Abstract
Heavy metal pollution is one of the most severe ecological problems with Lead (Pb) being present in high concentrations. Cyanobacteria growing in polluted sites have evolved different mechanisms to avoid metal toxicity and continue to contribute to the biological activity of the site. The present study was undertaken to understand the mechanism of Pb tolerance in a common diazotroph Desmonostoc muscorum as it tolerated Pb up to 20 mg L-1. During first 48 h, Pb toxicity in this organism resulted in 88% increase in superoxide radicals (SOR) which caused morphological alterations, 37–72% decrease in photosynthetic pigments leading to reduced growth of the organism. The cyanobacterium managed this toxicity by activating antioxidant enzymes superoxide dismutase (SOD) (187% increase) and peroxidase (POD) (181% increase). Simultaneously, increase in the levels of gluthathione (GSH) (53%) and proline (60%), and activity of glutathione reductase (GR) was observed. After 48 h, level of SOR, activities of SOD and POD decreased while the level of GSH and GR remained high. This indicated that Pb toxicity at initial level is managed by the organism by activating both SOD/POD and GSH/GR systems, while later on the organism adopts some other mechanism and is able to manage Pb toxicity by only GSH/GR system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Some heavy metals are essential micronutrients for all type of organisms but become toxic when present in high concentrations (Nagajyoti et al. 2010). Natural processes and anthropogenic activities such as mining of metal ores, fossil fuel combustion, wood preservation, production and application of fertilizers, pesticides, and herbicides add heavy metals into the environment (Pinto et al. 2003; Bazihizina et al. 2014; Hussein et al. 2019; Sirunyan et al. 2019). Excess release of heavy metals has become significant pollutants posing a severe threat to water and land ecosystems (Nagajyoti et al. 2010). Aquatic ecosystems are more prone to heavy metal pollutants than terrestrial ecosystems as large quantities of industrial effluents and municipal wastes containing hazardous chemicals and metals are being discharged to the aquatic environment (Ajitha et al. 2021; Tiwari et al. 2020). Non-biodegradable nature of heavy metals leads to their prolonged persistence in the environment (Mitra et al. 2021). Moreover, metallic pollutants get biomagnified through food chain leading to human health risks (Ali et al. 2019; Chakraborty and Mishra 2021; Shen et al. 2021).
Heavy metal toxicity leads to morphological, ultrastructural and metabolic changes in the microorganisms. Toxicity of heavy metals results from (i) displacement of essential metal cations from the active sites of the enzymes leading to impairment of enzyme activity and metabolic pathways (ii) reaction with low molecular weight compounds such as glutathione (GSH) and carboxyl, histidyl, thiol groups of proteins which may result in altered signaling pathways (iii) similarity to important biochemical functional groups mainly phosphate, thus when taken up replace these groups (iv) generation of reactive oxygen species (ROS) (DalCorso 2012). ROS such as the superoxide anion (O2−), singlet oxygen (1O2), hydroxyl radical (·OH) and the hydrogen peroxide (H2O2) pose a severe threat to the organisms when produced in larger amounts (Ahad and Syiem 2021). Acute metal toxicity may lead to death of the organisms.
Among various heavy metals, Pb being non-essential metal, is one of the most hazardous pollutants spread widely in air, water, soil, and food (Rahman and Singh 2019; Agustina and Tjahjaingsih 2021). Exhaust of automobiles, chimneys of factories, industries, mining, and smelting of Pb ores, finishing operations, fertilizers and pesticides are the major source of Pb pollution (Hu et al. 2019; Gottesfeld et al. 2019; Lim et al. 2019). Average concentration of Pb in the soil ranges from 10 to 100 ppm (Jarosławiecka and Piotrowska-Seget 2014) and from 0.45 to 14 ppm in the groundwater (Smedley et al. 2002).
It is a common observation that some of the microorganisms survive and grow in heavy metal contaminated sites, indicating thereby that they self-adapt to such environment. Microorganisms have evolved a number of different mechanisms for modulating heavy metal toxicity to adapt to changes in the concentration of metals in the environment (Khattar et al. 2015; Parveen et al. 2015; Boden et al. 2021). Extracellular barrier, active efflux of metal ions, extracellular and intracellular sequestration, biotransformation of metal ions are main mechanisms by which microorganisms protect themselves from heavy metal toxicity (Lima de Silva et al. 2012; Gonzatez-Heano and Ghneim-Herrera 2021). Cyanobacteria, an ancient group of photosynthetic prokaryotic microbes, are an important component of aquatic systems as well as agriculture fields (Goswami et al. 2019; Arias et al. 2020; Dutta and Bhadury 2020). These organisms play a fundamental role in the soil biological activity by the production of organic matter and release of oxygen (Qu et al. 2015; Munagamage et al. 2020). Cyanobacteria also build up soil fertility by excreting growth promoting substances such as hormones, vitamins, amino acids; by increasing water holding capacity and phosphate solubility, adding organic matter and even by fixing nitrogen (Tiwari et al. 2020; Bhat et al. 2021). Thus these organisms are very important from ecological point of view. Understanding of the mechanism of heavy metal tolerance in cyanobacteria is very significant as these organisms may be exploited for supporting the biological activity of heavy metal laden sites. Since mechanism of Pb tolerance in cyanobacteria is not fully known, the present study was aimed to understand the mechanism of Pb tolerance in the nitrogen-fixing cyanobacterium Desmonostoc muscorum. The selection of this organism was based on its omni-presence in terrestrial/aquatic systems. Although heavy metals such as cadmium, arsenic, nickel, cobalt, nickel have been shown to cause oxidative stress in cyanobacteria (Kashyap et al. 2021; Verma et al. 2021; Mo et al. 2022; Nowicka et al. 2022; Pandey et al. 2022, Patel et al. 2021; Qiu et al. 2022) but Pb caused oxidative stress in cyanobacteria is not reported. In this study it is demonstrated that initial toxic effect of Pb in Desmonostoc muscorum is the generation of ROS and the organism is successful in managing this effect by activating antioxidant defense system.
Materials and methods
Test organism and culture conditions
The cyanobacterium Desmonostoc muscorum PUPCCC 405.10 employed in the present study is an isolate from paddy fields of Punjab state of India and was propagated in slightly modified Chu-10 nutrient medium (Singh et al. 2015). Experimental cultures were maintained in a culture room at 28 ± 2 °C and illuminated with LED tube lights giving a photon flux 45 µmol m− 2 s− 1 on the surface of culture vessels for 14 h every day. The cultures were kept in actively dividing state by transferring these into fresh medium after every 6–8 days and actively dividing cultures were used for experiments and each experiment was repeated three times.
Determination of tolerance level to Pb
The tolerance level of Desmonostoc muscorum towards Pb was determined by monitoring its growth in graded concentration (4–28 mg L− 1) of Pb. The growth experiment was performed in 250 mL Erlenmeyer flasks containing 100 mL nutrient medium. Actively dividing stock cultures were washed by centrifugation and appropriate volumes were inoculated in flasks containing nutrient medium with graded concentrations of Pb so as to obtain an initial absorbance of the cultures 0.1 at 720 nm. Ten mL aliquots of the cultures were withdrawn at regular intervals of 2 days, extending up to 10 days, and the absorbance the of cultures was noted at 720 nm (A720) using UV-Visible spectrophotometer (Shimadzu 1280, Kyoto, Japan).
Estimation of photosynthetic pigments
At the desired time, known volumes of cultures were withdrawn, washed, suspended in 80% acetone and incubated in dark at 4 oC for 8 h. The contents were centrifuged and absorbance of the supernatant was noted at 665 nm, 645 nm and 450 nm for the determination of Chlorophyll a (Chl a) and carotenoids. The pellet obtained above was suspended in same volume of distilled water, agitated, centrifuged and absorbance of the supernatant was noted at 652 nm, 615 nm and 565 nm for the determination of phycobiliproteins.
Amount of Chl a was determined following Holm (1954) as per the following equation:
Amount of carotenoids was determined following Kratz and Myers (1955) as per the equation:
The equations given by Bennett and Bogorad (1973) were employed to calculate the amount of water soluble phycocyanin, allophycocyanin and phycoerythrin. The amounts of these pigments were summed up to get the amount of phycobiliproteins.
The amounts of pigments obtained above in µg mL− 1 were converted to µg mg− 1 dry biomass by dividing the amount of particular pigment with dry biomass (mg) present in one mL culture.
Extraction and determination of enzymatic and non- enzymatic antioxidants
At the desired time, thick cell suspension of control and Pb grown cultures was obtained by centrifugation (4000 g for 10 min), washed twice with double distilled, suspended in phosphate buffer (100 mM, pH 7.8) and disintegrated at 4 oC using sonicator (Soniprep 150, Sanyo, USA). The contents were centrifuged (4 oC) at 12,000 g for 10 min and the supernatant was used as cell free extract for the determination of amount of proteins, superoxide radicals, non-enzyme antioxidants and for the activity of enzymes.
Estimation of superoxide radicals
Content of superoxide radicals (SOR) was determined by following the method of Elstener and Heupel (1976). The assay mixture containing 3 mL phosphate buffer (65 mM, pH 7.8), 2 mL cell free extract, 0.1 mL hydroxylamine hydrochloride (10 mM) was incubated at room temperature for 30 min and then 0.2 mL sulphanilamide (8.5 mM) and 0.2 mL α-naphthylamine (3 mM) were added and mixed well. After 10 min, absorbance was recorded at 530 nm. Standard curve was prepared by using sodium nitrite.
Estimation of superoxide dismutase (EC 1.15.1.1) activity
The activity of superoxide dismutase (SOD) was measured as inhibition of photochemical reduction of nitro blue tetrazolium chloride (NBT) (Beauchamp and Fridovich 1971). The assay mixture for SOD activity was prepared by mixing 27 mL of 100 mM sodium phosphate buffer (pH 7.8), 1.5 mL methionine (3%), 1 mL NBT (0.014%), 1 mL Na2CO3 (23.4%) and 1.5 mL of 2 mM ethylenediamine tetraacetic acid (EDTA). To 2.7 mL of the above assay mixture, 0.1 mL cell free enzyme extract was added followed by the addition of 0.2 mL riboflavin (0.015%). After proper mixing, test tubes were illuminated with LED tube lights for 5 min. The enzyme activity was terminated by transferring these test tubes to dark. Test tubes containing reaction mixture with enzyme extract and kept in dark served as blank. Test tubes without enzyme extract and kept under light served as control. The absorbance of the solution was noted at 560 nm. One unit of SOD activity is the enzyme required to inhibit the reduction of NBT by 50% under light min− 1 mg− 1 protein.
Estimation of peroxidase (EC 1.11.1.7) activity
The method of Gahagan et al. (1968) was followed to determine the activity of peroxidase (POD). The assay mixture (3 mL) contained 50 mM phosphate buffer (pH 6.1), 3% (w/v) pyrogallol, 0.4% (w/v) H2O2 and 0.2 mL cell free enzyme extract. The reaction was started by the addition of H2O2 and change in absorbance of the assay mixture was recorded for 3 min. The enzyme activity was calculated using an extinction coefficient of 25.5 mM− 1 cm− 1. One unit of enzyme activity is defined as enzyme required to oxidize one µmol pyrogallol min− 1 mg− 1 protein.
Estimation of catalase (EC 1.11.1.6) activity
Catalase (CAT) activity was measured following the method of Aebi (1984). The reaction mixture (3 mL) in the cuvette contained 100 mM phosphate buffer (pH 7.0), 20 mmol H2O2 and 0.1 mL cell free extract. The substrate H2O2 was added at the last and immediately decrease in absorbance of the solution was noted at 240 nm at an interval of 30 s for 5 min using a UV-Vis spectrophotometer. Concentration of H2O2 was calculated using molar extinction coefficient 43.6 M− 1 cm− 1. One unit of the enzyme activity is defined as amount of enzyme required to decompose one µmol of H2O2 min− 1 mg− 1 protein.
Estimation of glutathione reductase (1.6.4.2) activity
Glutathione reductase (GR) activity was determined by monitoring decrease in amount of NADPH in assay mixture as NADPH gets oxidized while GR reducing glutathione as per the method of Carlberg and Mannervik (1985). The assay mixture was prepared in the cuvette by adding 2 mL of 100 mM phosphate buffer (pH 7.5), 200 µL of 2 mM NADPH (in 10 mM Tris-HCl, pH 7.0) and 80 µL cell free enzyme extract. The reaction was started by the quick addition and mixing of 200 µL of 20 mM glutathione disulphide (GSSG) and decrease in absorbance was recorded at 340 nm for 10 min. Decrease in the amount of NADPH was calculated using molar extinction coefficient 6220 M− 1 cm− 1. A unit of GR is defined as the amount of enzyme that catalyzes reduction of 1 µmol of NADPH min− 1 mg− 1 protein.
Estimation of ascorbate peroxidase (EC 1.11.1.11) activity
The activity of ascorbate peroxidase (APX) was estimated following the method of Nakano and Asada (1981). The assay mixture in a total volume of 3.0 mL contained 50 mM phosphate buffer (pH 7.0), 0.5 mM ascorbate, 0.1 mM hydrogen peroxide, 0.1 mM EDTA and 0.1 mL cell free enzyme extract. The reaction was started by the addition of enzyme extract. The activity of the enzyme was determined from the decrease in absorbance at 290 nm (an absorbance coefficient of 2.8 mM− 1 cm− 1) as ascorbate was oxidized. One unit of enzyme activity is defined as 1 nmol ascorbate oxidized min− 1 mg− 1 protein.
Estimation of reduced glutathione content
Amount of reduced glutathione (GSH) was estimated according to Ellman’s method (1959). The reaction mixture was prepared by mixing 3 mL of phosphate buffer (50 mM, pH 8.0), 0.2 mL cell free extract, and 0.5 mL of 5,5’-dithiobis-(2-nitrobenzoic acid) solution. Absorbance of coloured solution was noted at 412 nm. Glutathione (reduced) was used as standard.
Estimation of ascorbate content
Method of Oser (1979) was followed to determine ascorbate (AsA) content. The assay mixture contained 2 mL of 5% of sulfosalicylic acid, 2 mL of 2% sodium molybdate, 2 mL of 0.15 N H2SO4, 1 mL of 1.5 mM K2HPO4 and 1 mL of cell free extract. The contents were incubated at 80 °C in a water bath for 60 min and centrifuged at 12,000 g for 15 min. Absorbance of colour developed was recorded at 660 nm. Ascorbic acid was used to prepare the standard curve.
Estimation of proline content
The protocol given by Bates et al. (1973) was followed to measure proline content. Proline from the cells was extracted in 3% sulpho-salicylic acid by disintegrating the washed and concentrated cells using sonicator. One mL cell free extract obtained above was mixed with pre-prepared 2 mL ninhydrin reagent (3.75 g ninhydrin was dissolved in 90 mL glacial acetic acid, to this 24 mL ortho-phosphoric acid and 36 mL distilled water were added and mixed) and 2 mL glacial acetic acid. The contents were incubated in a boiling water bath for 1 h and the reaction was terminated by transferring and keeping the tubes in ice for 5 min. Then 4 mL toluene were added and vortexed for 1 min to transfer proline in the toluene layer. The contents were centrifuged at low speed and the upper pink toluene layer containing proline was pipetted out and its absorbance was noted at 520 nm using a UV-Vis spectrophotometer. Proline was used to obtain standard curve.
Isoenzyme profiling of SOD, POD and CAT
The separation of isoenzymes of the SOD, POD and CAT was performed in a vertical gel electrophoretic unit (Bio-rad, India) following Laemmli (1970). The isozymes of SOD, POD and CAT were stained according to the methods of Fornazier et al. (2002), Van Loon (1971) and Woodbury et al. (1971), respectively, as described by Verma and Prasad (2021). Photographs of stained gels were captured with Gel Doc (ImageQuantLAS500, GE Healthcare Bio–Science AB751 Uppasala, Sweden).
Estimation of protein content
Protein content was determined following Lowry et al. (1951).
Scanning electron microscopy analysis
Pb treated and untreated cells of the organism were used for Scanning Electron Microscopy (SEM) analyses (JSM, 6510LV, JEOL, Japan) following the method of Zhang et al. (2019) as described by Shen et al. (2021).
Chemicals
The chemicals used in the present study were procured from Sigma Aldrich, USA, SdFine Chemical Limited India and Merck India. The commercial grade Pb(NO3)2 was used as Pb. Stock solution of known concentration of lead nitrate was prepared and diluted appropriately to get desired concentration of only Pb in mg L− 1.
Statistical analysis
All the data are the average of three independent experiments ± Standard Deviation (SD). Data were statistically analyzed by applying ANOVA and Tukey’s honest significance difference test. All statistical analyses were tested against the probability value at 95% confidence level (p > 0.05) using GraphPad Prism 8.0 version 8.0 (www.graphpad.com).
Results and discussion
Tolerance limit
Tolerance limit of the test organism towards Pb was determined in terms of IC50 by studying its growth in graded concentrations of the metal (Fig. 1). In the presence of Pb, concentration-dependent decrease in growth of the organism was observed. On day 10, decrease in growth of the test organism by 14%, 31%, 41%, 50%, 69%, 84% and 95% was noted in presence of 4, 8, 12, 16, 20, 24 and 28 mg Pb L− 1, respectively. The organism survived in Pb up to 20 mg L− 1 (Fig. 1) but most of the cells in presence of 24 and 28 mg Pb L− 1 lysed with release of the pigments. Further it was observed that the organism experienced severe toxic effect of Pb during the first two days as no growth was observed, however the organism resumed growth after 2 days, though slowly, in all the tested concentrations of Pb. When growth data of day 6 was considered, it was noted that 12 mg Pb L− 1 caused 50% decrease in growth of the organism and this IC50 concentration was chosen to create experimental Pb stress for this organism. The growth inhibition in presence of Pb indicated that this metal is cytotoxic like other metals such as arsenate in Nostoc muscorum and Anabaena sp. (Dhuldhaj et al. 2018; Patel et al. 2018), zinc and mercury in Chlorella vulgaris (Ajitha et al. 2021), cadmium in Synechocystis sp. and Nostoc muscorum (Ahad and Syiem 2021; Shen et al. 2021) and chromium in Nostoc muscorum and Anabaena sp. (Tiwari et al. 2020). The growth data further indicated that the organism has the capacity to bear the Pb toxicity to a certain concentration of Pb, toxic effect on growth is more severe during first 2 days and later the organism is able to manage toxicity and resumes growth. The decrease in growth may be due to interaction with photosynthetic pigments, generation of reactive oxygen species, and interference with enzyme activity by replacing metal ion cofactors.
Photosynthetic pigments
IC50 concentration of Pb caused reduction in the content of Chl a, carotenoids and PBP of the test organism by 37%, 60% and 67%, respectively (Table 1). Pb caused decrease in Chl a may be due to the fact that heavy metal ions are known to replace essential metal ions from proteins/enzymes. Similarly Mg2+ from chlorophyll molecules might have been replaced with Pb leading to damage to chlorophyll molecules. Pb is also known to strongly inhibit chlorophyll biosynthesis, has the capacity to replace Mn2+ in PS II and inhibits many enzymes of the Calvin cycle, which ultimately leads to a decrease in photosynthetic rate and consequently growth of the organism (Sharma and Dubey 2005; DalCorso 2012; Baracho et al. 2019). Pb caused inhibition of carotenoids and plastoquinone synthesis has also been reported (Pourrut et al. 2011).
SOR content
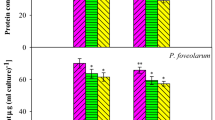
Heavy metals have been reported to target photosynthetic as well as respiratory activities of the organisms leading to the production of superoxide radicals (Ahad and Syiem 2021; Ren et al. 2021). Exposure of the test cyanobacterium to 12 mg Pb L− 1 resulted in continuous enhancement of SOR up to 48 h over control cultures (88% increase on 48 h), afterwards slow decline in SOR content was observed (Fig. 2a). Increase in SOR during initial hours indicated that Pb entered into the cells of the test organism, is highly reactive under cellular conditions leading to the generation of SOR. The results of growth experiment, photosynthetic pigments and SOR content indicated that the reduction in growth of the organism in presence of Pb was possibly as a result of degradation/decrease in synthesis of photosynthetic pigments by increased level of SOR. Similar reports on other microalgae and cyanobacteria with other heavy metals stress are available which support our results (Patel et al. 2018; Ajitha et al. 2021; Tiwari et al. 2020; Shen et al. 2021; Shivagangaiah et al. 2020; Kashyasp et al. 2021; Wang et al. 2021). Initial increase and then decrease in SOR content further indicated that the test organism experienced severe Pb toxicity during first 48 h and later somehow it was able to manage. This is also evident from the resumed growth of the organism after 2 days.
Effect of Pb on (a) SOR content and SOD activity, (b) POD and GR activity of Desmonostoc muscorum in basal medium (SOR and POD: Royal-blue diagonal box/diamond ♦; SOD and GR: green triangle ▲) supplemented with 12 mg Pb L− 1 (SOR and POD: maroon square ■; SOD: and GR: indigo cross x)
One Unit of SOD: Amount of enzyme required for 50% inhibition of reduction of NBT min− 1 mg− 1 protein
One Unit of POD: Amount of enzyme required to oxidize one µmol of pyrogallol min− 1 mg− 1 protein
One unit of GR: Amount of enzyme that catalyzes reduction of 1 µmol of NADPH min− 1 mg− 1 protein
All data are the mean values of three independent experiments ± SD. Data of control and 12 mg Pb L− 1 grown cultures at different times of experiment are significantly different at 95% confidence level (p < 0.05)
SEM analysis
SEM provides a direct observation of the cell(s) in which high magnification and resolving power facilitate the improved examination of morphological features and surface attachments (Shen et al. 2021). SEM analysis during the present study revealed that control cultures had intact integrated filamentous structures having healthy cells with smooth surface. Cells grown in 12 mg Pb L− 1 exhibited changed morphology with structural deformities, high floccules and rough cell surface (Fig. S1). These observations pointed that the test organism experienced severe stress caused by Pb resulting in cell deformities, probably due to lipid peroxidation caused by generation of SOR. The exterior morphology of Ulva lactuca and Synechocystis sp. treated with Cd2+ was irregular with some attachments aggregated on the outside of cell (Ghoneium et al. 2014; Shen et al. 2021). Pb toxicity affects biological morphology, growth and photosynthetic pigments thereby disrupting growth and development of plants and microbes (Leung et al. 2017; Etesami 2018; Li et al. 2019; Kushwaha et al. 2018; Shivagangaiah et al. 2021).
SOD, POD and CAT activity
SOD is one of the most important antioxidant enzymes, present in all sub-cellular compartments of aerobic organisms which are produced in high amounts in response to ROS-mediated oxidative stress (Das and Roychoudhury 2014). SOD mediates conversion of superoxide radicals into less toxic H2O2. SOD activity of the test cyanobacterium increased up to 48 h in presence of Pb with 187% increase on 48 h. The increased SOD activity is attributed to counter the oxidative stress caused by elevated generation of reactive oxygen species. After 48 h, decrease in SOD activity up to 120 h was noticed (Fig. 2a). The SOD played a significant role in scavenging SOR produced by Pb to convert it into less toxic radical in green microalgae (Kashyap et al. 2021). H2O2 produced by SOD is further converted into O2 and H2O by either POD or/and CAT (Patel et al. 2018; Nowicka 2022). In presence of Pb, POD activity in the test organism also increased up to 48 h (with 181% increase on 48 h over control cultures) and later decreased continuously up to 120 h (Fig. 2b). This increase in POD activity is in parallel with increase in SOD activity. Thus the test organism simultaneously detoxified H2O2 produced by the activity of SOD. CAT activity of control and Pb grown cultures, however, remained almost same during the course of experiment, being 82 U in control and 84 U in Pb grown cultures on 48 h. This observation indicated that CAT did not appear to play an important role in detoxification of H2O2 in this organism and only POD did this job. Most of the studies on metal stress and antioxidant enzymes have demonstrated that generally both POD and CAT activities are enhanced in response to oxidative stress (Verma and Prasad 2021; Verma et al. 2021; Pandey et al. 2022; Qiu et al. 2022) while some other workers have studied only SOD and CAT (Ahad and Syiem 2021; Kashyap et al. 2021; Mo et al. 2022). Increase or decrease of antioxidant enzymes in cyanobacteria depends upon the test organism and the nature of heavy metals (Danouche et al. 2020; Farooqui et al. 2017; Ajitha et al. 2021; Ahad and Syiem 2021). Most of the organisms have been reported to induce CAT to scavenge H2O2 (Chakraborty and Mishra 2021) but in Anabaena, Scenedesmus and Chlorella sp. CAT activity did not change in response to metal stress (Kashyap et al. 2021; Verma et al. 2021). The increase in the activity of SOD and POD of test organism was also confirmed by their activity on polyacrylamide gel (Fig. S2). Separation of enzymes on PAGE followed by staining of their activity on the gel revealed that there were two isoenzymes for SOD, while isoenzymes for POD and CAT were not observed. Bands of SOD and POD on gel corresponding to Pb grown culture were more intense compared to control cultures while no change in band intensity corresponding to CAT was observed. These observations supported the results obtained for these enzymes by biochemical analysis.
Glutathione, ascorbate content and gluthathione reductase activity
Glutathione is the major low-molecular-weight thiol in both prokaryotes and eukaryotes and represents as a major pool of non-protein reduced sulphur (Sirikhachornkit and Niyogi 2010, Nowicka 2022). Glutathione mainly exists in a reduced form (GSH) under cellular physiological conditions, while in oxidized conditions it is glutathione disulphide (GSSG). Glutathione reductase (GR) reduces GSSG back to GSH (Sirikhachornkit and Niyogi 2010). GSH also plays important role in antioxidant defense as it has the ability to scavenge H2O2, O2•−, 1O2 and organic radicals (Pikula et al. 2019; Tiwari et al. 2020). Cellular GSH content of the test organism was significantly higher in Pb grown cultures as compared to control ones. The GSH content continuously increased up to 120 h and was 65% higher in Pb grown cultures over control cultures at 120 h (Fig. 3). The activity of GR in the test organism increased by 59.37% in Pb containing cultures compared to the control cultures (Fig. 2b). The GR utilizes NAD(P)H to reduce GSSG to GSH and maintains an increased level of GSH in the cells (Ahad and Syiem 2021). High activity of GR in Pb grown cultures indicated its involvement in maintaining high levels of GSH. Its activity was positively correlated with enhanced GSH content under Pb exposure (Figs. 2b and 3). Ascorbic acid (AsA) is another low molecular weight antioxidant reported to help organisms in oxidative stress. At cellular physiological pH, AsA is predominantly present in the form of ascorbate anion which is an effective reducing agent able to directly scavenge free radicals and has role in regulation of photosynthesis (Sirikhachornkit and Niyogi 2010; Piotrowska-Niczyporuk et al. 2015). Ascorbate is also a cofactor of ascorbate peroxidase (APX), an H2O2 detoxifying enzyme. There was no significant difference between the levels of AsA of Pb grown and control cultures. Ascorbate-Glutathione cycle has also been reported to detoxify H2O2 (Ahmad et al. 2010). Non-enhancement of AsA in Pb grown cultures indicated that AsA has no direct role in scavenging of free radicals in this organism. It has been reported that the rate of the reaction catalysed by APX is higher than the rate of direct scavenging of H2O2 by ascorbate (Tamaki et al. 2021). APX activity was not observed in Pb grown and control cultures of the test organism. These observations suggest that Ascorbate-Glutathione cycle is not operative in this organism. APX is widely distributed in plants and eukaryotic algae but is absent in prokaryotes (Ishikawa and Shigeoka 2008; Gest et al. 2013). Organisms overcome the oxidative stress caused by heavy metals and exhibit growth by maintaining the level of AsA and GSH for efficient detoxification of highly toxic ROS component (Chattergee et al. 2018).
Effect of Pb on glutathione and proline content of Desmonostoc muscorum grown in basal medium (glutathione: green triangle ▲; proline: Royal-blue diagonal box/diamond ♦) supplemented with 12 mg Pb L− 1 (glutathione: indigo cross x; proline: maroon square ■)
All data are the mean values of three independent experiments ± SD. Data of control and 12 mg Pb L− 1 grown cultures at different times of experiment are significantly different at 95% confidence level (p < 0.05)
Proline content
Proline (Pro) is able to scavange 1O2 and radicals, inhibits lipid peroxidation, acts as an osmoregulator, metal chelator and molecular chaperon, balances cellular redox status, is considered to act as a regulatory molecule in activating physiological and/or molecular responses, enhances antioxidant response in organisms exposed to various stress factors (Verbruggen and Hermans 2008; Hossain et al. 2014; Rejeb et al. 2014; Zhang et al. 2019; Ahad and Syiem 2021). Proline content of the Pb grown cultures increased by 60% over control cultures at 120 h (Fig. 3). It is likely that Pro helped in enhancing antioxidant defense mechanism of the organism under Pb stress. Cyanobacteria such as Nostoc muscorum, Anabaena sp. Spirulina platensis and Westiellopsis sp. have been shown to produce proline in response to Cu2+, Pb2+, Zn2+ and As stress (Choudhary et al. 2007; Fatma et al. 2007; Patel et al. 2021).
Microorganisms defend themselves against ROS by stimulating SOD, POD, CAT, GR enzymes and by producing non-enzymatic antioxidants; glutathione, ascorbate and proline (Dhuldhaj et al. 2018; Zhang et al. 2019; Kashyap et al. 2021; Patel et al. 2021; Shen et al. 2021; Verma et al. 2021; Mo et al. 2022; Nowicka et al. 2022; Pandey et al. 2022; Qiu et al. 2022). In most of the above studies it has been shown that level of all of the above antioxidants is altered in response to metal stress, but the present study has demonstrated that glutathione/GR system more strongly helps the organism to manage Pb toxicity.
Conclusions
Observations and data analysis of the present study revealed that (i) Pb caused toxicity to the test organism as revealed by decrease in its growth. Severe effect on growth was observed during first 48 h, later the organism resumed growth. This indicated that the organism was able to manage Pb toxicity. (ii) Severe toxic effect of Pb during initial 48 h was due to high levels of SOR which affected photosynthetic pigments, which in turn led to decrease in growth. (iii) Deleterious effect of SOR was avoided by the test organism by producing high levels of SOD and POD which detoxified SOR. Simultaneously GSH/GR levels were enhanced which helped in detoxification of H2O2. (iv) After 48 h, the level of SOR decreased, the organism also reduced the level of SOD and POD but the levels of GSH and GR were kept high to detoxify decreased levels of SOR (Fig. 4). Decrease in the level of SOR after 48 h indicated that the test organism was somehow able to reduce the toxic effect of Pb which helped it to resume growth. The mechanism by which it reduces toxic effect of Pb after 48 h is being investigated.
Abbreviations
- A720 :
-
Absorbance at 720 nm
- APX:
-
Ascorbate peroxidase
- AsA:
-
Ascorbic acid
- CAT:
-
Catalase
- Chl a :
-
Chlorophyll a
- EDTA:
-
Ethylenediamine tetra-acetic acid
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- GSSG:
-
Glutathione disulphide
- H2O2 :
-
Hydrogen peroxide
- NADPH:
-
Nicotineamide adenine dinucleotide phosphate (reduced)
- NBT:
-
Nitroblue tetrazolium chloride
- POD:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SEM:
-
Scanning electron microscopy
- SOD:
-
Superoxide dismutase
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Agustina M, Tjahjaningsih W (2021) Assessment of heavy metal (pb) contents in canned crab products by atomic absorption spectrophotometry (AAS). Environ Earth Sci 679(1):012012. https://doi.org/10.1088/1755-1315/679/1/012012
Ahad RIA, Syiem MB (2021) Analyzing dose dependency of antioxidant defense system in the cyanobacterium Nostoc muscorum Meg 1 chronically exposed to Cd2+. Regul Toxicol Pharmacol 456(20):30250–30257. https://doi.org/10.1016/j.cbpc.2020.108950
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010) Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30:161–175. https://doi.org/10.3109/07388550903524243
Ajitha V, Sreevidya CP, Sarasan M, Park JC, Mohandas A, Singh ISB, Lee JS (2021) Effects of zinc and mercury on ROS-mediated oxidative stress-induced physiological impairments and antioxidant responses in the microalga Chlorella vulgaris. Environ Sci Pollut Res 28:32475–32492. https://doi.org/10.1007/s11356-021-12950-6
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence toxicity and bioaccumulation. J Chem 1–14. https://doi.org/10.1155/2019/6730305
Arias DM, Garcia J, Uggetti E (2020) Production of polymers by cyanobacteria grown in wastewater: current status, challenges and future perspectives. N Biotechnol 55:46–57. https://doi.org/10.1016/j.nbt.2019.09.001
Baracho DH, Silva JC, Lombardi AT (2019) The effects of copper on photosynthesis and biomolecules yield in Chlorolobion braunii. J Physiol 55(6):1335–1347. https://doi.org/10.1111/jpy.12914
Bates B, Waldern RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bazihizina N, Taiti C, Marti L, Rodrigo-Moreno A, Spinelli F, Giordano C, Caparrotta S, Gori M, Azzarello E, Mancuso S (2014) Zn2+ induced changes at the root level account for the increased tolerance of acclimated tobacco plants. J Exp Bot 65:4931–4942. https://doi.org/10.1093/jxb/eru251
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58(2):419–435. https://doi.org/10.1083/jcb.58.2.419
Bhat MA, Singh DP, Khattar JIS, Singh RS (2021) Toxicological effect of pendimethalin on some physiological parameters of the diazotrophic cyanobacterium Desmonostoc muscorum PUPCCC405.10. J Appl Biol Biotechnol 9(4):10–18. https://doi.org/10.7324/JABB.2021.9402
Boden JS, Konhauser KO, Robbins LJ, Sánchez-Baracaldo P (2021) Timing the evolution of antioxidant enzymes in cyanobacteria. Nat Commun 12(1):1–12. https://doi.org/10.1038/s41467-021-24396-y
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490. https://doi.org/10.1016/S0076-6879(85)13062-4
Chakraborty S, Mishra AK (2021) Effects of zinc toxicity on the nitrogen-fixing cyanobacteria Anabaena sphaerica ultrastructural, physiological and biochemical analyses. Environ Sci Pollut Res 28:33292–33306. https://doi.org/10.1007/s11356-021-12882-1
Chattergee P, Biswas S, Biswas AK (2018) Sodium chloride primed seeds modulate glutathione metabolism in legume cultivars under NaCl stress. Am J Plant Physiol 13:8–12. https://doi.org/10.3923/ajpp.2018.8.22
Choudhary M, Jetley UK, Abash Khan M, Zutshi S, Fatma T (2007) Effect of heavy metal stress on proline, malondialdehyde and superoxide dismutase activity in the cyanobacterium Spirulina platensis-S5. Ecotoxicol Environ Saf 66:204–209. https://doi.org/10.1016/j.ecoenv.2006.02.002
DalCorso G (2012) Heavy metal toxicity in plants. In: Furini A (ed) Plants and heavy metals. Springer, pp 1–25. https://doi.org/10.1007/978-94-007-4441-7_1
Danouche M, EI Ghachtouli N, El Baouchi A, El Arroussi H (2020) Heavy metals phycoremediation using tolerant green microalgae: enzymatic and non-enzymatic antioxidant systems for the management of oxidative stress. J Environ Chem Engg 8(5):104460. https://doi.org/10.1016/j.jece.2020.104460
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Science 2:53. https://doi.org/10.3389/fenvs.2014.00053
de Lima AA, de Carvalho MA, de Souza SA, Dias PM, da Silva Filho RG, de Meirelles Saramago CS, de Melo Bento CA, Hofer E (2012) Heavy metal tolerance (cr, ag and hg) in bacteria isolated from sewage. Braz J Microbiol 43(4):1620–1631. https://doi.org/10.1590/s1517-838220120004000047
Dhuldhaja U, Pandyab U, Singh S (2018) Anti-oxidative response of cyanobacterium Anabaena sp. strain PCC 7120 to arsenite as(III). J Microbiol 87:848–856. https://doi.org/10.1134/S0026261718060097
Dutta S, Bhadury P (2020) Effect of arsenic on exopolysaccharide production in a diazotrophic cyanobacterium. J Appl Phycol 32:2915–2926. https://doi.org/10.1007/s10811-020-02206-0
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxyl ammonium chloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620. https://doi.org/10.1016/0003-2697(76)90488-7
Etesami H (2018) Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: mechanisms and future prospects. Ecotoxicol Environ Saf 147:175–219. https://doi.org/10.1016/j.ecoenv.2017.08.032
Farooqui A, Suhail S, Zeeshan M (2017) Cadmium induced oxidative stress and biochemical responses in cyanobacterium Nostoc muscorum. Russ J Plant Physiol 64(1):124–132. https://doi.org/10.1134/S102144371701006X
Fatma T, Khan MA, Choudhary M (2007) Impact of environmental pollution on cyanobacterial proline content. J Appl Phycol 19(6):625–629. https://doi.org/10.1007/s10811-007-9195-2
Fornazier RF, Ferreira RR, Pereira GJG, Molina SMG, Smith RJ, Lea PJ, Azevedo RA (2002) Cadmium stress in sugarcane callus cultures: effect on antioxidant enzymes. Plant Cell Tissue Organ Culture 71:125–131. https://doi.org/10.1023/A:1019917705111
Gahagen HE, Holm RE, Abeles FB (1968) Effect of ethylene on peroxidase activity. Physiol Plant 21:1270–1279. https://doi.org/10.1111/j.1399-3054.1968.tb07358.x
Gest N, Gautier H, Stevens R (2013) Ascorbate as seen through plant evolution: the rise of a successful molecule? J Exp Bot 64:33–53. https://doi.org/10.1093/jxb/ers297
Ghoneim MM, El-Desoky HS, El-Moselhy KM, Amer A, Abou El-Naga EH, Mohamedein LI, Al-Prol AE (2014) Removal of cadmium from aqueous solution using marine green algae, Ulva lactuca. Egypt J Aquat Res 40(3):235–242. https://doi.org/10.1016/j.ejar.2014.08.005
González-Henao S, Ghneim-Herrera T (2021) Heavy metals in soils and the remediation potential of bacteria associated with the plant microbiome. Front Environ Sci 9:604216. https://www.frontiersin.org/articles/https://doi.org/10.3389/fenvs.2021.604216/full
Goswami S, Ahad RIA, Syiem MB (2019) Expression of copper toxicity in the rice-field cyanobacterium Anabaena oryzae Ind4. Eur Asian J Bio Sci 13(1):57–67. https://doi.org/10.1016/j.ejar.2014.08.005
Gottesfeld P, Tirima S, Anka SM, Fotso A, Nota MM (2019) Reducing lead and silica dust exposures in small-scale mining in northern Nigeria. Ann Work Expo Health 63(1):1–8. https://doi.org/10.1093/annweh/wxy095
Holm G (1954) Chlorophyll mutations in barley. Acta Agric Scand 4:457–471. https://doi.org/10.1080/00015125409439955
Hossain MA, Hoque MA, Burritt DJ, Fujita M (2014) Proline protects plants against abiotic oxidative stress: biochemical and molecular mechanisms. In: Ahmad P (ed) Oxidative damage to plants: Antioxidant networks and signaling. Academic Press, pp 477–522. https://doi.org/10.1016/B978-0-12-799963-0.00016-2
Hu J, Lin B, Yuan M, Lao Z, Wu K, Zeng Y, Fan H (2019) Trace metal pollution and ecological risk assessment in agricultural soil in Dexing Pb/Zn mining area China. Environ Geochem Health 41(2):967–980. https://doi.org/10.1007/s10653-018-0193-x
Hussein MH, Hamouda RA, Elhadary AM, Abuelmagd MA, Ali S, Rizwan M (2019) Characterization and chromium biosorption potential of extruded polymeric substances from Synechococcus mundulus induced by acute dose of gamma irradiation. Environ Sci Pollut Res 26:31998–32012. https://doi.org/10.1007/s11356-019-06202-x
Ishikawa T, Shigeoka S (2008) Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidise in photosynthesizing organisms. Biosci Biotechnol Biochem 72:1143–1154. https://doi.org/10.1271/bbb.80062
Jarosławiecka A, Piotrowska-Seget Z (2014) Lead resistance in micro-organisms. Microbiol 160(1):12–25. https://doi.org/10.1099/mic.0.070284-0
Kashyap M, Anand V, Ghosh A, Kiran B (2021) Superintending Scenedesmus and Chlorella sp. with lead and cobalt tolerance governed via stress biomarkers. Water Supply 21(5):2387–2399. https://doi.org/10.2166/ws.2021.065
Khattar JIS, Parveen S, Singh Y, Singh DP, Gulati A (2015) Intracellular uptake and reduction of hexavalent chromium by the cyanobacterium Synechocystis sp. PUPCCC 62. J Appl Phycol 27(2):827–837. https://doi.org/10.1007/s10811-014-0374-7
Kratz WA, Myers J (1955) Nutrition and growth of several blue green algae. Amer J Bot 42(3):282–287. https://doi.org/10.1002/j.1537-2197.1955.tb11120.x
Kushwaha A, Hans N, Kumar S, Rani R (2018) A critical review speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicol Environ Saf 147:1035–1045. https://doi.org/10.1016/j.ecoenv.2017.09.049
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Leung PTY, Yi AX, Ip JCH, Mak SST, Leung KMY (2017) Photosynthetic and transcriptional responses of the marine diatom Thalassiosira pseudonana to the combined effect of temperature stress and copper exposure. Mar Pollut Bull 124(2):938–945. https://doi.org/10.1016/j.marpolbul.2017.03.038
Li YP, Wang SL, Nan ZR, Zang F, Sun HL, Zhang Q (2019) Accumulation, fractionation and health risk assessment of fluoride and heavy metals in soil-crop systems in Northwest China. Sci Total Environ 663:307–314. https://doi.org/10.1016/j.scitotenv.2019.01.257
Lim LB, Priyantha N, Lu Y, Zaidi NAHM (2019) Adsorption of heavy metal lead using Citrus grandis (Pomelo) leaves as low-cost adsorbent. Desalin Water Treat 166:44–52. https://doi.org/10.5004/dwt.2019.24620
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Mitra A, Chatterjee S, Kataki S, Rastogi RP, Gupta DK (2021) Bacterial tolerance strategies against lead toxicity and their relevance in bioremediation application. Environ Sci Pollut Res 28:14271–14284. https://doi.org/10.1007/s11356-021-12583-9
Mo L, Yang Y, Zhao D, Qin L, Yuan B, Liang N (2022) Time-dependent toxicity and health effects mechanism of cadmium to three green algae. Int J Environ Res Public Health 19:10974. https://doi.org/10.3390/ijerph191710974
Munagamage T, Rathnayake IV, Pathiratne A, Megharaj M (2020) Comparison of sensitivity of tropical freshwater microalgae to environmentally relevant concentrations of cadmium and hexavalent chromium in three types of growth media. Bull Environ Contam Toxicol 105:397–404. https://doi.org/10.1007/s00128-020-02950-6
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216. https://doi.org/10.1007/s10311-010-0297-8
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nowicka B (2022) Heavy metal–induced stress in eukaryotic algae mechanisms of heavy metal toxicity and tolerance with particular emphasis on oxidative stress in exposed cells and the role of antioxidant response. Enviorn Sci Pollut Res 29:16860–16911. https://doi.org/10.1007/s11356-021-18419-w
Oser BL (1979) Hawks physiological chemistry. McGraw Hill N.Y. USA. pp 702–705. https://doi.org/10.1038/161830b0
Pandey N, Patel A, Tiwari S, Prasad SM (2022) Differential response of copper nanoparticles and ionic copper on growth, chlorophyll fluorescence, oxidative stress, and antioxidant machinery of two paddy field cyanobacteria. Ecotoxicol 31:933–947. https://doi.org/10.1007/s10646-022-02553-3
Parveen S, Khattar JIS, Singh DP (2015) The cyanobacterium Synechocystis sp. PUPCCC 62 a potential candidate for biotransformation of cr (VI) to cr (III) in the presence of sulphate. Environ Sci Pollut Res 22(14):10661–10668. https://doi.org/10.1007/s11356-015-4260-x
Patel A, Tiwari S, Prasad SM (2018) Toxicity assessment of arsenate and arsenite on growth, chlorophyll a fluorescence and antioxidant machinery in Nostoc muscorum. Ecotoxicol Environ Saf 157:369–379. https://doi.org/10.1016/j.ecoenv.2018.03.056
Patel A, Tiwari S, Prasad SM (2021) Arsenate and arsenite-induced inhibition and recovery in two diazotrophic cyanobacteria Nostoc muscorum and Anabaena sp. study on time-dependent toxicity regulation. Environ Sci Pollut Res 1–17. https://doi.org/10.1007/s11356-021-13800-1
Pikula KS, Zakharenko AM, Aruoja V, Golokhvast KS, Tsatsakis AM (2019) Oxidative stress and its biomarkers in microalgal ecotoxicology. Curr Opin Toxicol 13:8–15. https://doi.org/10.1016/j.cotox.2018.12.006
Pinto E, Sigaud-kutner TC, Leitao MA, Okamoto OK, Morse D, Colepicolo P (2003) Heavy metal–induced oxidative stress in algae. J Phycol 39(6):1008–1018. https://doi.org/10.1111/j.0022-3646.2003.02-193
Piotrowska-Niczyporuk A, Bajguz A, Talarek M, Bralska M, Zambrzycka E (2015) The effect of lead on the growth, content of primary metabolites, and antioxidant response of green alga Acutodesmus obliquus (Chlorophyceae). Environ Sci Pollut Res 22:19112–19123. https://doi.org/10.1007/s11356-015-5118-y
Pourrut B, Shahid M, Dumat C, Winterton P, Pinelli E (2011) Lead uptake, toxicity and detoxification in plants. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology. Springer, New York LLC, pp 113–136. https://doi.org/10.1007/978-1-4419-9860-6_4
Qiu C, Wang W, Zhang Y, Zhou GJ, Bi Y (2022) Response of antioxidant enzyme activities of the green microalga Chlorococcum sp. AZHB to Cu2+ and Cd2+ stress. Sustainability 14(16):10320. https://doi.org/10.3390/su141610320
Qu M, Li W, Zhang C, Huang B, Zhao Y (2015) Assessing the pollution risk of soil chromium based on loading capacity of paddy soil at a regional scale. Sci Rep 5:1–8. https://doi.org/10.1038/srep18451
Rahman Z, Singh VP (2019) The relative impact of toxic heavy metals (THMS) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg) and lead (Pb)) on the total environment: an overview. Environ Monit Asses 191:419. https://doi.org/10.1007/s10661-019-7528-7
Rejeb KB, Abdelly C, Savoure A (2014) How reactive oxygen species and proline face stress together. Plant Physiol Biochem 80:278–284. https://doi.org/10.1016/j.plaphy.2014.04
Ren R, Li Z, Zhang L, Zhou H, Jiang X, Liu Y (2021) Enzymatic and nonenzymatic antioxidant systems impact the viability of cryopreserved Paeonia suffruticosa pollen. Plant Cell Tissue Organ Culture 144(1):233–246. https://doi.org/10.1007/s11240-020-01794-6
Sharma P, Dubey RS (2005) Lead toxicity in plants. Brazilian J Plant Physiol 17:35–52. https://doi.org/10.1590/S1677-04202005000100004
Shen L, Chen R, Wang J, Fan L, Cui L, Zhang Y, Zeng W (2021) Biosorption behavior and mechanism of cadmium from aqueous solutions by Synechocystis sp. PCC6803. RSC Adv 11(30):18637–18650. https://doi.org/10.1039/D1RA02366G
Shivagangaiah CP, Sanyal D, Dasgupta S, Banik A (2021) Phycoremediation and photosynthetic toxicity assessment of lead by two freshwater microalgae Scenedesmus acutus and Chlorella pyrenoidosa. Physiol Plant 173(1):246–258. https://doi.org/10.1111/ppl.13368
Singh DP, Khattar JIS, Kaur G, Gupta M, Singh Y (2015) Effect of pretilachlor on nitrogen uptake and assimilation by the cyanobacterium Desmonostoc muscorum PUPCCC 405.10. Acta Physiol Plant 37:177. https://doi.org/10.1007/s11738-015-1923-7
Sirikhachornkit A, Niyogi KK (2010) Antioxidants and photo-oxidative stress responses in plants and algae. In: Rebeiz CA, Benning C, Bohnert HJ et al (eds) The chloroplast. Springer, Netherlands, Dordrecht, pp 379–396. https://doi.org/10.1007/978-90-481-8531-3_24
Sirunyan AM, Tumasyan A, Adam W, Ambrogi F, Asilar E, Bergauer T, Brandstetter J, Dragicevic M, Erö J, Del Valle AE, Flechl M (2019) Search for vector-like quarks in events with two oppositely charged leptons and jets in proton-proton collisions at √s = 13 TeV. Eur Phys J C 79:1–31. https://doi.org/10.1140/epjc/s10052-019-6855-8
Smedley PL, Nicolli HB, Macdonald DMJ, Barros AJ, Tullio JO (2002) Hygrogeochemistry of arsenic and other inorganic contitutents in ground waters from La Pampa. Argentina Appl Geochem 17:259–284. https://doi.org/10.1016/S0883-2927(01)00082-8
Tamaki S, Mochida K, Suzuki K (2021) Diverse biosynthetic pathways and protective functions against environmental stress of antioxidants in microalgae. Plants 10:1250. https://doi.org/10.3390/plants10061250
Tiwari S, Patel A, Prasad SM (2020) Phytohormone up-regulates the biochemical constituent, exopolysaccharide and nitrogen metabolism in paddy-field cyanobacteria exposed to chromium stress. BMC Microbiol 20:20. https://doi.org/10.1186/s12866-020-01799-3
Van Loon LC (1971) Tobacco polyphenoloxidase. A specific staining method indicating non-identity with peroxidase. Phytochemistry 10:503–507. https://doi.org/10.1016/S0031-9422(00)94689-2
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759. https://doi.org/10.1007/s00726-008-0061-6
Verma N, Prasad SM (2021) Regulation of redox homeostasis in cadmium stressed rice field cyanobacteria by exogenous hydrogen peroxide and nitric oxide. Sci Rep 11(1):1–15. https://doi.org/10.1038/s41598-021-82397-9
Verma N, Pandey A, Tiwari S, Prasad SM (2021) Calcium mediated nitric oxide responses: Acquisition of nickel stress tolerance in cyanobacterium Nostoc muscorum ATCC 27893. Biochem Biophys Rep 26:100953. https://doi.org/10.1016/j.bbrep.2021.100953
Wang S, Li Q, Huang S, Zhao W, Zheng Z (2021) Single and combined effects of microplastics and lead on the freshwater algae Microcystis aeruginosa. Ecotoxicol Environ Saf 208:111664. https://doi.org/10.1016/j.ecoenv.2020.111664
Woodbury W, Spencer AK, Stahmann MA (1971) An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem 44:301–305. https://doi.org/10.1016/0003-2697(71)90375-7
Zhang J, Li Q, Yv Z, Zhang J, Lu G, Lu Z, Dang Z, Guo C (2019) Bioaccumulation and distribution of cadmium by Burkholderia cepacia GYP1 under oligotrophic condition and mechanism analysis at proteome level. Ecotoxicol Environ Saf 176:162–169. https://doi.org/10.1016/j.ecoenv.2019.03.091
Acknowledgements
The authors are grateful to Head and Coordinator, FIST of DST, DRS-SAP-II of UGC, Department of Botany, Punjabi University, Patiala, for providing laboratory and other infrastructure facilities.
Funding
The authors did not receive any financial support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Davinder Pal Singh and Jasvirinder Singh conceived the idea, provided the general concept and inputs for each section of the manuscript, and drafted part of the manuscript. Minakshi Chandel, Kirti Sharma and Manzoor performed the experiments and wrote the manuscript after consulting the relevant literature. Jasvirinder and Davinder edited, compiled, and finalized the draft manuscript. Finally, all the authors read and approved it for publication.
Corresponding author
Ethics declarations
Financial or non-financial interests
There are no financial or non-financial interests.
Ethical approval
The present research did not involve any human participants and/or animals. No data/figure/table has been taken from any source which requires permission.
Informed consent
All the authors have gone through the contents of the manuscript and give their consent to submit it for publication.
Competing interests
The authors declare that there are no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chandel, M., Khattar, J.S., Singh, D.P. et al. Pb toxicity at initial level is managed by Desmonostoc muscorum PUPCCC 405.10 by activating antioxidant defense system. Biologia 78, 3725–3736 (2023). https://doi.org/10.1007/s11756-023-01535-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01535-y