDG = 12.5–21.2 μg min−1 mg−1 protein) (p < 0.05). A clear seasonal trend was thus observed as regard DG amylase and cellulase activities which were, on average, 2.9 and 1.5 times more elevated in summer than in winter, respectively. At the end of the study (autumn 2009), DG enzyme activities measured in mussels from the reference site were very similar to those recorded at the beginning of the study (autumn 2008) while, in mussels from the exposure sites, DG enzyme activities remained quite elevated, especially amylase ones. As regard site-to-site variations, significant differences were noticed in May–June (lower amylase and cellulase activities in mussels from the Ardre site) and in September–October (higher amylase activities in mussels from the exposure sites than in mussels from the reference site; higher cellulase activities in mussels from the upstream sites than in mussels from the downstream sites or the reference site). Over the monitoring period, DG enzyme activities were positively correlated with water temperature (r = 0.76–0.78; p < 0.05; n = 44—all sites considered) while no significant correlation was observed with chlorophyll levels (at least for the period during which chlorophyll data were available).
Throughout the study, specific activities of amylase were markedly higher than those of cellulase, whatever the organ considered: AmCS/CeCS = 2.8 ± 0.9 (mean ± SD, n = 218), AmDG/CeDG = 3.3 ± 1.7 (n = 351). As well, specific activities measured in the CS were markedly higher than those measured in the DG, whatever the enzyme considered: AmCS/AmDG = 14.6 ± 9.3 (n = 48), CeCS/CeDG = 13.9 ± 5.5 (n = 48). Otherwise, digestive carbohydrase activities were assayed at their optimal temperature activity, i.e. 25°C for amylase and 50°C for cellulase in D. polymorpha (Palais et al. 2010). However, as zebra mussels are temperate organisms, these temperatures are not truly relevant from an ecological point of view. Therefore, to be more representative of environmental conditions and to be comparable with literature data, amylase and cellulase activities were calculated over the range 10–20°C, using for this aim their Q10 temperature coefficient (Q X = multiplying factor by which enzyme activity increases every X°C increase), i.e. 1.45 for amylase and 1.28 for cellulase in D. polymorpha (Palais et al. 2010).
Principal component analysis (PCA)
The correlation circle and factorial maps obtained from the PCA performed on collected data are presented in Fig. 7 [some variables were not included in the PCA due to information redundancy with other variables (e.g. glycogen and lipid concentrations in the gonad highly correlated with those in the DG) or to partially lacking data (e.g. chlorophyll data unavailable in the last months of the study)]. F1 axis of the PCA appeared to be strongly associated with temperature and season (Fig. 7b: negative correlation with winter and spring, positive correlation with summer and fall), as well as with most of the biological responses (Fig. 7a: positive correlation with DG carbohydrase activities, GST activity and MT concentration, negative correlation with energy reserve concentration), except AChE activity and CS carbohydrase activities. On account of the monitored parameters, F1 axis especially discriminated mussels sampled at the reference site (Commercy site) from mussels sampled at the exposure sites (Bouy, Sept-Saulx, Fismes and Ardre sites). F2 axis of the PCA appeared to be strongly associated with site location (Fig. 7c: negative correlation with the Commercy, Bouy and Sept-Saulx sites, positive correlation with the Fismes and Ardre sites), as well as with metal exposure levels (Fig. 7a: positive correlation with Cu, Ni, Pb and Zn body metal concentrations). On account of the monitored parameters, F2 axis especially discriminated mussels transplanted in the lower Vesle river (Fismes site) from mussels transplanted in the upper Vesle river (Bouy and Sept-Saulx sites).
Correlation circle (a) and factorial maps (b, c) obtained from the principal component analysis performed on collected data. Active variables (biological variables—straight arrows in (a)): body metal concentrations (Cu, Ni, Pb, Zn), acetylcholinesterase activity in the gills (AChE), glutathione-S transferase activity in the digestive gland (GST), metallothionein concentration in the digestive gland (MT), carbohydrase activities in the crystalline style (CS amylase/endocellulase) and the digestive gland (DG amylase/endocellulase), glycogen and lipid concentrations in the digestive gland, condition index (CI) and mortality rate. Supplementary variables (physicochemical variables—dotted arrows in (a)): water pH, temperature, conductivity and nitrate concentration. Descriptive variables: ‘season’ (barycentres in (b)); ‘site’ (barycentres in (c)). Co Commercy site, Bo Bouy site, SS Sept-Saulx site, Fi Fismes site, Ar Ardre site, Wi winter season (December 2008 to February 2009), Sp spring season (March to May 2009), Su summer season (June to August 2009), Au autumn season (September to October 2009)
Discussion
Site contamination
Water pH, temperature and ion concentrations (Ca2+, NO3 −, PO4 3−) are environmental parameters known to regulate the distribution of zebra mussels within inland water bodies (Ramcharan et al. 1992; McMahon 1996). The values recorded for these parameters in the Vesle and Ardre rivers were consistent with the ecological requirements of the species (Ramcharan et al. 1992; McMahon 1996), indicating that exposure sites were suitable environments for zebra mussels. Nevertheless, mussels may have suffered from chemical stress as metallic and organic pollutants were detected in surface waters, the highest concentrations being recorded at the Fismes and Ardre sites, consistently with their hydrogeographic location downstream of agricultural and urban areas. Still, Priority (Hazardous) Substances detected in the Vesle and Ardre rivers (Ni, Pb; AMPA, atrazine, diuron, glyphosate, isoproturon, simazine; anthracene, fluoranthene) were at concentrations lower than the Environmental Quality Standards (European Directive 2008/105/EC).
Metal and PAH levels were assessed using time-integrated sampling systems (DGTs, SPMDs) which mainly sample the truly dissolved fraction of chemicals (‘free fraction’) and provide an insight of their potential bioavailability (Tusseau-Vuillemin et al. 2007). This explains the significant correlation obtained between labile metal concentrations in exposure waters and accumulated metal concentrations in exposed mussels. However, if DGTs and SPMDs are representative of the dissolved phase (waterborne exposure route), they are not representative of the particulate one (dietary exposure route): indeed, particle-associated pollutants do not readily diffuse into passive samplers while they may be ingested by filter-feeding bivalves (Prest et al. 1992; Björk 1995). Therefore, DGT and SPMD data records might have underestimated metal and organic exposure in the Vesle and Ardre rivers.
Mersch (1993) reported for D. polymorpha body metal concentration thresholds indicative of site contamination by Cu (12.0 μg g−1, dw), Ni (12.0 μg g−1, dw), Pb (0.5 μg g−1, dw) and Zn (110 μg g−1, dw). According to these values, the whole exposure sites were lowly contaminated by Cu while the Fismes site was also lowly contaminated by Ni, Pb and Zn. Body levels of these metals showed little seasonal variations throughout the study, obviously as a result of a dynamic balance between temporal fluctuations in metal bioavailability, uptake and depuration kinetics, and mussel body weight (Ruelas-Inzunza and Paez-Osuna 2000 and literature cited therein).
Core biomarker responses: evidences of a pollutant exposure
AChE activities measured in the gills of native zebra mussels (5.4–10.8 nmol min−1 mg−1 protein) were slightly higher than those previously reported for D. polymorpha (0.6–2.7 nmol min−1 mg−1 protein—gills or whole soft tissues) (Binelli et al. 2005; Faria et al. 2010) but were still in the range of cholinesterase activities reported for other bivalve species (1.5–80.0 nmol min−1 mg−1 protein—gills of Anodonta, Corbicula, Crassostrea, Mytilus and Ostrea sp.) (Escartin and Porte 1997; Mora et al. 1999; Valbonesi et al. 2003; Bocquené and Galgani 2004; Corsi et al. 2007). Neurotoxic pesticides such as carbamates, organophosphates and pyrethrins are known to exert an inhibitory effect on AChE activity (Bocquené and Galgani 2004; Binelli et al. 2005). And yet, despite the presence of pesticides in the Vesle and Ardre rivers, depressed AChE activities were never recorded in transplanted mussels, even at the most impacted sites. This may be related to the relative absence of neurotoxic chemicals in exposure waters and/or to the resistance of zebra mussels towards pesticide exposure (Dauberschmidt et al. 1997). Besides, timely inductions of AChE activity were noted during the study period, especially in late summer where transplanted mussels exhibited cholinesterase activities 2.6–5.9 times higher than those recorded in native mussels. Similar inductions have been reported for zebra mussels from Lake Lugano (Italy) (Binelli et al. 2005), probably as a result of Cu exposure as already noted in fish species (Dethloff et al. 1999; Romani et al. 2003). Enhanced AChE activity might be related to interactions of copper ions with AChE enzyme, substrate and coenzymes, resulting in protection of the enzyme against thermal inactivation, increased enzyme-substrate affinity or reduced energy activation (Jonsson and Aoyama 2010). As Cu levels in transplanted mussels (15.7–41.4 μg g−1 dw) were very similar to those recorded in mussels from Lake Lugano (17.5–36.2 μg g−1 dw) (Camusso et al. 2001), increased AChE activities noticed in transplanted mussels could be related to the Cu load of the Vesle and Ardre rivers. Nevertheless, as mussel AChE activities and Cu levels were not correlated with each other, some other external (e.g. water temperature: increased metabolic activities in summer) and/or internal (e.g. reproductive state: increased body Cu levels in post-spawned mussels) factors may have interfered in the activation of the cholinergic system (Escartin and Porte 1997; Najimi et al. 1997).
GST activities measured in the DG of native mussels (336.2–395.5 nmol min−1 mg−1 protein) were in the range of those previously reported for D. polymorpha (160.0–450.0 nmol min−1 mg−1 protein—whole soft tissues) (Osman et al. 2007; Binelli et al. 2010) and other bivalve species (22.0–1840.0 nmol min−1 mg−1 protein—DG of Anodonta, Corbicula, Crassostrea and Mytilus sp.) (reviewed in Le Pennec and Le Pennec 2003). From midsummer, GST activities exhibited an upstream–downstream gradient, the highest activities being recorded in mussels exposed at the Sept-Saulx, Fismes and Ardre sites, i.e. the sites most contaminated with PAHs and pesticide compounds. These observations are consistent with the well-documented role of GST in phase II biotransformation of organic pollutants and the reported increase in its activity as a function of water pollution (Roméo and Giambérini 2008; Contardo-Jara et al. 2009; Binelli et al. 2010). Besides, the midsummer increase in GST activities resulted very likely from an interaction with water temperature as mussels tend to accumulate more contaminants during periods of elevated temperature, especially as a result of increased filtration rates (Minier et al. 2006).
MT concentrations recorded in the DG of native mussels (572.3–676.2 μg g−1, ww) were in the range of those previously reported for D. polymorpha (120.0 μg g−1, ww—whole soft tissues) (Ivanković et al. 2009) although comparison with previous MT data was difficult owing to differences in expression of results. Still, MT concentrations measured in zebra mussels were consistent with those reported for other bivalve species (700–4,200 μg g−1, ww—DG of Chlamys, Crassostrea, Mytilus and Ruditapes sp.) (as reviewed in Amiard et al. 2006). MTs are metal-binding proteins generally considered to be involved in the detoxification and sequestration of metals present in toxic concentrations within cells (upregulation of MTs under metal stress) (Viarengo et al. 1997; Amiard et al. 2006). However, metal-binding to MTs is not the only detoxification process at play in mollusks and excess metals can also be incorporated in metal-rich insoluble granules (mineralization process) (Marigómez et al. 2002). Depending on the species, the relative importance of these two metal-sequestration systems may vary considerably (Amiard et al. 2006). Interestingly, in exposed mussels, MT concentrations were not correlated with accumulated metal levels (Cd, Cu, Ni, Pb and Zn considered alone or together). Moreover, mussel MT concentrations were very similar from an exposure site to another despite significant differences in metal exposure. These observations therefore suggest that, in D. polymorpha, MTs play only a very small role in the accumulation of part or all of the monitored metals, consistently with previous studies dealing with Cu (Ivanković et al. 2009) and Zn (Marie et al. 2006). Increased MT levels recorded in exposed mussels near the end of the study were obviously not related to changes in metal exposure and were probably more related to changes in protein metabolism (e.g. under nutritional stress—see discussion below) or any other confounding factors (e.g. presence of antibiotics, vitamins or herbicides in the water) (Mourgaud et al. 2002; Amiard et al. 2006 and literature cited therein).
Physiological responses: evidences of a nutritional stress
Glycogen and lipid concentrations recorded in native and transplanted mussels were consistent with those previously reported for D. polymorpha and other bivalve species (Palais et al. 2011 and literature cited therein). Energy reserve concentrations exhibited seasonal variations closely related to zebra mussel reproductive cycle, development of the gonad occurring in winter and early spring, maturation and release of gametes occurring in late spring and summer (reserve depletion), and resting of the gonad occurring in autumn (reserve restoration). Interestingly, reduced gonad development, delayed gamete maturation and/or asynchrony between male and female spawning events were noticed at the Bouy and Sept-Saulx sites (Palais et al. 2011). According to authors, these reproductive disturbances resulted very likely from the low food (chlorophyll) levels recorded in the upper Vesle river. Mussels exposed at the Bouy and Sept-Saulx sites may have thus suffered from a nutritional stress throughout the study, explaining the important weight losses and mortality rates recorded from mid-spring, as well as mussel inability to restore energy reserves in autumn.
At the downstream sites, Palais et al. (2011) noticed ‘normal’ gametogenic cycles in exposed male and female mussels. Still, at the Fismes and Ardre sites, mussel reproductive effort was obviously limited by environmental condition as, in comparison to the reference site, gonad tissue development was lower (as suggested by the lower body weights recorded in spring). Besides, as already noticed in mussels exposed at the upstream sites, mussels exposed at the downstream sites were unable to restore energy reserves in the months following the breeding season. These disturbances resulted very likely from reduced energy inputs (nutritional stress) and/or increased energy outputs (chemical stress). As chlorophyll data records were not available for autumn months, it is not possible to conclude about the potential decrease of mussel energy inputs at this time of the year. On the other hand, it is almost certain that energy outputs were high at this period owing to elevated water temperatures (increased filtration activity) and pollutant exposure (increased GST activity).
Digestive enzyme responses: integration of natural and man-made stressors
Carbohydrase activities determined in the CS of native mussels over the temperature range 10–20°C (AmCS = 166.2–448.2 μg min−1 mg−1 protein; CeCS = 39.2–78.1 μg min−1 mg−1 protein) were consistent with those previously reported for D. polymorpha (AmCS = 336.0–484.2 μg min−1 mg−1 protein; CeCS = 21.7–34.0 μg min−1 mg−1 protein; temperature: 10–20°C) (Palais et al. 2010) and other bivalve species (AmCS = 50.8–326.9 μg min−1 mg−1 protein; CeCS = 9.4–67.2 μg min−1 mg−1 protein; temperature: 18°C) (Brock and Brock 1989; Brock and Kennedy 1992). Similarly, enzyme activities determined in the DG of native mussels over the temperature range 10–20°C (AmDG = 9.6–60.0 μg min−1 mg−1 protein; CeDG = 4.3–8.1 μg min−1 mg−1 protein) were consistent with those already reported for D. polymorpha (AmDG = 14.8–24.1 μg min−1 mg−1 protein; CeDG = 1.4–2.3 μg min−1 mg−1 protein) (Palais et al. 2010) and other bivalve species (AmDG = 2.9–82.4 μg min−1 mg−1 protein; CeDG = 1.3–73.4 μg min−1 mg−1 protein) (Brock et al. 1986; Brock and Kennedy 1992). Besides, activity ratios calculated in the present study (AmCS/CeCS and AmDG/CeDG; AmCS/AmDG and CeCS/CeDG) were in the range of those previously reported for D. polymorpha (Palais et al. 2010).
According to literature data, digestive carbohydrase activity of bivalve species shows a great adaptability to variations in food availability, quantity and quality (Johnson et al. 1996; Ibarrola et al. 1998a, b; Labarta et al. 2002; Fernández-Reiriz et al. 2004; Albentosa and Moyano 2008; Bourgeault et al. 2010). However, if food availability exerts a primary control on digestive enzyme activities, the latter could also vary in response to other factors, both external (e.g. temperature) (Brock et al. 1986) and internal (e.g. reproductive state) (Lambert et al. 2008). As noticed in our study, these ‘regulation’ factors exhibit seasonal variations which, logically, should be reflected on digestive enzyme activities. Nevertheless, if seasonal amylase and cellulase activities were well recorded in the DG of mussels, no seasonal activities were recorded in their CS. Wong and Cheung (2001) noticed as well an absence of seasonal amylase and cellulase activities in the CS of Perna viridis (green mussel). CS enzyme activities are obviously not constitutive in D. polymorpha as site-to-site differences were noted in our study, indicating a regulation of CS activities by local environmental parameters. So, the absence of seasonal amylase and cellulase activities in the CS could be explained by adjustment processes of enzyme synthesis and secretion merely less sensitive in epithelial cells of the style sac than in digestive cells of the DG (Ibarrola et al. 1998b). The absence of seasonality in the CS activities could also be related to the innate nature of the style, i.e. a secretion organ whose weight does not vary according to seasons. On the contrary, the DG is an organ whose weight exhibit seasonal variations with a potential influence on digestive enzyme activities (Ibarrola et al. 1998a).
Several authors have reported a depression of digestive carbohydrase activities in invertebrate species exposed in vitro or in situ to chemical pollutants, especially heavy metals (Farris et al. 1994; Yan et al. 1996; De Coen and Janssen 1997; Milam and Farris 1998; Barfield et al. 2001; Chen et al. 2002; Le Bihan et al. 2004; Li et al. 2008; Dedourge-Geffard et al. 2009; Kalman et al. 2009; Boldina-Cosqueric et al. 2010; Bourgeault et al. 2010). Digestive enzyme response to chemical stressors may actually be rather complex (Fig. 8) and depends, among others, on exposure time, toxicant concentration and exposure route (Farris et al. 1994; De Coen and Janssen 1997). Toxic contaminants present in water may initially lead to a reduction of mussel energy inputs as a result of decreased food availability (reduced phytoplankton productivity) (Jantz and Neumann 1998) and/or decreased food intake (reduced filtration activity, increased valve-closure duration) (Borcherding 1992; Kraak et al. 1993). At this time, mussels may be compelled to activate or enhance their defense and repair mechanisms, leading to increased metabolic requirements (general adaptation syndrome—Selye 1976). As a result of decreased energy inputs and increased energy outputs, mussels might respond with an adjustment of their digestive processes (e.g. increased enzyme activities, increased gut passage time) so as to maximize dietary energy assimilation (De Coen and Janssen 1997; Allison et al. 1998). Moreover, metal contaminants ingested with food particles may start to interact with extracellular enzymes (e.g. binding to active site, modification of enzyme structure) resulting in (induction followed by) decline of their activity (Yan et al. 1996; De Coen and Janssen 1997; Milam and Farris 1998; Barfield et al. 2001; Chen et al. 2002; Le Bihan et al. 2004). If exposure persists or even worsens, detoxification systems of mussels may become unable to cope with entering toxicants which may then start to exert deleterious effects at the intracellular level, resulting for example in nervous disturbances (Moulton et al. 1996). Besides, toxicants may start to interfere with intracellular digestive enzyme activity through a down-regulation of enzyme hydrolytic properties (direct interaction) or expression processes (indirect interaction) (Le Bihan et al. 2004). In the end, under chemical stress, digestive enzyme responses obviously follow a bell-shaped curve, early induction of enzyme activities being followed by a decrease of the latter.
At upstream sites, mussels exhibited especially high digestive enzyme activities in spring and early summer, concurrently with the development and maturation of gametes. Besides, digestive cellulase activities recorded in mussels from the Bouy and Sept-Saulx sites were always higher or equal to those recorded in mussels from other sites. These digestive patterns were obviously related to an interaction between mussel reproductive investment and adverse nutritional conditions, mussels increasing the assimilation efficiency of both starch and cellulose derivatives in an attempt to provide sufficient energy to support gametogenesis. At the Fismes site, mussels exhibited especially high digestive amylase activities both inside (spring and early summer) and outside (late summer and autumn) the reproductive season. Amylase activities were obviously not induced to support the sole reproductive effort of mussels and, as the latter were continuously exposed to chemical stressors in the lower Vesle river, activity induction were also very likely related to investment in detoxification processes. At the Ardre site, digestive enzyme activities were always lower than those recorded at the Fismes site (as well as at the upstream sites) but were very similar to those recorded at the reference site. These activity levels were consistent with the higher chlorophyll a levels and lower pollution levels noted at the Ardre site than at the Fismes site: mussels exposed at the Ardre site had indeed probably less to invest in enzyme activity to meet their energy requirements. This assumption is at least admissible for the period of the year during which chlorophyll data records were available: in the Ardre river (as in the Vesle river), exposed mussels had indeed great difficulties to recover their initial (T 0) energy reserve levels, which suggests they suffered from low dietary energy inputs in autumn. This hypothesized autumnal nutritional stress could besides explain the especially high amylase activities recorded in all exposed mussels in September–October.
Biomonitoring perspectives
As indicated by the PCA, most of the biological parameters monitored in situ were sensitive to seasonal environmental factors such as temperature (Fig. 7a) as well as, very likely, other seasonal-dependant (a)biotic factors (e.g. filtration activity, reproductive state). Interestingly, a few parameters did not exhibit such sensitivity to seasonal confounding factors, as was the case for SC carbohydrase activities. SC amylase activity especially appeared to be more associated with site location and metal exposure level, allowing the discrimination of the most chemically impacted site (i.e. Fismes site) from the other exposure sites. Thus, the high discrimination potential of CS enzyme activities, in association with their low seasonal-dependency, makes these parameters very promising biomarkers to develop in biomonitoring studies using bivalves—or other organisms with a style—as sentinel species.
Synthesis and conclusion
The monitoring study performed on the Vesle river basin revealed no or little effect of chemical stressors on the biology of transplanted zebra mussels. Metal and organic pollutants were present in waters at concentrations low enough to exert no apparent deleterious effects on digestion and energy storage. These energy demanding processes were on the other hand seriously affected by a nutritional stress, especially evident at upstream exposure sites. Adverse food conditions recorded in the upper Vesle river had no apparent effect on the monitored core biomarkers (AChE, GST, MT) which responded more specifically to the (low) metallic and organic contamination levels recorded in the lower Vesle and Ardre rivers. Although AChE and GST responses were not especially alarming as regard water quality, they revealed the presence of bioavailable contaminants in surface waters of the Vesle river basin.
Digestive enzyme activities appeared as interesting monitoring tools as they responded both to the chemical stress detected at the downstream sites (responses consistent with those of core biomarkers) and to the nutritional stress detected at the upstream sites (responses consistent with those of energy reserve and reproductive cycles). Activity induction recorded in most of exposed mussels supported the idea of an energy optimizing strategy implementation in stressed mussels, the latter trying to compensate for either increased energy outputs (general adaptation syndrome) or decreased energy inputs (food scarcity). No activity repression was observed in any of exposed mussels, consistently with the low metallic and organic contamination levels recorded in the Vesle river basin.
Despite evident informative values, digestive enzyme responses may be quite hard to interpret as they integrate complex metabolic regulation processes. Digestive enzyme activities have thus to be monitored along with other, more specific, biomarkers so as to efficiently exploit the informative potential of their responses. Besides, the CS appeared as an interesting alternative to the DG for the study of digestive enzyme activities in sentinel bivalves: this organ is indeed easily sampled, devoted to sole digestive functions and exhibits no seasonal variations in its activity. Still, digestive enzyme sensitivity to potentially confounding biotic (e.g. individual life stage) and abiotic factors (e.g. food availability) remains to be precised in D. polymorpha.
References
Albentosa M, Moyano FJ (2008) Influence of nutritional stress on digestive enzyme activities in juveniles of two marine clam species, Ruditapes decussatus and Venerupis pullastra. J Sea Res 59:249–258
Allison N, Millward GE, Jones MB (1998) Particle processing by Mytilus edulis: effects on bioavailability of metals. J Exp Mar Biol Ecol 222:149–162
Amiard JC, Amiard-Triquet C, Barka S, Pellerin J, Rainbow PS (2006) Metallothioneins in aquatic invertebrates: their role in metal detoxification and their use as biomarkers. Aquat Toxicol 76:160–202
Bacchetta R, Mantecca P (2009) DDT polluted meltwater affects reproduction in the mussel Dreissena polymorpha. Chemosphere 76:1380–1385
Baines SB, Fisher NS, Cole JJ (2007) Dissolved organic matter and persistence of the invasive zebra mussel (Dreissena polymorpha) under low food conditions. Limnol Oceanogr 52:70–78
Barfield ML, Farris JL, Black MC (2001) Biomarker and bioaccumulation responses of asian clams exposed to aqueous cadmium. J Toxicol Environ Health A 63:495–510
Bernfeld P (1955) Amylases, α and β. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 1. Academic Press, New York, pp 149–158
Bervoets L, Voets J, Covaci A, Chu S, Qadah D, Smolders R, Schepens P, Blust R (2005) Use of transplanted zebra mussels (Dreissena polymorpha) to assess the bioavailability of microcontaminants in Flemish surface waters. Environ Sci Technol 39:1492–1505
Binelli A, Ricciardi F, Riva C, Provini A (2005) Screening of POP pollution by AChE and EROD activities in zebra mussels from the Italian Great Lakes. Chemosphere 61:1074–1082
Binelli A, Cogni D, Parolini M, Provini A (2010) Multi-biomarker approach to investigate the state of contamination of the R. Lambro/R. Po confluence (Italy) by zebra mussel (Dreissena polymorpha). Chemosphere 79:518–528
Björk M (1995) Bioavailability and uptake of hydrophobic organic contaminants in bivalve filter-feeders. Ann Zool Fenn 32:237–245
Bocquené, G., Galgani, F., 2004. Méthodes d’analyse en milieu marin – Les marqueurs biologiques des effets de polluants : l’acétylcholinestérase. Ifremer, Plouzané
Boldina-Cosqueric I, Amiard JC, Amiard-Triquet C, Dedourge-Geffard O, Métais I, Mouneyrac C, Moutel B, Berthet B (2010) Biochemical, physiological and behavioural markers in the endobenthic bivalve Scrobicularia plana as tools for the assessment of estuarine sediment quality. Ecotoxicol Environ Saf 73:1733–1741
Borcherding J (1992) Another early warning system for the detection of toxic discharges in the aquatic environment based on valve movements of the freshwater mussel Dreissena polymorpha. In: Neumann D, Jenner HA (eds) The zebra mussel Dreissena polymorpha: ecology, biological monitoring and first applications in the water quality management–Limnologie aktuell, vol 4. Gustav Fischer Verlag, pp 127–146
Bourgeault A, Gourlay-Francé C, Vincent-Hubert F, Palais F, Geffard A, Biagianti-Risbourg S, Pain-Devin S, Tusseau-Vuillemin MH (2010) Lessons from a transplantation of zebra mussels into a small urban river: an integrated ecotoxicological assessment. Environ Toxicol 25:468–478
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brdička A (1933) Polarographic studies with the dropping mercury method. A new test for proteins in the presence of cobalt salts in ammoniacal solution of ammonium chloride. Collect Czechoslov Chem Commun 5:112–128
Brock A, Brock V (1989) Kinetics of amylase, cellulase and laminarinase in four commercially important bivalves. In: Klekowski RZ, Styczyńska-Jurewicz E, Falkowski L (eds) Proceedings of the 21st European Marine Biology Symposium, Gdańsk, 14-19 September 1986. Zakład Narodowy im. Ossolińskich, pp 31–40
Brock V, Kennedy VS (1992) Quantitative analysis of crystalline style carbohydrases in five suspension- and deposit-feeding bivalves. J Exp Mar Biol Ecol 159:51–58
Brock V, Kennedy VS, Brock A (1986) Temperature dependency of carbohydrase activity in the hepatopancreas of thirteen estuarine and coastal bivalve species from the North American east coast. J Exp Mar Biol Ecol 103:87–101
Calow P, Sibly R (1990) A physiological basis population processes: ecotoxicological implications? Funct Ecol 4:283–288
Camusso M, Balestrini R, Binelli A (2001) Use of zebra mussel (Dreissena polymorpha) to assess trace metal contamination in the largest Italian subalpine lakes. Chemosphere 44:263–270
Carr RS, Neff JM (1984) Quantitative semi-automated enzymatic assay for tissue glycogen. Comp Biochem Physiol B 77:447–449
Chen Z, Mayer LM, Weston DP, Bock MJ, Jumars PA (2002) Inhibition of digestive enzyme activities by copper in the guts of various marine benthic invertebrates. Environ Toxicol Chem 21:1243–1248
Contardo-Jara V, Krueger A, Exner HJ, Wiegand C (2009) Biotransformation and antioxidant enzymes of Dreissena polymorpha for detection of site impact in watercourses of Berlin. J Environ Monitor 11:1147–1156
Corsi I, Pastore AM, Lodde A, Palmerini E, Castagnolo L, Focardi S (2007) Potential role of cholinesterases in the invasive capacity of the freshwater bivalve Anodonta woodiana (Bivalvia: Unionacea): a comparative study with the indigenous species of the genus Anodonta sp. Comp Biochem Physiol C 145:413–419
Coughlan J (1969) Estimation of filtering rate from clearance of suspensions. Mar Biol 2:356–358
Dauberschmidt C, Dietrich DR, Schlatter C (1997) Esterases in the zebra mussel Dreissena polymorpha: activities, inhibition, and binding to organophosphates. Aquat Toxicol 37:295–305
Davison W, Zhang H (1994) In situ speciation measurements of trace components in natural waters using thin-film gels. Nature 367:546–548
De Coen WM, Janssen CR (1997) The use of biomarkers in Daphnia magna toxicity testing. II. Digestive enzyme activity in Daphnia magna exposed to sublethal concentrations of cadmium, chromium and mercury. Chemosphere 35:1053–1067
De Coen WM, Janssen CR, Giesy JP (2000) Biomarker applications in ecotoxicology: bridging the gap between toxicology and ecology. In: Persoone G, Janssen C, De Coen WM (eds) New microbiotests for routine toxicity screening and biomonitoring. Kluwer Academic, Plenum Publishers, New York, pp 13–25
de Lafontaine Y, Gagné F, Blaise C, Costan G, Gagnon P, Chan HM (2000) Biomarkers in zebra mussels (Dreissena polymorpha) for the assessment and monitoring of water quality of the St Lawrence River (Canada). Aquat Toxicol 50:51–71
Dedourge-Geffard O, Palais F, Biagianti-Risbourg S, Geffard O, Geffard A (2009) Effects of metals on feeding rate and digestive enzymes in Gammarus fossarum: an in situ experiment. Chemosphere 77:1569–1576
Dethloff GM, Schlenk D, Hamm JT, Bailey HC (1999) Alterations in physiological parameters of rainbow trout (Oncorhynchus mykiss) with exposure to copper and copper/zinc mixtures. Ecotoxicol Environ Saf 42:253–264
DREAL Champagne-Ardenne (2010) Eaux et milieu aquatiques, Qualité des eaux, Eaux de surface, Les données sur la qualité de l’eau en Champagne-Ardenne en 2008. http://www.champagne-ardenne.developpement-durable.gouv.fr
Ellman GL, Courtney KD, Andres jr V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Escartin E, Porte C (1997) The use of cholinesterase and carboxylesterase activities from Mytilus galloprovincialis in pollution monitoring. Environ Toxicol Chem 16:2090–2095
Faria M, Carrasco L, Diez S, Riva MC, Bayona JM, Barata C (2009) Multi-biomarker responses in the freshwater mussel Dreissena polymorpha exposed to polychlorobiphenyls and metals. Comp Biochem Physiol C 149:281–288
Faria M, López MA, Díez S, Barata C (2010) Are native naiads more tolerant to pollution than exotic freshwater bivalve species? An hypothesis tested using physiological responses of three species transplanted to mercury contaminated sites in the Ebro River (NE, Spain). Chemosphere 81:1218–1226
Farris J, Grudzien J, Belanger S, Cherry D, Cairns J (1994) Molluscan cellulolytic activity responses to zinc exposure in laboratory and field stream comparisons. Hydrobiologia 287:161–178
Fernández-Reiriz MJ, Labarta U, Navarro JM (2004) Feeding and digestive response of Argopecten purpuratus to short-term variation in food quality and quantity. Aquaculture 237:347–364
Frings CS, Fendley TW, Dunn RT, Queen CA (1972) Improved determination of total serum lipids by the sulfophosphovanillin. Clin Chem 18:673–674
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Huckins JN, Tubergen MW, Manuweera GK (1990) Semi-permeable membrane devices containing model lipid: a new approach to monitoring the bioavailability of lipophilic contaminants and estimating their bioconcentration potential. Chemosphere 20:533–552
Ibarrola I, Larretxea X, Iglesias JIP, Urrutia MB, Navarro E (1998a) Seasonal variation of digestive enzyme activities in the digestive gland and the crystalline style of the common cockle Cerastoderma edule. Comp Biochem Physiol A 121:25–34
Ibarrola I, Navarro E, Iglesias JIP (1998b) Short-term adaptation of digestive processes in the cockle Cerastoderma edule exposed to different food quantity and quality. J Comp Physiol B 168:32–40
Ivanković D, Pavičić J, Beatović V, Klobučar RS, Klobučar GI (2009) Inducibility of metallothionein biosynthesis in the whole soft tissue of zebra mussels Dreissena polymorpha exposed to cadmium, copper, and pentachlorophenol. Environ Toxicol 25:198–211
Jantz B, Neumann D (1998) Growth and reproductive cycle of the zebra mussel in the River Rhine as studied in a river bypass. Oecologia 114:213–225
Johnson MA, Paulet YM, Donval A, Le Pennec M (1996) Histology, histochemistry and enzyme biochemistry in the digestive system of the endosymbiont-bearing bivalve Loripes lucinalis (Lamarck). J Exp Mar Biol Ecol 197:15–38
Jonsson CM, Aoyama H (2010) Effect of copper on the activation of the acid phosphatase from the green algae Pseudokirchneriella subcapitata. Biometals 23:93–98
Kalman J, Palais F, Amiard JC, Mouneyrac C, Muntz A, Blasco J, Riba I, Amiard-Triquet C (2009) Assessment of the health status of populations of the ragworm Nereis diversicolor using biomarkers at different levels of biological organisation. Mar Ecol Prog Ser 393:55–67
Kraak MH, Lavy D, Toussaint M, Schoon H, Peeters WH, Davids C (1993) Toxicity of heavy metals to the zebra mussel (Dreissena polymorpha). In: Nalepa TF, Schloesser DW (eds) Zebra mussels: biology, impacts and control. CRC Press, Boca Raton, pp 491–502
Labarta U, Fernández-Reiriz M, Navarro J, Velasco A (2002) Enzymatic digestive activity in epifaunal (Mytilus chilensis) and infaunal (Mulinia edulis) bivalves in response to changes in food regimes in a natural environment. Mar Biol 140:669–676
Lambert C, Moal J, Le Moullac G, Pouvreau S (2008) Mortality risks associated with physiological traits of oysters during reproduction. In: Samain JF, McCombie H (eds) Summer mortality of Pacific oyster Crassostrea gigas. The Morest Project. Editions Quae, Versailles, pp 63–106
Le Bihan E, Perrin A, Koueta N (2004) Development of a bioassay from isolated digestive gland cells of the cuttlefish Sepia officinalis L. (Mollusca Cephalopoda): effect of Cu, Zn and Ag on enzyme activities and cell viability. J Exp Mar Biol Ecol 309:47–66
Le Pennec G, Le Pennec M (2003) Induction of glutathione-S-transferases in primary cultured digestive gland acini from the mollusk bivalve Pecten maximus (L.): application of a new cellular model in biomonitoring studies. Aquat Toxicol 64:131–142
Li N, Zhao Y, Yang J (2008) Effects of water-borne copper on digestive and metabolic enzymes of the giant freshwater prawn Macrobrachium rosenbergii. Arch Environ Contam Toxicol 55:86–93
Marie V, Gonzalez P, Baudrimont M, Bourdineaud JP, Boudou A (2006) Metallothionein response to cadmium and zinc exposures compared in two freshwater bivalves, Dreissena polymorpha and Corbicula fluminea. Biometals 19:399–407
Marigómez I, Soto M, Carajaville MP, Angulo E, Giambérini L (2002) Cellular and subcellular distribution of metals in molluscs. Microsc Res Tech 56:358–392
McMahon RF (1996) The physiological ecology of the zebra mussel, Dreissena polymorpha, in North America and Europe. Am Zool 36:339–363
Mersch J (1993) Modalités d’utilisation de la moule zébrée Dreissena polymorpha en tant qu’indicateur biologique de la contamination des écosystèmes d’eau douce par les métaux lourds – Comparaison avec un autre type d’organisme sentinel, les mousses aquatiques. Thèse de doctorat. Université de Metz, France
Milam CD, Farris JL (1998) Risk identification associated with iron-dominated mine discharges and their effect upon freshwater bivalves. Environ Toxicol Chem 17:1611–1619
Minier C, Abarnou A, Jaouen-Madoulet A, Le Guellec AM, Tutundjian R, Bocquené G, Leboulenger F (2006) A pollution-monitoring pilot study involving contaminant and biomarker measurements in the Seine Estuary, France, using zebra mussels (Dreissena polymorpha). Environ Toxicol Chem 25:112–119
Mora P, Michel X, Narbonne JF (1999) Cholinesterase activity as a potential biomarker in two bivalves. Environ Toxicol Pharmacol 7:253–260
Morton BS (1983) Feeding and digestion in Bivalvia. In: Saleuddin ASM, Wilbur KM (eds) The Mollusca. Academic Press, New York, pp 65–147
Moulton CA, Fleming WJ, Purnell CE (1996) Effects of two cholinesterase-inhibiting pesticides on freshwater mussels. Environ Toxicol Chem 15:131–137
Mourgaud Y, Martinez E, Geffard A, Andral B, Stanisiere JY, Amiard JC (2002) Metallothionein concentration in the mussel Mytilus galloprovincialis as a biomarker of response to metal contamination: validation in the field. Biomarkers 7:479–490
Najimi S, Bouhaimi A, Daubèze M, Zekhnini A, Pellerin J, Narbonne JF, Moukrim A (1997) Use of acetylcholinesterase in Perna perna and Mytilus galloprovincialis as a biomarker of pollution in Agadir Marine Bay (South of Morocco). Bull Environ Contam Toxicol 58:901–908
Osman AM, Van Den Heuvel H, Van Noort PCM (2007) Differential responses of biomarkers in tissues of a freshwater mussel, Dreissena polymorpha, to the exposure of sediment extracts with different levels of contamination. J Appl Toxicol 27:51–59
Palais F, Jubeaux G, Dedourge-Geffard O, Biagianti-Risbourg S, Geffard A (2010) Amylolytic and cellulolytic activities in the crystalline style and the digestive diverticulae of the freshwater bivalve Dreissena polymorpha (Pallas, 1771). Molluscan Res 30:29–36
Palais F, Mouneyrac C, Dedourge-Geffard O, Giambérini L, Biagianti-Risbourg S, Geffard A (2011) One-year monitoring of reproductive and energy reserve cycles in transplanted zebra mussels (Dreissena polymorpha). Chemosphere 83:1062–1073
Prest HF, Jarman WM, Burns SA, Weismueller T, Martin M, Huckins JN (1992) Passive water sampling via Semi-Permeable Membrane Devices (SPMDs) in concert with bivalves in the Sacramento/San Joaquin River delta. Chemosphere 25:1811–1823
Ramcharan CW, Padilla DK, Dodson SI (1992) Models to predict potential occurrence and density of the zebra mussel, Dreissena polymorpha. Can J Fish Aquat Sci 49:2611–2620
Romani R, Antognelli C, Baldracchini F, De Santis A, Isani G, Giovannini E, Rosi G (2003) Increased acetylcholinesterase activities in specimens of Sparus auratus exposed to sublethal copper concentrations. Chem Biol Interact 145:321–329
Roméo M, Giambérini L (2008) Historique. In: Amiard JC, Amiard-Triquet C (eds) Les biomarqueurs dans l’évaluation de l’état écologique des milieux aquatiques. Lavoisier, Tec & Doc, Paris, pp 17–54
Rouxel-David E, Cordonnier G, Dachy S (2002) Synthèse des études menées sur le basin versant du champ captant de Couraux (Marne). Rapport BRGM/RP-51739-FR
Ruelas-Inzunza JR, Paez-Osuna F (2000) Comparative bioavailability of trace metals using three filter-feeder organisms in a subtropical coastal environment (Southeast Gulf of California). Environ Pollut 107:437–444
Schneider DW (1992) A bioenergetics model of zebra mussel, Dreissena polymorpha, growth in the Great Lakes. Can J Fish Aquat Sci 49:1406–1416
SCOR-UNESCO (1966) Determination of photosynthetic pigments in sea-water. UNESCO Monographs on Oceanographic Methodology, no. 1
Selye H (1976) Stress in health and disease. Butterworth Publishers, Boston
Thompson JAJ, Cosson RP (1984) An improved electrochemical method for the quantification of metallothioneins in marine organisms. Mar Environ Res 11:137–152
Tusseau-Vuillemin MH, Gourlay C, Lorgeoux C, Mouchel JM, Buzier R, Gilbin R, Seidel JL, Elbaz-Poulichet F (2007) Dissolved and bioavailable contaminants in the Seine river basin. Sci Total Environ 375:244–256
Valbonesi P, Sartor G, Fabbri E (2003) Characterization of cholinesterase activity in three bivalves inhabiting the North Adriatic sea and their possible use as a sentinel organisms for biosurveillance programmes. Sci Total Environ 312:79–88
Viarengo A, Ponzano E, Dondero F, Fabbri R (1997) A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic molluscs. Mar Environ Res 44:69–84
Voets J, Talloen W, de Tender T, van Dongen S, Covaci A, Blust R, Bervoets L (2006) Microcontaminant accumulation, physiological condition and bilateral asymmetry in zebra mussels (Dreissena polymorpha) from clean and contaminated surface waters. Aquat Toxicol 79:213–225
Wong WH, Cheung SG (2001) Feeding rates and scope for growth of green mussels, Perna viridis (L.) and their relationship with food availability in Kat O, Hong Kong. Aquaculture 193:123–137
Xuereb B, Chaumot A, Mons R, Garric J, Geffard O (2009) Acetylcholinesterase activity in Gammarus fossarum (Crustacea Amphipoda): intrinsic variability, reference levels, and a reliable tool for field surveys. Aquat Toxicol 93:225–233
Yan T, Teo LH, Sin YM (1996) Effects of metals on alpha-amylase activity in the digestive gland of the green mussel, Perna viridis L. Bull Environ Contam Toxicol 56:677–682
Zorita I, Ortiz-Zarragoitia M, Soto M, Cajaraville MP (2006) Biomarkers in mussels from a copper site gradient (Visnes, Norway): an integrated biochemical, histochemical and histological study. Aquat Toxicol 78:S109–S116
Acknowledgments
This PhD work was supported by grants from the ‘Région Champagne-Ardenne’ (Contrat de Projets Etat-Région, programme AQUAL) and from the ‘Programme Interdisciplinaire de Recherche sur l’Environnement de la Seine’ (PIREN-Seine). The authors gratefully acknowledge Mrs. Françoise Gourdon, Mrs. Antoinette Meyer, Mrs. Laurence Delahaut, M. Antoine Portelette and M. Ali Jaffal for kind help and support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Palais, F., Dedourge-Geffard, O., Beaudon, A. et al. One-year monitoring of core biomarker and digestive enzyme responses in transplanted zebra mussels (Dreissena polymorpha). Ecotoxicology 21, 888–905 (2012). https://doi.org/10.1007/s10646-012-0851-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-012-0851-1