Abstract
Effect of nitric oxide donor (sodium nitroprusside, SNP, 500 μM) or hydrogen peroxide scavenger (dithiothreitol, DTT, 500 μM) on cadmium (Cd) or copper (Cu) uptake (150 μM solutions) and toxicity using Scenedesmus quadricauda was studied. Combined treatments (Cd or Cu + DTT or SNP) usually ameliorated metal-induced toxicity at the level of pigments, proteins, and mineral nutrients in comparison with respective metal alone. Viability tests (MTT and TTC) showed the lowest values preferentially in Cu treatments, indicating higher toxicity in comparison with Cd. Cd showed low impact on amino acids while strong Cu-induced depletion was mitigated by DTT and SNP. Amount of ROS and NO showed the most pronounced responses in SNP variants being rather reciprocal than parallel and regulated ascorbate peroxidase activity. Blot gel analyses of hsp70 protein did not reveal extensive changes after given exposure period. Phenols were elevated by DTT alone while all Cu treatments revealed depletion. Total Cu content decreased while total Cd content increased in metal + SNP or metal + DTT. Subsequent experiment using lower Cd, SNP or DTT doses (10 and 100 μM) revealed concentration-dependent impact on Cd uptake. Overall, DTT was found to be more suitable for the amelioration of metal-induced toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reactive oxygen species (ROS) are un-avoidable products of aerobic metabolism being not only negative stress agents if present in excess but also important regulators of various metabolic processes (Mittler 2002; Kováčik et al. 2009, 2010a). Nitric oxide (NO) has gained increasing interest as an essential signaling molecule in living organisms including plants (Arasimowicz and Floryszak-Wieczorek 2007).
Contamination of environment by different toxic substances including heavy metals represents serious health risk. Plants including algae could not only serve as a food source but may also be a valuable and inexpensive tool for refining of polluted localities. Numerous studies reported the effect of heavy metals on the induction of ROS and NO in plants. Algae, being often microscopic and thus under-estimated objects, produce important part of Earth’s organic biomass. Unlike vascular plants, there exists very limited number of studies focused on the production of ROS and NO in algae. However, the involvement of both ROS and NO in response to wound (Ross et al. 2006) and metal exposure (Zhang et al. 2008) has been observed. Algae are also potent metal-accumulating agents (Kováčik et al. 2011; Zheng et al. 2011) but numerous responses to metal excess and potential manipulation of oxidative balance (aimed to increase stress tolerance) are almost unknown (Kováčik et al. 2010b).
Various ROS/NO modulators are frequently used in the physiological and toxicological studies with plants. For example, sodium nitroprusside (SNP) is the usual NO donor which typically exhibits protective effect but may be toxic in higher concentrations, including studies with algal species (Qian et al. 2009; Zhang et al. 2009). Unlike NO, modulators of ROS production have only occasionally been tested, e.g., the generation of superoxide by methyl viologen (Tanaka et al. 2011). To our knowledge, direct use of H2O2 modulators in algal physiology is not known. Dithiothreitol (DTT) is a ROS level-reducing compound in vascular plants which reduced not only H2O2 but also superoxide level (Kováčik et al. 2009).
Heavy metals usually reduce basic physiological parameters in algae and vascular plants such as the accumulation of chlorophylls, free amino acids and proteins being more pronounced after exposure to Cu than to Cd excess (Kováčik et al. 2010a, b, 2011). Algae are also relatively tolerant to higher Cd concentrations (40–100 μM) in terms of growth or cytoskeletal alterations (Cepák et al. 2002; Bišová et al. 2003; Přibyl et al. 2005) while Cu in general shows higher toxicity (Macken et al. 2009). In contrast to basic cellular molecules, phenolic metabolites are metal-induced compounds with chelating or antioxidative effect (Kováčik and Klejdus 2008) but their accumulation in algae in response to metallic stress has only rarely been studied (Kováčik et al. 2010b).
Regulation of metal uptake by compounds affecting oxidative balance in algae has not yet been studied, providing the main stimulus for the present research. Scenedesmus quadricauda is a widely distributed freshwater green algal species producing high biomass and revealed considerable tolerance to metal excess including copper (Cu) and cadmium (Cd) in our previous studies (Kováčik et al. 2010b, 2011). We therefore applied higher Cu and Cd concentrations (150 μM) for the present investigation to study the eventual protective effect of ROS (DTT) and NO (SNP) modulators against metal-induced damage. Preliminary experiments with low SNP and DTT doses showed no visible impact on Scenedesmus cells (data not shown) under tested experimental conditions therefore 500 μM was selected for the main physiological assays. Because lower SNP doses are usually used in algal research (Qian et al. 2009) and because Cd accumulation showed interesting accumulation pattern in 150 μM concentration, subsequent experiment with lower SNP or DTT doses (10 and 100 μM) focused on Cd uptake was conducted using also lower exogenous Cd concentrations (10 and 100 μM Cd solutions). Many data in terms of algal biology are presented here for the first time.
Material and methods
Scenedesmus culture, experimental design, and statistics
S. quadricauda (Turp.) Bréb. (Chlorophyta, Chlorophyceae), strain UTEX 76 (originated from The University of Texas, Austin) was cultured under sterile conditions in liquid ‘Trebouxia medium’ (Ahmadjian 1993; Kováčik et al. 2011) in cultivation room with controlled temperature (25/20 °C day/night) at PAR ~30 μmol m−2 s−1 during 4–5 weeks after inoculation (from stock culture maintained on the same medium solidified with 1 % agar). Because this liquid medium contains casein hydrolysate and glucose, cells were washed two times with HEPES buffer before treatments. Then the biomass was harvested by centrifugation (5 min, 3,000 rpm) and re-suspended in 5 mM HEPES buffer (pH 6.5) in order to achieve 0.2 g FW/50 mL of buffer. Exposure was realized using 50 mL volume of experimental solutions in screw-cap tubes (Sarstedt, Nümbrecht, Germany) and Cd or Cu ions (in the form of chlorides) were applied in a final concentration of 150 μM (diluted from 5 mM stock solutions). Radical regulators (SNP or DTT) were applied in a final concentration of 500 μM. Lower SNP or DTT doses (10 and 100 μM) were used in a subsequent experiment focused on Cd uptake using also lower Cd concentrations (10 and 100 μM Cd solutions). The pH was checked to be 6.0 ± 0.1 in all treatments. After 24 h of exposure to these treatments (slightly shaken on orbital shaker), samples were centrifuged (5 min, 3,000 rpm) and biomass was extracted with respective solvents as mentioned below for the determination of parameters in fresh material. Processing of samples was done using cold mortar and pestle to achieve complete cell disruption. For quantification of amino acids and mineral nutrients, samples were dried at 70 °C for 12 h.
ANOVA followed by a Tukey’s test (MINITAB Release 11, Minitab Inc., State College, PA, USA) was used to evaluate the significance of differences (P < 0.05). Three individual 50-mL tubes were assessed for each variant and each parameter (then n = 3 in all tables/figures), thus the whole experiment included 306 tubes and 61.2 g FW of algal biomass. Two independent repetitions of the whole experiment were performed in order to check reproducibility. Spectrophotometry for below-mentioned parameters was carried out with spectrophotometer Uvi Light XTD 2 (Secomam, ALES Cedex, France).
Assay of pigments and phenolic metabolites
Photosynthetic pigments were extracted using DMSO (0.2 g FW/15 mL, final dilution) and calculated according to equations by Wellburn (1994). Total soluble phenols were extracted with 80 % methanol by homogenization with inert sand. Calculation was based on the calibration curve prepared with gallic acid using the Folin–Ciocalteu method (detection at 750 nm); sum of flavonols was measured in the same methanol supernatants using AlCl3 method and quercetin-constructed calibration curve (detection at 420 nm, Kováčik et al. 2012a).
Quantification of nitrogenous metabolites and Western blot of proteins
Soluble proteins were extracted with 50 mM potassium phosphate buffer (pH 7.0) containing 1 % (w/v) insoluble polyvinylpolypyrrolidone and quantified according to Bradford (1976) using bovine serum albumin as standard (detection at 595 nm). Free amino acids were quantified after stirred extraction using a computer-controlled commercially available IKA Werke 50 device (IKA®Labortechnik, Staufen, Germany) connected to Soxhlet apparatus. The measurement of amino acid concentrations was performed using an HP 1100 liquid chromatograph (Hewlett Packard, Waldbronn, Germany) with fluorometric detector FLD HP 1100 and using pre-column derivatization with o-phthalaldehyde and 9-fluorenylmethyl chloroformate as described in detail previously (Kováčik et al. 2009).
For western blot analysis, the algal cells were collected by centrifugation at 400 g for 20 min. at 4 °C, washed twice in ice-cold phosphate buffered saline solution/PBS (P5368, Sigma-Aldrich, Saint Louis, MO, USA) and lysed in lysis buffer (100 mM Tris–HCl, pH 7.4, 1 % SDS (w/v), 10 % glycerol (v/v)) supplemented with a protease inhibitor cocktail (P2714, Sigma-Aldrich, Saint Louis, MO, USA) and phosphatase inhibitor cocktail (P78420, Sigma-Aldrich, Saint Louis, MO, USA) for 10 min on ice. The cell lysates were sonicated on ice, three times for 60 s in a 50 % duty cycle pulse mode, with 60 s cooling intervals between (Branson sonifier), centrifuged (10,000×g for 15 min) and only supernatants were collected for further processing. Protein concentration was determined using detergent compatible protein assay (500-0001, BioRad Laboratories Inc., Hercules, CA, USA). All samples were diluted to concentrations of 20 μg mL−1 and supplemented with 0.01 % bromophenol blue (v/v) and 1 % 2-mercaptoethanol (v/v). Protein extracts (20 μl) were then separated with sodium dodecyl sulfate–polyacrylamide gel and transferred onto polyvinylidene fluoride/PVDF membrane (Millipore, Bedford, MA, USA) in a transfer buffer (192 mM glycine, 25 mM Tris and 10 % (v/v) methanol). After 1 h of membrane blocking at room temperature in 5 % non-fat milk (w/v) in tris-buffered saline/TBS (20 mM Tris–HCl, pH = 7.6; 150 mM NaCl; 0.05 % TWEEN 20 (v/v), pH = 7.4), the PVDF membrane blots were incubated overnight at 4 °C with primary antibody: anti-HSP70 (mouse monoclonal IgG1, MA3-006, Affinity BioReagents, Golden, Colorado, USA, 1:5,000). After 20 min washing in wash buffer (TBS, see above), membranes were incubated with appropriate horseradish-conjugated secondary antibody for 1 h at room temperature (anti-mouse IgG F (AB′) 2, 1:10,000, PI-31461, Pierce, Rockford, Illinois, USA). Detection of antibody reactivity was performed with a chemiluminescence detection kit ECL + (PI-32106, Amersham Biosciences, Little Chalfont, UK) and visualized on X-ray films (Foma Slovakia, Skycov, Slovakia). Equal sample loading was verified by immunodetection of α-tubulin (AS10680, 1:1000, Agrisera AB, Vännäs, Sweden) and staining of PVDF membranes with 0.1 % amidoblack (v/v) in mixture of acetic acid, methanol, and water (1:3:6, v/v/v) for non-specific visualization of proteins.
Assay of ROS, NO, peroxidase activity, and viability tests
Amount of ROS was measured using the flow cytometry. Algal cells were collected by centrifugation (400 g for 5 min), washed with Hank’s buffered salt solution/HBSS, stained with 200 nM dihydrorhodamine-123 (DHR-123; Fluka, Buchs, Switzerland) and kept on ice for 15 min. Samples were subsequently analyzed using a flow cytometer (BD FACSCalibur, Becton Dickinson, San Jose, CA, USA). ROS production was quantified as the ratio of the fluorescence median intensity in experimental groups compared to the particular controls (Kello et al. 2010).
NO content was measured using the Griess reagent in homogenates prepared with Na-acetate buffer (50 mM, pH 3.6) as the formation of nitrite detected at 540 nm (Kováčik et al. 2009). Calculation was done using NaNO2-constructed calibration curve. Results were expressed as % of increase in comparison with “control–no metal” being considered as 100 %.
Ascorbate peroxidase (APX, EC 1.11.1.11) activity was assayed in the homogenates prepared with 50 mM potassium phosphate buffer mentioned above and assayed as the oxidation of ascorbate at 290 nm (Kováčik et al. 2009).
Viability was assayed by TTC [2,3,5-triphenyltetrazolium chloride] (Bačkor and Fahselt 2005) and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Bačkorová et al. 2011) tests. They measure the reduction of tetrazolium salts to respective formazan derivatives by various dehydrogenases in living tissues (TTC test) or by mitochondrial reductase in the inner membrane (MTT test). Technically, both these methods should give similar results, indicating living algal cells. Reaction mixture for TTC and MTT contained 5 and 2 mg cells/mL, respectively (other details and reagents as described in Bačkor and Fahselt 2005; Bačkorová et al. 2011).
Quantification of Cd, Cu, and mineral nutrients
Samples for the quantification of mineral nutrients were prepared as described elsewhere: dry material was kept overnight in HNO3 and H2O2 mixture (10 + 10 mL, Suprapur, Merck) and next day evaporated to dryness at 90 °C in a water bath (5–6 h). Dry residue was dissolved in 5 % HNO3 and diluted to final volume of 8 mL. For intracellular content, samples were washed with 2 mM Na2-EDTA for 10 min before drying. Cu and Cd were determined at Cu λ max = 324.8 nm and Cd λ max = 228.8 nm and other elements as reported earlier (Kováčik et al. 2010a, b, 2012a, b). Accuracy of mineralization and measurement was checked using reference plant material as reported previously (Kováčik et al. 2012b).
Results and discussion
Assimilation pigments and phenols
Parallel decrease in chlorophyll a, chlorophyll b and significant changes in the chlorophyll a pheophytinization (A 435/A 415) after metals application (150 μM) suggest a direct impact on chlorophyll biosynthesis and chlorophyll destruction (Table 1). Photosynthesizing plants including non-vascular organisms such as lichens and algae are more sensitive to the excess of Cu in comparison with Cd (Bačkor et al. 2007). This may be evoked by higher potential of Cu to induce ROS over-production and/or by higher Cu accumulation. Modulators alone also affected chlorophylls and SNP showed more negative effect in comparison with DTT. This effect of SNP has been previously reported in Chlorella vulgaris where 100 μM SNP enhanced while 20 μM SNP ameliorated herbicide-induced decrease in chlorophylls (Qian et al. 2009). However, depletion we found in response to 500 μM was considerably lower in comparison with the mentioned 100 μM SNP indicating species-specific differences. Carotenoids play an important role in the function and integrity of the chloroplast thylakoid membrane and their accumulation was less affected in comparison with the sum of chlorophylls mainly in Cd variants (Table 1). Overall, despite partially negative effects, DTT showed protection against metal-induced changes to assimilation pigments suggesting the usability of this compound also in the research of algal physiology. We note absence of visible damage to cell morphology in the light microscope (data not shown).
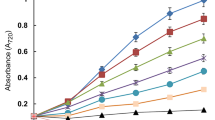
Phenolic metabolites are abundant organic compounds in vascular plants but little is known about their quantitative changes in algae. Our previous study showed depletion in 25 μM Cu-exposed Scenedesmus (Kováčik et al. 2010b) which is in accordance with the present results (Fig. 1). SNP had no effect either in control or Cd variant but prevented Cu-induced depletion. Significance of this phenomenon is unclear but it may be assumed that this stimulation is mediated by the highest content of ROS just in this treatment because stress-induced ROS accumulation including Cu excess stimulates accumulation of phenols (Kováčik et al. 2010a). Changes in Cu + DTT treatment are in accordance with this assumption because amount of ROS was lower than in Cu alone (see below). Besides, DTT alone stimulated increase in phenols and sum of flavonols, agreeing with our previous investigation where DTT-induced NO participation in this process was observed (Kováčik et al. 2009). However, present data did not reveal involvement of NO in this DDT-induced stimulation, suggesting both concentration-dependent and species-specific differences. Further studies using UPLC-MS techniques will be conducted in order to study in detail the profile of individual flavonoids in Scenedesmus.
Accumulation of total soluble phenols and AlCl3-reactive flavonoids (sum of flavonols) in Scenedesmus quadricauda cells exposed to different treatments for 24 h (Cu copper/150 μM, Cd cadmium/150 μM, SNP sodium nitroprusside/500 μM, DTT dithiothreitol/500 μM). Data are means ± SDs (n = 3). Values within graph, followed by the same letter(s), are not significantly different according to Tukey’s test (P < 0.05)
Free amino acids, soluble proteins and Western blot
Pool of free amino acids is mainly affected by their biosynthesis and by protein synthesis/catabolism. This was also confirmed in the algae and inhibitors of GS and GOGAT evoked changes to respective amino acids and ammonium after short-term exposure (Alhama et al. 1998). Strong decrease in both sum of amino acids and soluble proteins in Cu alone (Table 2) confirms negative impact of this metal on the metabolism in general and attenuation of nitrogen metabolism. We note such extreme decrease of individual amino acids was not observed in 25 μM Cu-exposed Scenedesmus (Kováčik et al. 2010b). Recently, Tanaka et al. (2011) reported that the generation of superoxide by methyl viologen (MV) caused increase in proline and alanine in MV-tolerant marine Chlamydomonas strain W80 while MV-sensitive C. reinhardtii showed several times lower accumulation. Our present data indicate that among control variants, individual amino acids were depleted by Cu alone and increase in ROS was observed (cf. Table 2 and Fig. 3). Further changes in combined treatments could not be simply related to changes in ROS because endogenous pool of metals was also affected. For example, decrease in Cu uptake in combined treatments may be a reason for restoration of amino acids in comparison with Cu alone (cf. Table 2 and Fig. 4). Other mechanisms in terms of nitrogenous metabolism could also contribute to various Cd and Cu impact on amino acids observed here. For example, GS but not GOGAT enzyme was affected by 100 μM Cd in Chlamydomonas reinhardtii (Devriese et al. 2001).
In the present study, amino acids were the least affected or even stimulated in SNP variants, suggesting potential regulation role of NO. In terms of NO effect, a study using Cu-exposed C. reinhardtii revealed that Cu-induced proline accumulation is a concentration-dependent (stimulation only up to 5 μM Cu) and low SNP (10 μM) enhanced Cu-induced stimulation (Zhang et al. 2008). Our results also indirectly confirm that Cu dose we used (150 μM) is toxic enough allowing to study the effect of SNP and DTT on Cu toxicity and both these compounds ameliorated Cu-induced proline decrease.
Blot gel analyses of hsp70 protein (Fig. 2) did not reveal considerable impact if metals alone and combined treatments are compared, suggesting difference in comparison with amino acid quantitative changes. On the contrary, Cu and Cd showed different impact on the hsp70 protein in the unicellular green alga Trebouxia erici (Bačkor et al. 2006) and species-specific variation is assumed. In accordance with our present results (Fig. 2), mRNA level of hsp70 in algal species Ulva pertusa (Ulvales, Chlorophyta) was only slightly affected after thalli exposure to Cu, Cd, or Pb up to 24 h (as we did) and longer exposure time was needed to observe significant increase (Tominaga et al. 2010). This suggests potential but not essential participation of this particular hsp protein in algal tolerance to metallic stress.
Western blot analyses of hsp70 protein in Scenedesmus quadricauda cells exposed to different treatments for 24 h. Tubulin was used as an indicator of accuracy of protein loading. Other details are as in the Fig. 1
Stress-related parameters
ROS are formed in response to various abiotic stresses, including excess of heavy metals in non-vascular organisms (Dávila Costa et al. 2011; Kováčik et al. 2011; Zheng et al. 2011). The Cu-induced ROS accumulation and enhanced peroxidase activity we observed (Fig. 3) confirm stronger pro-oxidative properties of this element in comparison with Cd; higher Cu toxicity was observed also in other algal species (Macken et al. 2009). We therefore modulated toxicity of these two metals with different redox properties using ROS and NO modulators and several interesting findings emerged. Firstly, SNP alone stimulated oxidative stress (increase in ROS and APX activity) concomitantly with the release of NO (Fig. 3). Similar, a dose-dependent stimulation of ROS accumulation in response to SNP (in combination with herbicides) has been found in C. vulgaris, where 20 μM SNP reduced H2O2 while 100 μM SNP increased H2O2, ROS and NO content (Qian et al. 2009). One possible reason for the negative effect of high SNP is the reaction of excess NO with superoxide forming peroxynitrite or with O2 forming N2O3: both these compounds are powerful cytotoxic species (Qian et al. 2009). Secondly, changes to ROS/NO level could only hardly be discussed without metal quantification and this comparison allow us to conclude that especially in SNP variants, ROS/NO balance is related to metal uptake. For example, increase in Cd uptake in Cd + SNP did not enhance ROS accumulation owing to increase in NO. On the other hand, intracellular Cu content was not affected in Cu + SNP but ROS were strongly accumulated, leading to low NO amount (cf. Figs. 3 and 4). In accordance with our results but in inverse relation, low SNP co-applied with low Cu concentration reduced ROS accumulation in Chlamydomonas (Zhang et al. 2008). Our data also indicate different interaction of Cd and Cu with SNP. Other gaseous molecules may also reduce ROS accumulation such as the carbon monoxide in C. reinhardtii (Zheng et al. 2011).
Quantitative changes of ROS, nitric oxide (NO) and ascorbate peroxidase (APX) activity in Scenedesmus quadricauda cells exposed to different treatments for 24 h. Other details are as in the Fig. 1
Uptake of copper (Cu) and cadmium (Cd) by Scenedesmus quadricauda cells exposed to different treatments for 24 h. Other details are as in the Fig. 1
To mitigate the oxidative stress caused by ROS, organisms developed efficient antioxidant systems to scavenge them, including APX. It has been suggested that APX may function as a modulator of H2O2 level for signaling of metabolic changes (Mittler 2002). Recently, strong increase in APX activity was found in MV-tolerant marine Chlamydomonas strain W80 while MV-sensitive C. reinhardtii showed depleted activity in response to ROS generation by MV (Tanaka et al. 2011). Increase in APX we observed mainly in SNP treatments (Fig. 3) is another indication that Scenedesmus is more tolerant to metal-induced oxidative damage in comparison with other algae tested up to date and that NO is involved in the APX regulation. In accordance, desert cyanobacterium Nostoc sp. exposed to UV-B radiation (Wang et al. 2008) showed usually higher induction of antioxidative enzymes including APX if SNP was applied in higher concentration (0.5 mM as we did). Additionally, NO donor alone (DEANO, diethylamine nitric oxide) stimulated peroxidase activity in control marine chlorophycean alga Dasycladus vermicularis (Ross et al. 2006) agreeing well with our results if Cd and Cd + SNP treatments are compared (Fig. 3). Compensatory changes to ROS/NO level suggested above have been observed to regulate not only antioxidative enzymes, e.g., in chamomile roots under Cu excess (Kováčik et al. 2009, 2010a). Taken together, there exists growing evidence that ROS/NO balance modulates enzymatic activities in various plant species but such mechanism in algae is reported here for the first time. For complexity, it should also be noted that several studies found parallel increase in ROS and NO and it has been suggested that NO emission acts synergistically with oxygen intermediates to increase oxidative cell death (de Pinto et al. 2002).
Viability assays (such as MTT and TTC) are widely used to identify living cells in the physiological studies (Bačkor and Fahselt 2005) or to study the effect of bioactive metabolites on cancer cells (Bačkorová et al. 2011). Unfortunately, correlation between these assays and metal excess is only poorly known. It has been suggested that decrease in MTT value in Al-exposed tobacco cell cultures could be related to increase in ROS because of the impediment of an electron flow of mitochondrial inner membrane (detected by MTT reduction, Yamamoto et al. 2002). This assumption is partially in agreement with our data, e.g., in all SNP variants where the lowest MTT value but the highest ROS amounts were recorded (cf. Table. 1 and Fig. 3). With respect to fast antioxidative responses and eventual complex interaction between chemicals and algae in the experimental solutions, direct correlation between ROS and MTT was not observed in all treatments. TTC test showed no direct correlation with ROS amount, however, both MTT and TTC values were the lowest preferentially in Cu treatments, suggesting decrease in viability as also shown by depleted content of chlorophylls and amino acids.
Cu/Cd accumulation and mineral nutrients
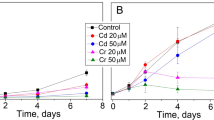
Excess of heavy metals including Cu (25 μM) and Cd (10 and 100 μM) showed non-significant impact on several mineral nutrients such as K, Na, Mg, Fe, and partially Ca under identical exposure conditions (Kováčik et al. 2010b, 2011). This encouraged us to use higher metal concentrations to verify the protective role of applied modulators also on these parameters (Table 3). K+ accumulation showed a correlation with metal uptake and changes to Mg content indicated that despite reduction of chlorophylls, Mg ions are not released from the cells. Ca acts in a number of signal transduction pathways as a second messenger including responses of algae to ROS and NO (Ross et al. 2006). Its decrease highlights the harmful effect of Cu under tested experimental condition. Besides, metal-induced Ca depletion was ameliorated in combined treatments, confirming the protective role of applied modulators. Fe as a component of several ROS scavenging enzymes (including peroxidases) was stimulated mostly in metal + SNP variants, indicating Fe uptake from the treatment solution (Table 3). This trend was also visible in terms of Na increase in metal + SNP variants (both Fe and Na are components of SNP). Despite these trends, ROS and NO content differed in Cd and Cu treatments, confirming metal-specific responses. It was interesting to find that intracellular Cu content was similar in both Cu and Cu + SNP treatments (Fig. 4). SNP usually reduces uptake of metals such as Cu in vascular plants (Zhang et al. 2009) and similar observation has been partially confirmed in the present experiment (Fig. 4). On the other hand, both SNP and DTT elevated Cd uptake but we found no data from any plant species which we could discuss in detail. For this reason, subsequent verification of this finding was done using lower DTT or SNP concentrations and also lower Cd doses (Fig. 5). It was found that only combination of 100 μM of exogenous Cd and 100 μM of DTT or SNP enhanced Cd uptake by ca. 30 % while 100 μM DTT even slightly reduced Cd uptake at 10 μM exogenous Cd concentration (Fig. 5). These data indicate concentration-dependent effect on metal uptake being rather affected by exogenous concentration of the given metal. However, strong increase of adsorbed Cd content in response to 500 μM DTT or SNP (Fig. 4) suggests combined effect on Cd accumulation. In accordance, Matricaria chamomilla cultured hydroponically with 600 μM of SNP and 60 μM of Cd also showed considerable increase in shoot Cd content (J. Kováčik, unpublished results). Data in Cu and Cd variants alone also indicate that Cu is more accumulated in Scenedesmus cells (ca. five times more in comparison with Cd). Another comparison shows that Cd amounts were only slightly higher (Fig. 4) in comparison with 100 μM Cd-exposed cells (Kováčik et al. 2011, c.f. also Figs. 4 and 5) while Cu amount strongly increased if 150 μM Cu (Fig. 4) and 25 μM Cu are compared (Kováčik et al. 2010b). These data confirm that Cd and Cu accumulation relies on different uptake mechanisms. For example, cell wall in Chlamydomonas has been suggested as an important barrier against metal uptake (Macfie and Welbourn 2000). This is in accordance with our data because only up to 50 % of metal content was present in intracellular fraction.
Total Cd content related to lower exogenous Cd concentrations (10 or 100 μM) and co-applied modulators (10 or 100 μM of SNP or DTT) after 24 h of exposure. Statistical details are as in the Fig. 1
Conclusions
To sum up, Cu showed more pronounced toxic effects in comparison with Cd owing to strong enhancement of oxidative parameters despite the fact that Cu was not present in higher amounts than Cd in some combined treatments (e.g.. Cd + SNP vs. Cu + SNP). Modulation of enzymatic activities such as the APX activity by ROS/NO balance in algae is reported here for the first time. SNP revealed partially negative effects such as the reduction in chlorophyll content. DTT showed rather protective impact and altered Cd adsorption more pronouncedly than SNP, suggesting the use for wastewater treatments and for further study of ROS metabolism in algae.
References
Ahmadjian V (1993) The lichen symbiosis. Wiley, New York
Alhama J, López-Ruiz A, Dize J, Garciá-Fernández JM (1998) Effect of carbon and nitrogen availability on intracellular amino acids and ammonium pools in the green alga Monoraphidium braunii. J Plant Physiol 153:529–533
Arasimowicz M, Floryszak-Wieczorek J (2007) Nitric oxide as a bioactive signaling molecule in plant stress responses. Plant Sci 172:876–887
Bačkor M, Fahselt D (2005) Tetrazolium reduction as an indicator of environmental stress in lichens and isolated bionts. Environ Exp Bot 53:125–133
Bačkor M, Gibalová A, Buďová J, Mikeš J, Solár P (2006) Cadmium-induced stimulation of stress-protein hsp70 in lichen photobiont Trebouxia erici. Plant Growth Regul 50:159–164
Bačkor M, Váczi P, Barták M, Buďová J, Dzubaj A (2007) Uptake, photosynthetic characteristics and membrane lipid peroxidation levels in the lichen photobiont Trebouxia erici exposed to copper and cadmium. Bryologist 110:100–107
Bačkorová M, Bačkor M, Mikeš J, Jendželovský R, Fedoročko P (2011) Variable responses of different human cancer cells to the lichen compounds parietin, atranorin, usnic acid and gyrophoric acid. Toxicol in Vitro 28:37–44
Bišová K, Hendrychová J, Cepák V, Zachleder V (2003) Cell growth and division processes are differentially sensitive to cadmium in Scenedesmus quadricauda. Folia Microbiol 48:805–816
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cepák V, Zobačová M, Zachleder V (2002) The effect of cadmium ions on the cell cycle of the green flagellate Chlamydomonas noctigama. Arch Hydrobiol/Algol Studies 106:117–129
Dávila Costa JS, Albarracín VH, Abate CM (2011) Responses of environmental Amycolatopsis strains to copper stress. Ecotox Environ Safe 74:2020–2028
de Pinto MC, Tommasi F, De Gara L (2002) Changes in the antioxidant systems as part of the signaling pathway responsible for the programmed cell death activated by nitric oxide and reactive oxygen species in tobacco bright-yellow 2 cells. Plant Physiol 130:698–708
Devriese M, Tsakaloudi V, Garbayo I, León R, Vilchez C, Vigara J (2001) Effect of heavy metals on nitrate assimilation in the eukaryotic microalga Chlamydomonas reinhardtii. Plant Physiol Biochem 39:443–448
Kello M, Mikeš J, Jendželovský R, Kovaľ J, Fedoročko P (2010) PUFAs enhance oxidative stress and apoptosis in tumour cells exposed to hypericin-mediated PDT. Photochem Photobiol Sci 9:1244–1251
Kováčik J, Klejdus B (2008) Dynamics of phenolic acids and lignin accumulation in metal-treated Matricaria chamomilla roots. Plant Cell Rep 27:605–615
Kováčik J, Klejdus B, Bačkor M (2009) Nitric oxide signals ROS scavenger-mediated enhancement of PAL activity in nitrogen-deficient Matricaria chamomilla roots: side effects of scavengers. Free Radical Biol Med 46:1686–1693
Kováčik J, Grúz J, Klejdus B, Štork F, Marchiosi R, Ferrarese-Filho O (2010a) Lignification and related parameters in copper-exposed Matricaria chamomilla roots: role of H2O2 and NO in this process. Plant Sci 179:383–389
Kováčik J, Klejdus B, Hedbavny J, Bačkor M (2010b) Effect of copper and salicylic acid on phenolic metabolites and free amino acids in Scenedesmus quadricauda (Chlorophyceae). Plant Sci 178:307–311
Kováčik J, Klejdus B, Štork F, Hedbavny J, Bačkor M (2011) Comparison of methyl jasmonate and cadmium effect on selected physiological parameters in Scenedesmus quadricauda (Chlorophyta, Chlorophyceae). J Phycol 47:1044–1049
Kováčik J, Grúz J, Klejdus B, Štork F, Hedbavny J (2012a) Accumulation of metals and selected nutritional parameters in the field-grown chamomile anthodia. Food Chem 131:55–62
Kováčik J, Klejdus B, Štork F, Hedbavny J (2012b) Physiological responses of Tillandsia albida (Bromeliaceae) to long-term foliar metal application. J Hazard Mater 239–240:175–182
Macfie SMP, Welbourn M (2000) The cell wall as a barrier to uptake of metal ions in the unicellular green alga Chlamydomonas reinhardtii (Chlorophyceae). Arch Environ Contam Toxicol 39:413–419
Macken A, Giltrap M, Ryall K, Foley B, McGovern E, McHugh B, Davoren M (2009) A test battery approach to the ecotoxicological evaluation of cadmium and copper employing a battery of marine bioassays. Ecotoxicology 18:470–480
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Přibyl P, Cepák V, Zachleder V (2005) Cytoskeletal alterations in interphase cells of the green alga Spirogyra decimina in response to heavy metals exposure: I. The effect of cadmium Protoplasma 226:231–240
Qian H, Chen W, Li J, Wang J, Zhou Z, Liu W, Fu Z (2009) The effect of exogenous nitric oxide on alleviating herbicide damage in Chlorella vulgaris. Aquat Toxicol 92:250–257
Ross C, Küpper FC, Jacobs RS (2006) Involvement of reactive oxygen species and reactive nitrogen species in the wound response of Dasycladus vermicularis. Chem Biol 13:353–364
Tanaka S, Ikeda K, Miyasaka H, Shioi Y, Suzuki Y, Tamoi M, Takeda T, Shigeoka S, Harada K, Hirata K (2011) Comparison of three Chlamydomanas strains which show distinctive oxidative stress tolerance. J Biosci Bioeng 112:462–468
Tominaga H, Coury DA, Amano H, Kakinuma M (2010) Isolation and characterization of a cDNA encoding a heat shock protein 70 from a sterile mutant of Ulva pertusa (Ulvales, Chlorophyta). Ecotoxicology 19:577–588
Wang G, Chen K, Chen L, Hu C, Zhang D, Liu Y (2008) The involvement of the antioxidant system in protection of desert cyanobacterium Nostoc sp. against UV-B radiation and the effects of exogenous antioxidants. Ecotox Environ Safe 69:150–157
Wellburn AR (1994) The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolutions. J Plant Physiol 144:307–313
Yamamoto Y, Kobayashi Y, Rama Devi S, Rikiishi S, Matsumoto H (2002) Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol 128:63–72
Zhang LP, Mehta SK, Liu ZP, Yang ZM (2008) Copper-induced proline synthesis is associated with nitric oxide generation in Chlamydomonas reinhardtii. Plant Cell Physiol 49:411–419
Zhang Y, Han X, Chen X, Jin H, Cui X (2009) Exogenous nitric oxide on antioxidative system and ATPase activities from tomato seedlings under copper stress. Sci Hortic 123:217–223
Zheng Q, Meng Q, Wei YY, Yang ZM (2011) Alleviation of copper-induced oxidative damage in Chlamydomonas reinhardtii by carbon monoxide. Arch Environ Contam Toxicol 61:220–227
Acknowledgments
This work was supported by the Slovak Grant Agency VEGA (1/1238/12) and the CEITEC—Central European Institute of Technology with research infrastructure supported by the project CZ.1.05/1.1.00/02.0068 financed from European Regional Development Fund. The authors thank Dr. Jaromír Mikeš for help with the assay of ROS by flow cytometry.
Disclosure statement
The authors declare that there are no conflicts of interest.
Role of the funding source
Sponsors had no involvement in the present study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Štork, F., Bačkor, M., Klejdus, B. et al. Changes of metal-induced toxicity by H2O2/NO modulators in Scenedesmus quadricauda (Chlorophyceae). Environ Sci Pollut Res 20, 5502–5511 (2013). https://doi.org/10.1007/s11356-013-1541-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1541-0