Abstract
The response of purifying capability, enzyme activity, nitrification potentials, and total number of bacteria in the rhizosphere in December to wetland plants, substrates, and earthworms was investigated in integrated vertical flow constructed wetlands (IVFCW). The removal efficiency of total nitrogen (TN), NH4–N, chemical oxygen demand (COD), and total phosphorus (TP) was increased when earthworms were added into IVFCW. A significantly average removal efficiency of N in IVFCW that employed river sand as substrate and in IVFCW that employed a mixture of river sand and Qing sand as substrate was not found. However, the average removal efficiency of P was higher in IVFCW with a mixture of river sand and Qing sand as substrate than in IVFCW with river sand as substrate. Invertase activity in December was higher in IVFCW that used a mixture of river sand and Qing sand as substrate than in IVFCW which used only river sand as substrate. However, urease activity, nitrification potential, and total number of bacteria in December was higher in IVFCW that employed river sand as substrate than in IVFCW with a mixture of river sand and Qing sand as substrate. The addition of earthworms into the integrated vertical flow constructed wetland increased the above-ground biomass, enzyme activity (catalase, urease, and invertase), nitrification potentials, and total number of bacteria in December. The above-ground biomass of wetland plants was significantly positively correlated with urease and nitrification potentials (p < 0.01). The addition of earthworms into IVFCW increased enzyme activity and nitrification potentials in December, which resulted in improving purifying capability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Enzyme activity is usually related to the general condition of soil microbial populations. Plants, microorganisms, and animal activities all result in the accumulation of a variety of enzymes (Zhou et al. 2005). The mineralization of organic matter is mainly carried out by microbes both under aerobic and anaerobic conditions. The high molecular weight organic matter can be decomposed into small sub-units by intracellular or extracellular enzymes (Singh and Kumar 2008). Enzymes such as cellulase, protease, urease, phosphatase, and phenol oxidase are widely distributed in peatlands, salt marshes, and wetlands (Zhang et al. 2010). Duarte et al. (2008) demonstrated that enzyme activity in soil was affected by many factors, including biological factors and abiotic factors (pH, organic matter content, depth profiles, etc.).
Enzymes in constructed wetland systems play an important role in pollutant removal. Urease can indicate the level of removal of N from a constructed wetland (Huang et al. 2012). In a constructed wetland, Cui et al. (2013) and Wu et al. (2001) found significant correlations between the activities of urease and the removal rates of total nitrogen (TN) and NH4 +–N. Shackle et al. (2006) suggested that enzyme activity could be modified by adjusting the quantity and quality of carbon supply to maximize the efficiency of wastewater treatment in constructed wetlands, and exogenous enzyme supplement to constructed wetlands could enhance the biodegradation processes. However, Kong et al (2009) showed that the removal of TN and NO3 − and chemical oxygen demand (COD) were significantly correlated with enzyme activity in only a few constructed wetlands. Wu et al. (2001) also found that there was not a significant correlation between phosphatase activity and removal efficiency of total phosphorus (TP). Therefore, the previous findings showed some differences in the effects of enzyme activity on pollutant removal.
Plants can influence soil enzyme activity by excreting exogenous enzymes and releasing exudates and oxygen into the rhizosphere (Singh and Kumar 2008). Plants can indirectly mediate enzyme activities in wetlands through controlling above-ground and below-ground litter quantity and quality as well as the microclimate (Caravaca et al. 2005). Neori et al. (2000) also demonstrated that plants can reactivate the free enzymes which may be inactivated and preserved by tannins and other chemicals in the bulk anaerobic soil by oxygenating the anaerobic substrate by its expanding root system. Zhang et al. (2010) indicated that higher plant species richness improved the removal efficiencies of NH4–N and NO3–N from the post-treatment wastewater. Plant species richness strongly affected the activity of some enzymes, such as cellulase, urease, and acid phosphatase. Therefore, enzyme activities are influenced by wetland plants.
Constructed wetlands are widely used to purify wastewater because they have many advantages, such as the low cost of operation and maintenance, the aesthetics of the environment, and the potential to provide a wildlife habitat. However, constructed wetlands have some limitations including the low hydraulic loading rate and requirement for large land areas. Therefore, integrated vertical flow constructed wetlands (IVFCW) were developed to overcome these limitations and have been used to purify wastewater in Europe and China (Perfler et al. 1999; Chang et al. 2012). However, there is little information on enzyme activity and nitrification potentials in the rhizosphere of wetland plants in IVFCW. Therefore, the objectives of this study were to investigate the response of enzyme activity and nitrification potentials to wetland plants, substrates, and earthworms and to study the correlations between enzyme activity and pollutant removal efficiency in IVFCW.
Material and methods
Materials
River sand and Qing sand were obtained from a local building supply company, and the fractional distribution and exchanged calcium are shown in Table 1. Rice straw, ground to powder, was used as organic matter. Uniform and healthy rhizomes of Canna indica, Iris japonica, Acorus calamus L., Phragmites australis, Zizania caducifolia, and Typha angustifolia were used in this study because they are often found planted in constructed wetlands in order to help purifying wastewater. These wetland plants were collected from a field in Pukou District, Nanjing, China. Earthworms (Eisenia fetida, Savigny, 1828) were purchased from a local farm market. E. fetida was chosen because it was widely used in vermifiltration (Taylor et al. 2003) and has been shown to process organic wastes with great efficiency (Edwards and Bater 1992).
Experimental setup
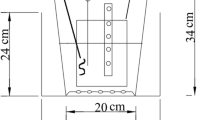
The IVFCW was designed in Nanjing University of Information Science & Technology. It comprised two parallel influent and effluent chambers. The influent chamber had dimensions 60 × 60 × 90 cm (length × width × height), and the effluent chamber was 60 × 60 × 60 cm (length × width × height) (Fig. 1). Both the influent and effluent chambers were divided into three uniform cells by nylon mesh; the mesh prevented plant roots from penetrating into adjacent cells (Fig. 1).
Four types of IVFCW were designed. (1) The IVFCW without plants (IVFCW I)—the 97:3 (v/v) mixture of river sand and organic matter as substrate was added to the influent chamber with 80-cm depth and effluent chamber with 50-cm depth, respectively. (2) The IVFCW with wetland plants (IVFCW II)—the same quantity of substrate was added into the influent chamber and effluent chamber of IVFCW, respectively, and then four C. indica, four I. japonica, and five A. calamus were separately planted into three cells in the influent chamber and four P. australis, five Z. caducifolia, and four T. angustifolia were also separately planted into three cells in the effluent chamber. (3) The IVFCW with wetland plants and earthworms (IVFCW III)—the same quantity of river sand and plants were added into IVFCW, and then earthworms with a density 6 g L−1 for the upper layer (30-cm depth) were also added into the influent chamber of the constructed wetland. (4) The IVFCW employed a mixture of river sand, Qing sand, and organic matter as substrate (IVFCW IV)—firstly, river sand and Qing sand (V R/V Q = 1:1) was prepared, and then it was mixed with organic matter (V M/V O = 97:3) as the tested substrate. The tested substrate was added into the influent and effluent chambers to maintain the same substrate depth as the previously designed IVFCW. The same quantity of wetland plants and earthworms were also added into IVFCW.
Synthetic domestic wastewater was used in the experiment for minimizing the fluctuation of influent pollutant concentrations. The synthetic wastewater was prepared using (NH4)2SO4, glucose, peptone, and KH2PO4. The average COD, TN, NH4–N, and TP were 221, 21, 14, and 2.8 mg L−1, respectively. All IVFCW were fed with synthetic wastewater at a rate of 20 L day−1 giving a hydraulic retention time of 2 days. Water was sampled from the constructed wetland after 2 days to analyze NH4 +, NO3 −, and TN from January to December 2013.
Measurement of biomass of above-ground wetland plants
The six wetland plants were harvested in December 2013 and divided into below-ground and above-ground plants. The above-ground wetland plants were washed with deionized water and dried at 60 °C for 48 h to a constant weight in a forced air cabinet. The following equations were used to determine the above-ground biomass of wetland plants:
where B is the above-ground biomass (kg m−2), M is the dry weight of above-ground wetland plants (g plant−1), and D is the density of wetland plants (plant m−2).
Measurement of enzyme activity, total number of bacteria, and nitrification potentials in the rhizosphere
Three uniformly distributed sites were chosen in each cell of the influent and effluent chambers for rhizospheric substrate sampling in December 2013. The below-ground plants in each sampling site were excavated and substrate released from the roots by hand. Small roots were removed to obtain substrate in the rhizosphere. The substrate in the rhizosphere of six wetland plants in different constructed wetlands was taken to determine enzyme activity, nitrification potentials, and total number of bacteria.
Catalase and urease activities were measured following the method of Li et al. (2008) and Guan et al. (1983), respectively. Briefly, 5 g wetland plant rhizospheric substrate was mixed with 5 mL of 0.3 % H2O2 and 40 mL distilled water and the reaction mixture was incubated at 25 °C for 30 min. To stop the reaction, 5 mL of 1.5 mol L−1 H2SO4 was added to the solution, and then the mixture was filtered. The 25-mL filtrate was titrated by 0.02 mol L−1 KMnO4 solution. The dissipative volume of KMnO4 solution was used to denote the catalase activity. The catalase activity was expressed as KMnO4 mL g−1 substrate day−1. To determine urease activity, 2 mL toluene, 20 mL of pH 6.7 citrate buffer, and 10 mL 10 % urea were added to 10.0 g wetland plant rhizospheric substrate, and then the mixture was incubated at 37 °C for 24 h. The mixture was filtered, and then 1 mL filtrate was mixed with 4 mL sodium phenol and 3 mL sodium hypochlorite to obtain indophenol blue, which was measured at 578 nm. A control without urea was used with each sample. The urease activity was expressed as mg NH4 +–N g−1 day−1.
Invertase activity was determined according to Cang et al. (2009). A 15-mL amount of 8 % sucrose was used as the substrate; 5 g of the wetland plant rhizospheric substrate from the experiment was mixed with 0.5 mL toluene and 5 mL phosphate buffer of pH 5.5 and incubated at 37 °C for 24 h. The glucose produced was extracted and determined with 3,5-dinitrosalicylic acid colorimetric method (DNS method) at 508 nm. Assays without rhizospheric substrate from the experiment and without sucrose were made at the same time as controls. A buffer solution of 5 mL invertase and 15 mL 8 % sucrose was mixed and then incubated at 37 °C for 5 min. The glucose produced was determined by the DNS method, and the invertase activity was expressed as mg glucose g−1 soil day−1.
The total number of bacteria was determined by the methods of Liang et al. (2003). Nitrification potentials were carried out as described by Xu et al. (2013). Briefly, 100 g of substrate was put in 500-mL Erlenmeyer flasks containing 100 mL of pH 7.2 of incubation solution in M (volume ratio was 3:7:30 for 0.2 KH2PO4, 0.2 K2HPO4, and 0.05 (NH4)2SO4). Erlenmeyer flasks were plugged by absorbent cotton and were rotarily shaken at 25 °C (200 rpm). The concentration of NO3 − after 48 h was monitored by analyzing 10-mL samples from incubation using an ultraviolet spectrophotometer. Nitrification potentials were analyzed using Eq. 2
where N is the nitrification potential (mg kg−1 h−1), C 2 and C 1 are the concentration of NO3 − after 48-h incubation of solution and initial concentration of substrate (mg L−1), respectively, V 1 and V 2 represent the volume of incubation solution and water volume of substrate (L), T is the incubation time, and M is the dry weight of substrate (g).
Pollutant removal efficiency
Water samples were taken between 15 January 2013 and 15 December 2013 from the inlet and outlet. Analysis was performed immediately to determine COD, TN, NH4 +–N, and TP according to standard methods (APHA, AWWA and WEF 1992). Pollutant removal efficiency was calculated by Eq. 3
where R is the removal efficiency and C inf and C eff are the influent and effluent concentrations in mg L−1, respectively.
Statistical analysis
All experimental data were calculated using Microsoft Excel 2007 and analyzed with the SPSS 12.0.
A one-way analysis of variance (ANOVA) was conducted for enzyme activity in different constructed wetlands. In this analysis, catalase, urease, invertase, and nitrification potentials were the dependent variables, respectively. The different constructed wetlands were the independent variables. To detect the statistical significance of differences between means of treatments (p < 0.05), the Tukey test was performed. The correlation between biomass, enzyme activity, and nitrification potentials in the rhizosphere was also analyzed.
Results and discussion
Biomass of wetland plants
The above-ground biomass of C. indica, I. japonica, A. calamus L., P. australis, Z. caducifolia, and T. angustifolia in IVFCW III was 2.40 times, 2.73 times, 6.29 times, 1.38 times, 1.32 times, and 3.20 times as much as in IVFCW II, respectively (Fig. 2). Similarly, the above-ground biomass of C. indica, I. japonica, A. calamus L., P. australis, Z. caducifolia, and T. angustifolia in IVFCW IV was 2.54 times, 2.70 times, 7.01 times, 1.37 times, 1.12 times, and 4.05 times as much as in IVFCW II, respectively. Results showed that the addition of earthworms into IVFCW increased the above-ground biomass of wetland plants. There was not a significant difference between IVFCW III and IVFCW IV for the above-ground biomass of wetland plants. These findings showed that the above-ground biomass of wetland plants was not greatly influenced by type of substrate.
Biomass of above-ground wetland plants in influent and effluent chamber of different IVFCW. CI, IJ, AC, PA, ZC, and TA represent C. indica, I. japonica, A. calamus L., P. australis, Z. caducifolia, and T. angustifolia, respectively. CW II, CW III, and CW IV are integrated vertical flow constructed wetlands II, III, and IV, respectively
Purifying capability of integrated vertical flow constructed wetland
The removal efficiency of COD, TN, NH4–N, and TP is shown in Fig. 3. According to Fig. 3a, the removal efficiency of COD from January to December followed the order of IVFCW with wetland plants and earthworms (IVFCW IV and IVFCW III) > IVFCW with wetland plants (IVFCW II) > IVFCW without wetland plants (IVFCW I). Results showed that earthworms can increase the removal efficiency of COD. As shown in Fig. 3a, the removal efficiency of COD from July to December was higher in IVFCW that employed river sand as substrate (IVFCW III) than in IVFCW that employed a mixture of sand and Qing sand as substrate (IVFCW IV).
According to Fig. 3b, c, the average removal efficiency of NH4–N and TN was 72 and 76.0; 77.4 and 81.3; 82.6 and 85.4; and 83.3 and 84.6 %, respectively, for IVFCW I, IVFCW II, IVFCW III, and IVFCW IV. The removal efficiency of TN and NH4–N followed the order of IVFCW IV and IVFCW III > IVFCW II > IVFCW I. According to Fig. 3d, the average removal efficiency of TP was 79.6, 87.9, 90.3, and 93.2 %, respectively, for IVFCW I, II, III, and IV. Therefore, the removal efficiency of TP followed the order of IVFCW IV > IVFCW III > IVFCW II > IVFCW I. Results suggested that the removal efficiency of TP was influenced by earthworms and type of substrates. Earthworms increased the wetland plants’ biomass (Fig. 2) and uptake of P, resulting in enhanced removal efficiency of TP. In addition, the removal efficiency of TP was also influenced by the type of substrate, and the removal efficiency of TP was higher in IVFCW IV with a mixture of sand and Qing sand as substrate than in IVFCW III with river sand as substrate. Zhu et al. (1997) demonstrated P can be adsorbed and precipitated by reaction with calcium, iron, and aluminum. The higher removal efficiency of IVFCW IV could be attributed to Qing sand with higher calcium content because higher calcium is related to higher P sorption (Table 1).
Enzyme activity in the rhizosphere of wetland plants
According to Fig. 4i, catalase activity in December in the rhizosphere of wetland plants was obviously higher in the influent chamber than in the effluent chamber of IVFCW. Catalase is a common enzyme found in nearly all living organisms (microorganisms, plants, and animal chambers). Catalase is exposed to oxygen, where it functions to catalyze the decomposition of hydrogen peroxide to water and oxygen (Ling et al. 2010). Dissolved oxygen in permanently saturated wetland beds is usually low. Therefore, the occurrence of higher catalase activity in the influent chamber than in the effluent chamber could be attributed to that chamber’s higher oxygen content (Fig. 1). As shown in Fig. 4i, catalase activity in December in the rhizosphere of the tested wetland plants was not significantly different in the influent chamber or in the effluent chamber. These findings agreed with Zhang et al. (2010) who found that species richness did not significantly impact catalase activity in the substrate and plant roots or their debris were probably not an important source of catalase activity in subsurface vertical flow constructed wetlands. As shown in Fig. 4i, catalase activity of influent chamber was significantly higher in IVFCW IV or IVFCW III than in IVFCW II (p < 0.05), which could be related to higher oxygen due to higher porosity produced by earthworm’s activities.
Catalase (i), urease (ii), and invertase (iii) activity in December in the rhizosphere of wetland plants in IVFCW. CI, IJ, AC, PA, ZC, and TA represent C. indica, I. japonica, A. calamus L., P. australis, Z. caducifolia, and T. angustifolia, respectively. CW I, CW II, CW III, and CW IV are integrated vertical flow constructed wetlands I, II, III, and IV, respectively. CK is constructed wetland without wetland plants. Data are means ± SD. Different lower case and capital letters in the same column indicate significant differences in different constructed wetland treatments in influent chamber or effluent chamber (p < 0.05)
Urease activity in December in the rhizosphere of different wetland plants in IVFCW is shown in Fig. 4ii. Urease activity in the rhizosphere was obviously higher in the influent chamber with C. indica than in the effluent chamber with P. australis and Z. caducifolia (Fig. 4ii). Hoult and McGarity (1986) indicated that the type, height, and age of the pasture sward could influence urease activity through litter input into soil. In this study, the effect of wetland plant type on urease activity depended on pollutant concentration in IVFCW. The urease activity in the rhizosphere of wetland plants was obviously influenced by the higher pollutant concentration in the influent chamber. Urease activity in the influent chamber followed the order C. indica > I. japonica > A. calamus. According to Fig. 4ii, the addition of earthworms into IVFCW significantly increased urease activity (p < 0.05). Our results agreed with Kong et al. (2009) who reported that enzymes activities were significantly positively correlated with root activity in Vetiveria zizanioides and P. australis wetlands, but not in Hymenocallis littoralis wetlands. Therefore, it can be seen that the influence of earthworms on urease activity in the rhizosphere varied by type of wetland plants.
Invertase activity in December in the rhizosphere of wetland plants is shown in Fig. 4iii. The order of ranking of invertase activity in the influent chamber was that of C. indica > I. japonica > A. calamus. Zhang et al. (2010) reported that invertase activities were significantly higher in plots with 8 and 16 plant species than in the control plots, but were not different between plots with 1 to 4 species. Therefore, invertase activity was influenced by the type of wetland plants and plant species richness. As shown in Fig. 4iii, invertase activity significantly increased when earthworms were added into IVFCW (p < 0.05). Ebersberger et al. (2003) reported that invertase activity increased significantly in the elevated CO2 treatment, which was attributed to the input of plant-derived invertase and carbon. Invertase catalyzes the hydrolysis of sucrose to glucose and fructose and has been extensively studied because of its widespread distribution in plants and soil microorganisms (Ross 1983). Zhang et al. (2010) demonstrated that invertase activity tended to increase with species richness and the decomposition of carbohydrates in the substrate of the constructed wetland was improved by species richness. Our results indicated that the increased invertase activity could be related to the higher biomass of wetland plants due to improved growth with earthworms present (Fig. 2).
According to Fig. 4, catalase, urease, and invertase activities in December in the influent chamber were significantly higher in IVFCW IV and IVFCW III than in IVFCW I (p < 0.05), and urease and invertase activities were not significantly different between the influent chamber and the effluent chamber for IVFCW without wetland plants (IVFCW I). Our results demonstrated that catalase, urease, and invertase activities in IVFCW with wetland plants were higher than in IVFCW without wetland plants. Similarly, Zhang et al. (2010) demonstrated that urease activity was significantly higher in planted wetlands than in the unplanted wetlands. Results from December showed that catalase and invertase activities followed the order of IVFCW IV > IVFCW III > IVFCW II > IVFCW I and the order of ranking of urease activity was that of IVFCW III > IVFCW IV > IVFCW II > IVFCW I (Fig. 4).
Nitrification potentials and total number of bacteria in the rhizosphere of wetland plants
Nitrification potentials in December are shown in Fig. 5i. The order of ranking of nitrification potentials in the rhizosphere of wetland plants was that of C. indica > Z. caducifolia > A. calamus L., T. angustifolia, P. australis, and I. japonica, and nitrification potentials were obviously higher in the rhizosphere of C. indica and Z. caducifolia than in the rhizosphere of I. japonica and P. australis. The addition of earthworms increased nitrification potentials in the rhizosphere except for I. japonica in IVFCW with a mixture of river sand and Qing sand as substrate (Fig. 5i). Similarly, the addition of earthworms into IVFCW also increased the total number of bacteria in the influent chamber (Fig. 5ii). Wetland plant roots could release oxygen from the aerenchyma to the rhizosphere, which is termed radial oxygen loss (ROL). Lai et al. (2012) found that photosynthetic rate was positively correlated with total biomass, leaf biomass, and root biomass and ROL was positively correlated with photosynthetic rate. Higher nitrification potentials and total number of bacteria in the rhizosphere in IVFCW III than in IVFCW II were found (Fig. 5i, ii), which was attributed to higher above-ground biomass of wetland plants in IVFCW.
Nitrification potentials (i) and total number of bacteria (ii) in the rhizosphere of wetland plants in IVFCW. CI, IJ, AC, PA, ZC, and TA represent C. indica, I. japonica, A. calamus L., P. australis, Z. caducifolia, and T. angustifolia, respectively. IVFCW I, IVFCW II, IVFCW III, and IVFCW IV are integrated vertical flow constructed wetlands I, II, III, and IV, respectively. CK is constructed wetland without wetland plants. Data are means ± SD. Different lower case and capital letters in the same column indicate significant differences in different constructed wetland treatments in influent chamber or effluent chamber (p < 0.05)
According to Figs. 4 and 5, invertase activity was higher in IVFCW IV with a mixture of river sand and Qing sand as substrate than in IVFCW III with river sand as substrate. However, urease activity, nitrification potentials, and total number of bacteria were lower in IVFCW IV than in IVFCW III. According to Table 1, the particle size distribution (>1 mm) for the mixture of river sand and Qing sand and for river sand only was 53.6 and 31.4 %, respectively. Therefore, the higher invertase activity in IVFCW IV was related to the higher coarse fraction (> 1 mm) in the mixture of river sand and Qing sand. However, the finer particle sized substrate enhanced urease activity and nitrification potentials and benefited bacterial growth in the rhizosphere of wetland plants in IVFCW III.
Statistical analysis indicated that the above-ground biomass of wetland plants was significantly positively correlated with catalase, urease, invertase, nitrification potentials, and total number of bacteria in the rhizosphere in December (p < 0.05) (Table 2). Catalase activity was significantly correlated with urease activity in the rhizosphere (p < 0.01). In addition, a significantly positive correlation between nitrification potentials and urease and invertase activity in the rhizosphere was found (p < 0.01). The total number of bacteria was also significantly correlated with urease activity and nitrification potentials in the rhizosphere (p < 0.01).
Catalase, invertase, and urease activities were related to redox conditions for aerobic metabolism, carbon, and nitrogen cycling, respectively. Zhou et al. (2005) reported that the significantly higher enzymatic activities, such as higher catalase and urease activities, lead to the more effective removal of different organic pollutants in constructed wetlands. Therefore, the removal efficiency of COD and N in December was improved by increased catalase, invertase, and urease activities due to the addition of earthworms into IVFCW (Fig. 4). Chang et al. (2012) indicated that the oxygen in the wetland bed was not adequate for nitrification and that more oxygen improved nitrification in IVFCW. It could be concluded that the removal efficiency of N was higher in IVFCW III than in IVFCW II, which was attributed to different nitrification potentials (Fig. 5).
Conclusions
The removal efficiency of TN, NH4–N, COD, and TP was increased when earthworms were added into IVFCW. There is no significant difference between the removal efficiency of TN and NH4–N in IVFCW with river sand as substrate and IVFCW with a mixture of sand and Qing sand as substrate. However, the removal efficiency of TP was higher in IVFCW with the mixture of sand and Qing sand as substrate than in IVFCW with just river sand as substrate. Therefore, the removal rate of N was not influenced by the type of substrate, but the removal rate of P was mainly influenced by the type of substrate. Catalase and urease activities in the rhizosphere in December were higher in the influent chamber than in the effluent chamber of IVFCW. However, invertase activity in the rhizosphere in December was not significantly different between the influent and effluent chambers. Urease and invertase activities in the rhizosphere in December followed the order of C. indica > I. japonica > A. calamus in the influent chamber, and urease and invertase activities were significantly higher in IVFCW with wetland plants than in CK (p < 0.05). The order of ranking of nitrification potentials in the rhizosphere of wetland plants was that of C. indica > Z. caducifolia > A. calamus L., T. angustifolia, P. australis, and I. japonica. The addition of earthworms into IVFCW increased the above-ground biomass, enzyme activity (catalase, urease, and invertase), and nitrification potentials. The removal efficiency of TN, NH4–N, COD, and TP was increased when earthworms were added into IVFCW.
References
APHA, AWWA and WEF (1992) Standard methods for the examination of water and wastewater, 18th edn. American Public Health Association, AWWA and WEF, Washington
Cang L, Zhou DM, Wang QY, Wu DY (2009) Effects of electrokinetic treatment of a heavy metal contaminated soil on soil enzyme activities. J Hazard Mater 172:1602–1607
Caravaca F, Alguacil MM, Torres P, Roldán A (2005) Plant type mediates rhizospheric microbial activities and soil aggregation in a semiarid Mediterranean salt marsh. Geoderma 124:375–382
Chang JJ, Wu SQ, Dai YR, Liang W, Wu ZB (2012) Treatment performance of integrated vertical-flow constructed wetland plots for domestic wastewater. Ecol Eng 44:152–159
Cui LH, Ying O, Gu WJ, Yang WZ, Xu QL (2013) Evaluation of nutrient removal efficiency and microbial enzyme activity in a baffled subsurface-flow constructed wetland system. Bioresour Technol 146:656–662
Duarte B, Reboreda R, Cacador I (2008) Seasonal variation of extracellular enzymatic activity (EEA) and its influence on metal speciation in a polluted salt marsh. Chemosphere 73:1056–1063
Ebersberger D, Niklaus PA, Kandeler E (2003) Long term CO2 enrichment stimulates N-mineralisation and enzyme activities in calcareous grassland. Soil Biol Biochem 35:965–972
Edwards CA, Bater JE (1992) The use of earthworms in environmental management. Soil Biol Biochem 24:1683–1689
Guan SY, Zhang DS, Zhang ZM (1983) Soil enzyme and research methods. Agriculture Press, Beijing
Hoult EH, McGarity JW (1986) The measurement and distribution of urease activity in a pasture system. Plant Soil 93:359–366
Huang L, Gao X, Liu M, Du G, Guo JS, Ntakirutimana T (2012) Correlation among soil microorganisms, soil enzyme activities, and removal rates of pollutants in three constructed wetlands purifying micro-polluted river water. Ecol Eng 46:98–106
Kong L, Wang YB, Zhao LN, Chen ZH (2009) Enzyme and root activities in surface-flow constructed wetlands. Chemosphere 76:601–608
Lai WL, Zhang Y, Chen ZH (2012) Radial oxygen loss, photosynthesis, and nutrient removal of 35 wetland plants. Ecol Eng 39:24–30
Li ZG, Luo YM, Teng Y (2008) Research methods of soil and environmental microorganism. Science Press, Beijing
Liang W, Wu ZB, Cheng SP, Zhou QH, Hu HY (2003) Roles of substrate microorganisms and urease activities in wastewater purification in a constructed wetland system. Ecol Eng 21:191–195
Ling DJ, Huang QC, Ouyang Y (2010) Impacts of simulated acid rain on soil enzyme activities in a latosol. Ecotoxicol Environ Saf 73:1914–1918
Neori A, Reddy KR, Ciskova-Koncalova H, Agami M (2000) Bioactive chemicals and biological-biochemical activities and their functions in rhizospheres of wetland plants. Bot Rev 66:351–378
Perfler R, Laber J, Langergraber G, Haberl R (1999) Constructed wetlands for rehabilitation and reuse of surface waters in tropical and subtropical areas—first results from small-scale plots using vertical flow beds. Water Sci Technol 40(3):155–162
Ross DJ (1983) Invertase and amylase activities as influenced by clay-minerals, soil-clay fractions and topsoils under grassland. Soil Biol Biochem 15:287–293
Shackle V, Freeman C, Reynolds B (2006) Exogenous enzyme supplements to promote treatment efficiency in constructed wetlands. Sci Total Environ 361:18–24
Singh DK, Kumar S (2008) Nitrate reductase, arginine deaminase, urease and dehydrogenase activities in natural soil (ridges with forest) and in cotton soil after cetamiprid treatments. Chemosphere 71:412–418
Taylor M, Clarke WP, Greenfield PF (2003) The treatment of domestic wastewater using small-scale vermicompost filter beds. Ecol Eng 21:197–203
Wu ZB, Chen HR, Cheng SP (2001) Primary studies on the purification efficiency of phosphorus by means of constructed wetland system. Acta Hydrobiol Sinica 25:28–35
Xu DF, Li YX, Alan H, Guan YD (2013) Effect of earthworm Eisenia fetida and wetland plants on nitrification and denitrification potentials in vertical flow constructed wetland. Chemosphere 92:201–206
Zhang CB, Wang J, Liu WL, Zhu SX, Liu D, Chang SX, Chang J, Ge Y (2010) Effects of plant diversity on nutrient retention and enzyme activities in a full-scale constructed wetland. Bioresour Technol 101:1686–1692
Zhou QH, Wu ZB, Cheng SP, He F, Fu GP (2005) Enzymatic activities in constructed wetlands and dibutyl phthalate (DBP) biodegradation. Soil Biol Biochem 37:1454–1459
Zhu T, Jenssen PD, Maechlum T, Krogstad T (1997) Phosphorus sorption and chemical characteristics in treatment wetlands. Water Sci Technol 35:103–108
Acknowledgments
This work was supported by the Natural Science Foundation of Jiangsu Province (Grant No. BK20141477), Jiangsu Key Laboratory of Agricultural Meteorology (Grant No. JKLAM2012 01) National Natural Science Foundation of China (Grant No. 40901257), Scientific Research Foundation for the Returned Overseas Chinese Scholars (2014s048), Scientific Research Foundation of Nanjing University of Information Science and Technology, and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Xu, D., Gu, J., Li, Y. et al. Purifying capability, enzyme activity, and nitrification potentials in December in integrated vertical flow constructed wetland with earthworms and different substrates. Environ Sci Pollut Res 23, 273–281 (2016). https://doi.org/10.1007/s11356-015-5734-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5734-6