Abstract
This study investigates biomass, density, photosynthetic activity, and accumulation of nitrogen (N) and phosphorus (P) in three wetland plants (Canna indica, Typha augustifolia, and Phragmites austrail) in response to the introduction of the earthworm Eisenia fetida into a constructed wetland. The removal efficiency of N and P in constructed wetlands were also investigated. Results showed that the photosynthetic rate (P n), transpiration rate (T r), and stomatal conductance (S cond) of C. indica and P. austrail were (p < 0.05) significantly higher when earthworms were present. The addition of E. fetida increased the N uptake value by above-ground of C. indica, T. augustifolia, and P. australis by 185, 216, and 108 %, respectively; and its P uptake value increased by 300, 355, and 211 %, respectively. Earthworms could enhance photosynthetic activity, density, and biomass of wetland plants in constructed wetland, resulting in the higher N and P uptake. The addition of E. fetida into constructed wetland increased the removal efficiency of TN and TP by 10 and 7 %, respectively. The addition of earthworms into vertical flow constructed wetland increased the removal efficiency of TN and TP, which was related to higher photosynthetic activity and N and P uptake. The addition of earthworms into vertical flow constructed wetland and plant harvests could be the significantly sustainable N and P removal strategy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nutrient enrichment of surface water bodies by nitrogen (N) and phosphorus (P) has become an important water quality issue. Eutrophication of water bodies may result in the growth of harmful algal blooms resulting in reduced dissolved oxygen and disturbance to the normal functioning of the aquatic system. Different management approaches have therefore been taken to try to protect sensitive water bodies from eutrophication. In Europe, the Urban Wastewater Treatment Directive and Nitrates Directive provide a legislative framework setting maximum permitted concentrations for N and P in discharges to aquatic systems.
In this paper, we focus on N and P uptake by wetland plants in constructed wetlands. Constructed wetlands can be used for domestic wastewater, industrial wastewater, and agricultural runoff treatment (Lu et al. 2009). The key processes for nutrient removal in constructed wetlands include microbial conversion, decomposition, plant uptake, sedimentation, volatilization, and adsorption–fixation reactions (Tchobanoglous 1993). Wetland plants will remove nutrients through uptake to support biomass accumulation, and through fixation of inorganic and organic particulates (Brix 1994; Huett et al. 2005). However, the capacity to remove nutrients will decrease over time through saturation of P sorption sites (Tanner et al. 1995). Therefore, the potential for P removal in constructed wetland is finite (Howard-Williams 1985) unless the accumulated nutrients can be removed further.
Nutrient removal capacity in a constructed wetland could be enhanced through harvesting of plant shoots (Wathugala et al. 1987; Huett et al. 2005). N and P absorbed by the rhizome or roots in a constructed wetland are transported into the stems and/or leaves of wetland plants. The harvest of stems and leaves will therefore remove N and P from the system (Markéta et al. 2009). Periodic re-harvesting will continue the process of nutrient removal (Markéta et al. 2009; Zhu et al. 2011) and reduce nutrient recycling on plant death (Zhou and Wang 2010; Zhu et al. 2011). Increasing N and P uptake by plants to enhance the nutrient removal rate in a constructed wetland is therefore a potentially important management process.
The scientific basis of this approach has been discussed previously in the literature. Maddison et al. (2009) found that the annual N and P uptake value by shoots of cattail (Typha latifolia) in wetland varied from 14.0 to 30.4 g N m−2 and from 1.9 to 5.6 g P m−2 in autumn and from 3.8 to 5.2 g N m−2 and from 0.5 to 0.6 g P m−2 in winter. The N and P uptake value was higher in autumn than in winter which could be related to factors promoting better plant growth in autumn. Wu et al. (2011) demonstrated that the nutrient uptake by plants ranged from 14 to 52 % of N removal, and accordingly 11 to 34 % of P removal in constructed microcosm wetlands. Therefore, the N and P removal rate in constructed wetland was influenced by N and P uptake value by wetland plants.
Another potential approach to facilitate the removal of N and P is the introduction of earthworms. Earthworms play an important role in ecological systems because they can breakdown a wide range of organic materials, including sewage sludge (Kwon et al. 2009). Consequently, earthworms are used in filtration systems to purify wastewater, a process which has been termed vermifiltration (Taylor et al. 2003). Several studies have been conducted to evaluate the use of vermifiltration in domestic wastewater treatment (Sinha et al. 2008), municipal wastewater treatment (Yang and Zhao 2008), and swine wastewater treatment processes (Li et al. 2008), as well as in simultaneous sludge reduction processes (Zhao et al. 2010). Research results indicate that earthworms convey a purification benefit.
Earthworms are also used in constructed wetlands to treat wastewater. Li et al. (2011) found that constructed wetlands with added earthworms removed 2–5 % more N and 12 % more P than constructed wetlands without added earthworms. Nuengjamnong et al. (2011) reported that earthworms helped reduce sludge production by 40 % on the surface of constructed wetlands, which lowered the cost of draining the wetland and treating the sludge. Davison et al. (2005) proposed that intentional introduction of earthworms may offer a natural alternative for cleaning clogged substrates in horizontal subsurface flow treatment wetlands. This research indicates that the purifying capacity and function of constructed wetland were influenced by the addition of earthworms. However, less attention has been given to plant growth when earthworms were added into constructed wetland. Moreover, the interactions between earthworms and plants, which are very important for understanding purifying mechanisms involved in earthworms, have not been fully investigated. The objectives of this study were to (1) determine the response of wetland plants in constructed wetland to earthworms; (2) assess the changes in N and P uptake efficiency by wetland plants in the presence of earthworms in constructed wetland, and (3) assess the removal efficiency of N and P in constructed wetland with addition of earthworms
Material and methods
Materials
Sand with a pH of 7.12 was obtained from a local building supply company. The sand’s fractional distributions of 0–0.25, 0.25–0.50, 0.50–1.0, 1.0–2.0, and 2.0–5.0 mm were 19, 32, 25, 13, and 11 % (w/w), respectively. The rice straw was used as organic matter, and was cut to <1-cm pieces. The total carbon, nitrogen, phosphorus, and potassium of rice straw were 337, 7, 0.4, and 15.8 mg kg−1, respectively. Uniform and healthy seedlings, about 5.0 − 8.0 cm tall, of Phragmites austrail, Typha augustifolia, and Canna indica were collected from a field in Pukou District, Nanjing, China. P. australis, T. augustifolia, and C. indica were chosen because they are the main macrophyte used in constructed wetlands to purify wastewater (Cristina et al. 2007; Xu et al. 2009; Vymazal 2011). Earthworms (Eisenia fetida, Savigny, 1828) were purchased from a local farm market. E. fetida was chosen because it was widely used in vermifiltration (Taylor et al. 2003) and has been shown to process organic wastes with great efficiency (Edwards and Bater 1992). The average weight of each E. fetida earthworm was about 0.4 g.
Design of earthworm vertical flow constructed wetlands
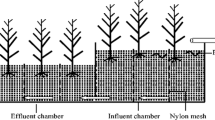
The three vertical flow constructed wetlands were made of iron plate. Each constructed wetland was 80 × 70 × 50 cm (length × width × height), and divided into three uniform cells by nylon mesh to evaluate plant (three wetland plants) biomass per square meter (Fig. 1). The mesh prevents the plant roots from penetrating into another cell, but earthworms could crawl among three cells (cell 1, 2, and 3). The 0.5-in. polypropylene pipe with holes was used to ensure uniform distribution of the influent, and the inflow rates of influent were adjusted by flow control valve to avoid water-saturated medium (Fig. 1). The tested substrate was prepared with mixing 97 % washed sand with 3 % organic matter (v/v). Three kinds of constructed wetlands were designed: (1) the tested substrate was uniformly placed into the constructed wetland to the same volume for each cell, and the height of substrate in the constructed wetland was 40 cm. The 5 seedlings of C. indica, 8 seedlings of T. augustifolia, and 12 seedlings of P. australis were put into cells 1, 2, and 3 of constructed wetland, respectively (Fig. 1a); (2) the same quantity of substrate and plants were added into constructed wetland, and then E. fetida (32 g L−1) were added (Fig. 1b); (3) constructed wetland with only substrate, to act as a control (Fig. 1c). A few earthworms died early in the experiment and these were removed. The remainder survived for the duration of the experiment, which was undertaken from March to November, 2011.
The vertical flow constructed wetlands were established and began to receive interval inflow of wastewater every 2 days in March 2011. All constructed wetlands were fed with aquaculture wastewater at a rate of 14 L day−1. The chemical oxygen demand (COD), total nitrogen (TN), ammonia nitrogen (NH4–N), nitrate nitrogen (NO3–N), total phosphorous (TP), suspended solid (SS), and pH of the wastewater were 31.2, 2.29, 0.031, 1.53, 0.10, 27.2, and 7.98 mg L−1, respectively, meaning the water was classed as eutrophic. The effluent control valve was closed after adding the influent into constructed wetland in November 2011. The effluent was collected by opening control valve after 2 day, which gives a hydraulic retention time of 2 days. The TN, TP, COD, and volume of the effluent were analyzed.
Analytical methods
The P. australis, T. augustifolia, and C. indica leaf photosynthetic rate (P n), transpiration rate (T r), and stomatal conductance (S cond) were measured with an LI-6400 portable photosynthesis system (LI-COR, Inc., Lincoln, NE). To ensure similar measuring conditions, the P n, T r, and S cond were analyzed at the same leaf positions on each plant and then averaged.
Each wetland plant was directly counted to determine plant density. The P. australis, T. augustifolia, and C. indica were harvested, and divided into roots, stems and/or leaves according to their physical characteristics. Each part of the wetland plants was washed with deionized water, and dried at 60 °C for 48 h to a constant weight in a forced air cabinet. The following equations were used to determine the total biomass of wetland plants:
where B is total biomass (kilogram per square meter); M is dry weight of tissue of wetland plants including roots, stems, and leaves (gram per plant); and D is the density of wetland plants (plant per square meter).
The roots, stems, and leaves samples were sub-sampled and ground to powder for the determination of the N and P concentrations. Subsamples of the dried roots, stems, and leaves (0.2 g) were digested with H2SO4–H2O2 at 260 °C. The N concentration in the digest was then determined by the Kjeldahl method, while the P concentration was determined at a wavelength of 700-nm spectrophotometer (Lu 1999). The following equations were used to determine the N and P uptake values by wetland plants:
where T is N and P uptake values (gram per square meter); M is dry weight of tissue of wetland plants including roots, stems, and leaves (gram per plant); C is tissue N and P concentration (gram per kilogram); and D is the density of wetland plants (plant per square meter).
The TN and TP concentration of wastewater were analyzed by according to Standard Methods (APHA et al. 1992). The COD was measured by open reflux method using standard 0.004167 M K2Cr2O7 followed by titrate with standardized 0.025 M ferrous ammonium sulfate due to low concentration COD in wastewater in this study (APHA, AWWA, and WEF, 1992). The removal efficiency was calculated using Eq. 3:
where R is removal efficiency (in percent), I v is initial volume (in liter), I c is initial concentration (in milligram per liter), E v is remaining volume (in liter), Ec is remaining concentration (in milligram per liter).
Statistical analysis
Statistical analysis was performed using the SPSS 12.0. A one-way analysis of variance (ANOVA) was conducted for constructed wetland with earthworms, and without earthworms. In this analysis, P n, T r, S cond, N and P uptake, and removal efficiency of TN, TP, and COD were the dependent variables, respectively, and constructed wetland with and without earthworms was the independent variable. To detect the statistical significance of differences (p < 0.05) between means of treatments, the Tukey test was performed.
Results and discussion
Weight and density of wetland plants
Dry weight of roots, stems, and leaves are shown in Table 1. The addition of E. fetida into the constructed wetland significantly increased roots dry weight of C. indica and P. australis (p < 0.05), and the increased rate of three species C. indica, T. augustifolia, and P. australis was 22, 53, and 37 %, respectively. Compared with wetland plants in a constructed wetland without E. fetida, the addition of E. fetida into the constructed wetland significantly (p < 0.05) increased the stems dry weight of C. indica and P. australis by 168 and 39 %. Similarly, the addition of E. fetida increased dry weight of leaves by 100, 45, and 9 %, respectively for C. indica, T. augustifolia, and P. australis. Chaoui et al. (2003) showed that earthworm casts can improve soil porosity, and thus provide a better root growth medium. So, the increase in dry weight of wetland plants following the addition of E. fetida may be related to a better root growth substrate. The above-ground dry weight of three wetland plants, C. indica, T. augustifolia, and P. australis planted in the constructed wetland with and without E. fetida, was 23.72 and 10.13 g plant−1, 13.98 and 9.62 g plant−1, and 11.95 and 9.29 g plant−1. So, C. indica had the highest value of dry weight of above-ground plant, and that of P. australis was the lowest.
The addition of E. fetida into the constructed wetland increased plant density by 18, 59, and 44 %, respectively for C. indica, T. augustifolia, and P. australis (Table 1). The total biomass of three wetland plants, C. indica, T. augustifolia, and P. australis planted in the constructed wetland with and without E. fetida, was 1.2 and 0.5 kg m−2, 2.4 and 1.0 kg m−2, and 4.3 and 2.3 kg m−2, respectively. The addition of E. fetida into the constructed wetland increased total biomass by 140, 140, and 87 %, respective for C. indica, T. augustifolia, and P. australis. Although dry weight of per P. australis had the lowest, its density was the highest resulting in the highest total biomass among three wetland plants (Table 1). So, the plant density clearly affected the plant total biomass.
Photosynthetic characteristics of wetland plants
The P n, T r, and S cond of wetland plants was shown in Table 2. The addition of E. fetida into constructed wetland significantly (p < 0.05) increased P n of C. indica and P. australis by 38 and 35 %, respectively; its T r significantly (p < 0.05) increased by 28 and 16 %, respectively; and its S cond significantly (p < 0.05) increased by 62 and 78 %, respectively. In terms of P n and T r, T. augustifolia had the highest value, and those of C. indica was the lowest. Blair et al. (1997) found that the addition of earthworms increased the soil NO3-N concentration over a 2-year period in inorganically fertilized plots. Eriksen-Hamel and Whalen (2007) reported that there was a significant linear increase in soil mineral-N (NO3–N + NH4–N) and microbial biomass N concentrations in the 0–15-cm depth of enclosures with more earthworms, and soybean grain-N yield was significantly greater in enclosures with the largest earthworm populations than the control which had no earthworms added. In addition, Chaoui et al. (2003) demonstrated that earthworm casts have high N contents which suggest that they would be good sources of plant N. N is one of the main constituent of chlorophyll. The periphyton growth was positively correlated with concentrations of chlorophyll a and total nitrogen (Mattila and Räisänen 1998). Therefore, the addition of E. fetida into constructed wetland increased the P n and T r, which may be related with higher leaf N content due to the higher available mineral-N from earthworm activity.
Nitrogen and phosphorus concentration of wetland plants
Nitrogen concentration of three wetland plants was shown in Table 3. The addition of E. fetida into the constructed wetland significantly increased N concentration of leaves of C. indica and P. australis (p < 0.05), and N concentration of roots and leaves increased by 85 and 13 %, 121 and 35 %, and 44 and 30 %, respectively for C. indica, T. augustifolia, and P. australis. Similarly, N concentration of stems of C. indica and P. australis increased by 10 and 8 % when E. fetida were added into the constructed wetland. So, the increased rate of N concentration followed the order of roots > leaves > stems. The N concentration in the roots, stems, and leaves was quite varied with species, and C. indica had the highest N concentration in roots, stems, and leaves, but in terms of roots, P. australis had the lowest N concentration (Table 3).
Phosphorus concentration of three wetland plants was shown in Table 3. The addition of E. fetida into the constructed wetland significantly increased leaves P concentration of three wetland plants (p < 0.05). The P concentration of roots and leaves increased by 74 and 92 %, 66 and 95 %, and 26 and 47 %, respectively for C. indica, T. augustifolia, and P. australis. Similarity, P concentration of stems of C. indica and P. australis also increased 9 and 88 % when E. fetida were added into constructed wetland. The P concentration of roots, stems, and leaves was also quite varied with species. In terms of leaves, C. indica had the highest P concentration, and that of T. augustifolia had the lowest (Table 3).
Grass species have critical N and P concentration of around 20 and 2 g kg−1, respectively (Pinkerton et al. 1997). Results in this study showed that the N concentration of leaves and roots of C. indica and T. augustifolia in constructed wetlands with E. fetida were higher than those of Pinkerton et al. (1997) (Table 3). Roots P concentration of T. augustifolia, leaves P concentration of C. indica and P. australis in constructed wetland with E. fetida were also higher than those of Pinkerton et al. (1997) (Table 3). These results demonstrated enhanced N and P uptake by wetland plants through addition of earthworms into constructed wetlands.
Nitrogen and phosphorus uptake value by wetland plants
The N and P uptake value by different wetland plants was shown in Table 4. The addition of E. fetida into constructed wetland increased N and P uptake value by roots, stems, and leaves of C. indica by 165 and 147 %, 250 and 239 %, and 169 and 359 %, respectively. It increased N and P uptake value by roots and leaves of T. augustifolia by 448 and 314 %, and 216 and 355 %, respectively. Similarly, it increased N and P uptake value by roots, stems, and leaves of P. australis by 186 and 148 %, 115 and 278 %, and 104 and 132 %, respectively.
The above-ground plant (sum of stems and/or leaves) was regularly harvested to enhance the removal rate of N and P. Otherwise the N and P may be recycled and returned to the roots-rhizomes promoting further shoot growth in the spring. The N and P uptake value by above-ground of C. indica, T. augustifolia, and P. australis in constructed wetland without addition of E. fetida were 8.1 and 0.57 g m−2, 14.7 and 0.58 g m−2, and 20.7 and 2.08 g m−2, respectively. However, the N and P uptake value by above-ground of C. indica, T. augustifolia, and P. australis in the constructed wetland with addition of E. fetida were 23.1 and 2.28 g m−2, 46.4 and 2.64 g m−2, and 43.0 and 6.47 g m−2, respectively. Therefore, the N and P uptake value by three wetland plants were higher in constructed wetland with earthworms than that of without earthworms. Maddison et al. (2009) found that the annual N and P uptake value by shoots of the common cattail (Typha latifolia) varied from 14.0 to 30.4 g N m−2 and from 1.9 to 5.6 g P m−2 in autumn and from 3.8 to 5.2 g N m−2 and from 0.5 to 0.6 g P m−2 in winter. So, the N and P uptake valve by above-ground of P. australis in constructed wetland with E. fetida in this study were higher than those of Maddison et al. (2009). Lantze et al. (1998) reported that the harvesting of tops accounted for 18–19 % N and P removal, and annual or biennial plant harvests are a significant nutrient removal strategy and the only sustainable removal mechanism. The addition of E. fetida into the constructed wetland increased the N uptake value by above-ground of C. indica, T. augustifolia, and P. australis by 185, 216, and 108 %, respectively; its P uptake value by 300, 355, and 211 %, respectively. So, addition of E. fetida obviously enhanced N and P uptake value of wetland plants compared with constructed wetland without addition of E. fetida.
Removal efficiency of COD, N, and P by constructed wetland
The removal efficiency of COD, TN, and TP were shown in Table 5. The removal efficiency of COD and TN were significantly different (p < 0.05), and addition of E. fetida into the constructed wetland increased the removal efficiency of COD by 11 %. Earthworms could increase the dissolved oxygen concentration, which was attributed to the porous cast aggregates and earthworm burrows within the medium (Taylor et al. 2003). The photosynthetic characteristics of wetland plants influenced oxygen-evolving activities in horizontal flow subsurface constructed wetlands (Huang et al. 2010). So, the higher removal efficiency of COD in constructed wetland with earthworms than that of without earthworms could contribute to higher oxygen concentrations in vertical flow constructed wetland because of earthworm burrows and higher leaf photosynthetic rates of C. indica, T. augustifolia, and P. australis resulting from the addition of E. fetida (Table 2).
The addition of E. fetida into the constructed wetland increased the removal efficiency of TN by 10 %. The removal mechanisms for N in constructed wetland include uptake by plants and other living organisms, ammonification, nitrification, denitrification, ammonia volatilization, and cation exchange for ammonium (Brix 1993). Adler et al. (1996) have reported that the mainly removal mechanisms for N in wetlands are biological denitrification of N by microbes. David and Gary (2009) showed denitrification rates increased by >400 % in the earthworm treatments compared with the control. This suggests that the removal efficiency of TN was higher in constructed wetland with earthworms than that of without earthworms because of a higher denitrification rate resulting from E. fetida activity. In addition, the total N uptake value (sum of roots, stems and/or leaves) by C. indica, T. augustifolia, and P. australis planted in the constructed wetland with and without E. fetida was 30 and 10.7 g m−2, 69.4 and 18.9 g m−2, and 63.0 and 27.7 g m−2, respectively. Consequently, we attribute the greater removal of TN in the constructed wetland with E. fetida to higher denitrification rates and significant uptake of N by C. indica, T. augustifolia, and P. australis.
The addition of E. fetida into constructed wetland increased the removal efficiency of TP by 7 %. Iron plaque is commonly observed on the surface of wetland plant roots (Crowder and MacFie 1986). Fe plaque formation increased P uptake, through enhancing the diffusion of P into the roots of wetland plants (Xu et al. 2009). Oxygen in constructed wetlands is transferred from the leaves to the roots of plants by the processes of molecular diffusion and convection, which was influenced by photosynthetic characteristics (Huang et al. 2010). The total P uptake value by C. indica, T. augustifolia, and P. australis planted constructed wetland with and without E. fetida were 2.75 and 0.76 g m−2, 4.96 and 1.14 g m−2, and 8.82 and 2.81 g m−2, respectively. Therefore, the addition of E. fetida into constructed wetland increased the removal efficiency of TP (Table 5), which could be contribute to the higher P uptake due to greater O2 released from higher P n of C. indica, T. augustifolia, and P. australis in constructed wetland with earthworms present. However, P was removed in constructed wetland through substrate adsorption, chemical precipitation, bacterial action, plant, and algal uptake and incorporation into organic matter. Of these, substrate adsorption plays the most important role (Xu et al. 2006). Therefore, the addition of E. fetida into constructed wetland was not significantly increased the removal efficiency of P (Table 5) because P was mainly removed in constructed wetland by substrate adsorption.
Conclusions
The addition of E. fetida into vertical flow constructed wetland increased roots, stems, and leaves dry weight, plant density and biomass of wetland plants. The addition of earthworms also increased leaf P n, T r, and S cond of three wetland plants. The N uptake value by above-ground of C. indica, T. augustifolia, and P. australis in constructed wetland with and without E. fetida was 23.1 and 8.1 g m−2, 46.4 and 14.7 g m−2, and 43.0 and 20.7 g m−2, respectively. The P uptake value by above-ground of C. indica, T. augustifolia, and P. australis in constructed wetland with and without E. fetida was 2.28 and 0.57 g m−2, 2.64 and 0.58 g m−2, and 6.47 and 2.08 g m−2, respectively. The addition of E. fetida increased the removal efficiency of TN and TP, which could be related to the higher photosynthetic activity, N and P uptake.
References
Adler PR, Summerfelt ST, Glenn DM, Takeda F (1996) Evaluation of a wetland system designed to meet stringent phosphorus discharge requirements. Water Environ Res 68:836–840
APHA, AWWA, WEF (1992) Standard methods for the examination of water and wastewater, 18th edn. American Public Health Association, Washington, DC
Blair JM, Parmelee RW, Allen MF, McCartney DA, Stinner BR (1997) Changes in soil N pools in response to earthworm population manipulations in agroecosystems with different N sources. Soil Biol Biochem 29:361–367
Brix H (1993) Wastewater treatment in constructed wetlands: system design, removal processes, and treatment performance. In: Moshiri GA (ed) Constructed wetlands for water quality improvement. Lewis Publishers, Boca Raton, pp 9–22
Brix H (1994) Functions of macrophytes in constructed wetlands. Water Sci Technol 29:71–78
Chaoui HI, Zibilske LM, Ohno T (2003) Effects of earthworm casts and compost on soil microbial activity and plant nutrient availability. Soil Biol Biochem 35:295–302
Cristina SCC, António OSSR, Paula MLC (2007) Constructed wetland systems vegetated with different plants applied to the treatment of tannery wastewater. Water Res 41:1790–1798
Crowder A, MacFie SM (1986) Seasonal deposition of ferric hydroxide plaque on roots of wetland plants. Can J Bot 64:2120–2124
David MC, Gary AL (2009) Biological and physical effects of non-native earthworms on nitrogen cycling in riparian soils. Soil Biol Biochem 41:2230–2235
Davison L, Headley TR, Pratt K (2005) Aspects of design, structure and performance and operation of reed beds—eight years’ experience in northeastern New South Wales, Australia. Water Sci Technol 51:129–138
Edwards CA, Bater JE (1992) The use of earthworms in environmental management. Soil Biol Biochem 24:1683–1689
Eriksen-Hamel NS, Whalen JK (2007) Impacts of earthworms on soil nutrients and plant growth in soybean and maize agroecosystems. Agric Ecosyst Environ 120:442–448
Howard-Williams C (1985) Cycling and retention of nitrogen and phosphorus in wetlands: a theoretical and applied perspective. Freshw Biol 15:391–431
Huang J, Wang SH, Yan L, Zhong QH (2010) Plant photosynthesis and its influence on removal efficiencies in constructed wetland. Ecol Eng 36:1037–1043
Huett DO, Morris SG, Smith G, Hunt N (2005) Nitrogen and phosphorus removal from plant nursery runoff in vegetated and unvegetated subsurface flow wetlands. Water Res 39:3259–3272
Kwon YT, Lee CW, Yun JH (2009) Development of vermicast from sludge and powdered oyster shell. J Clean Prod 17:708–711
Lantze IR, Heritage AD, Pistillo G, Mitchell DS (1998) Phosphorus removal rates in bucket size planted wetlands with a vertical hydraulic flow. Water Res 32:1280–1286
Li YS, Robin P, Cluzeau D, Bouché M, Qiu JP, Laplanche A, Hassouna M, Morand P, Dappelo C, Callarec J (2008) Vermifiltration as a stage in reuse of swine wastewater: monitoring methodology on an experimental farm. Ecol Eng 32:301–309
Li HZ, Wang S, Ye JF, Xu ZX, Jin W (2011) A practical method for the restoration of clogged rural vertical subsurface flow constructed wetlands for domestic wastewater treatment using earthworm. Water Sci Technol 63:283–290
Lu RK (1999) Agrochemical analyzed method of soil. Agricultural Science and Technology Press of China, Beijing
Lu SY, Zhang PY, Jin XC, Xiang CS, Gui M, Zhang J, Li FG (2009) Nitrogen removal from agricultural runoff by full-scale constructed wetland in China. Hydrobiologia 621:115–126
Maddison M, Soosaar K, Mauring T, Mander Ü (2009) The biomass and nutrient and heavy metal content of cattails and reeds in wastewater treatment wetlands for the production of construction material in Estonia. Desalination 246:120–128
Markéta SM, Adam P, Jan K (2009) Biomass production and nutrient accumulation in Sparganium emersum Rehm. After sediment treatment with mineral and organic fertilizers in three microcosm experiments. Aquat Ecol 43:903–913
Mattila J, Räisänen R (1998) Periphyton growth as an indicator of eutrophication; an experimental approach. Hydrobiologia 377:15–23
Nuengjamnong C, Chiarawatchai N, Polprasert C, Otterpohl R (2011) Treating swine wastewater by integrating earthworms into constructed wetlands. J Environ Sci Health, Part A: Tox Hazard Subst Environ Eng 46:800–804
Pinkerton A, Smith FW, Lewis DC (1997) Pasture species. In: Reuter DJ, Robinson JB (eds) Plant analysis: an interpretation manual. CSIRO Publishing, Collingwood
Sinha RK, Bharambe G, Chaudhari U (2008) Sewage treatment by vermifiltration with synchronous treatment of sludge by earthworms: a low-cost sustainable technology over conventional systems with potential for decentralization. Environmentalist 28:409–420
Tanner CC, Clayton JS, Upsdell MP (1995) Effect of loading rate and planting on treatment of dairy farm wastewaters in constructed wetlands—II. Removal of nitrogen and phosphorus. Water Res 29:27–34
Taylor M, Clarke WP, Greenfield PF (2003) The treatment of domestic wastewater using small-scale vermicompost filter beds. Ecol Eng 21:197–203
Tchobanoglous G (1993) Constructed wetlands and aquatic plant systems: research, design, operation and monitoring issues. In: Moshiri GA (ed) Constructed wetlands for water quality improvement. Lewis Publishers, Boca Raton, FL, pp 23–34
Vymazal J (2011) Plants used in constructed wetlands with horizontal subsurface flow: a review. Hydrobiologia 674:133–156
Wathugala AG, Suzuki T, Kurihara Y (1987) Removal of nitrogen, phosphorus and COD from waste water using sand filtration system with Phragmites australis. Water Res 21:1217–1224
Wu H, Zhang J, Li P, Zhang J, Xie H, Zhang B (2011) Nutrient removal in constructed microcosm wetlands for treating polluted river water in northern China. Ecol Eng 37:560–568
Xu DF, Xu JM, Wu JJ, Akmal M (2006) Studies on the phosphorus sorption capacity of substrates used in constructed wetland systems. Chemosphere 63:344–352
Xu DF, Xu JM, He Y, Huang PM (2009) Effect of iron plaque formation on phosphorus accumulation and availability in the rhizosphere of wetland plants. Water Air Soil Pollut 200:79–87
Yang J, Zhao LM (2008) Wastewater treatment performance of earthworm biofilter with filter media of quartz sand and ceramic pellet. The 2nd international conference on bioinformatics and biomedical engineering, Shanghai, China, pp 3031–3034
Zhao LM, Wang YY, Yang J, Xing MY, Li XW, Yi DH, Deng DH (2010) Earthworm–microorganisms interactions: a strategy to stabilize domestic wastewater sludge. Water Res 44:2572–2582
Zhou XH, Wang GX (2010) Nutrient concentration variations during Oenanthe javanica growth and decay in the ecological floating bed system. J Environ Sci 22:1710–1717
Zhu LD, Li ZH, Ketola T (2011) Biomass accumulations and nutrient uptake of plants cultivated on artificial floating beds in China’s rural area. Ecol Eng 37:1460–1466
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 40901257), Jiangsu Key Laboratory of Agricultural Meteorology (grant nos. KYQ1206 and JKLAM2012 01) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Xu, D., Li, Y. & Howard, A. Influence of earthworm Eisenia fetida on removal efficiency of N and P in vertical flow constructed wetland. Environ Sci Pollut Res 20, 5922–5929 (2013). https://doi.org/10.1007/s11356-013-1860-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1860-1