Abstract
The use of irrigation water containing cyanobacterial toxins may generate a negative impact in both yield and quality of agricultural crops causing significant economic losses. We evaluated the effects of microcystins (MC) on the growth, nodulation process and nitrogen uptake of a Faba bean cultivar (Vicia faba L., Fabaceae), particularly the effect of MC on rhizobia-V. faba symbiosis. Three rhizobial strains (RhOF4, RhOF6 and RhOF21), isolated from nodules of local V. faba were tested. The exposure of rhizobia to MC showed that the toxins had a negative effect on the rhizobial growth especially at the highest concentrations of 50 and 100 μg/l. The germination of faba bean seeds was also affected by cyanotoxins. We registered germination rates of 75 and 68.75% at the toxin levels of 50 and 100 μg/l as compared to the control (100%). The obtained results also showed there was a negative effect of MC on plants shoot, root (dry weight) and total number of nodules per plant. Cyanotoxins exposure induced a significant effect on nitrogen assimilation by faba bean seedlings inoculated with selected rhizobial strains RhOF6 and RhOF21, while the effect was not significant on beans seedling inoculated with RhOF4. This behavior of tolerant rhizobia-legumes symbioses may constitute a very important pathway to increase soil fertility and quality and can represent a friendly biotechnological way to remediate cyanotoxins contamination in agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global climate changes may contribute to the increasing eutrophication of aquatic ecosystems and particularly of lakes and reservoirs. As a result, cyanobacterial blooms become more frequent and ubiquitous in brackish and fresh waters. Several cyanobacterial genera including Microcystis, Nodularia, Anabaena, Oscillatoria and Nostoc are able to produce a variety of cyanotoxins (Haider et al. 2003) classified principally into two main groups, hepatotoxins and neurotoxins (Sivonen and Jones 1999). This way, the use of surface waters containing cyanobacterial blooms and their toxins for irrigation could be a threat for both the quality and the plant crop yield (Pflugmacher et al. 2006; Peuthert et al. 2007; Crush et al. 2008; Saqrane et al. 2009; Saqrane and Oudra 2009). Several studies have reported that the exposure to cyanobacteria aqueous extracts induced a significant reduction of the germination rate of terrestrial plant such as Lens esculenta, Zea mays, Triticum durum, Pisum sativum and Medicago sativa (Chen et al. 2004; Pflugmacher et al. 2006; Saqrane et al. 2008; El Khalloufi et al. 2011). In addition to the inhibitory effect on seed germination, cyanotoxins could also have a negative impact on the growth, development and photosynthetic process of the seedling (Ko’s et al. 1995; Kurki-Helasmo and Meriluoto 1998; McElhiney et al. 2001; Pflugmacher 2002; Pflugmacher et al. 2006; Crush et al. 2008; Saqrane et al. 2009). Changes in the antioxidative network of enzymes such as superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) as well as on phenolic compounds due to exposure to cyanobacterial toxins was observed in different plants (Asada 1992; Polle 2001; Chen et al. 2004; Saqrane et al. 2007).
The effect of cyanotoxins on aquatic and terrestrial plants has been previously reported by several authors (reviewed by Saqrane and Oudra 2009), and there is an original report related to the effect of cyanotoxins (microcystins) on leguminous plants especially on infection and nodule initiation of alfafa (El Khalloufi et al. 2011). The MC exposure affected the alfalfa biology and physiology as well as the symbiotic bacteria survival. Leguminous-rhizobia symbiosis is a highly integrated system, soil-based stress may act on the symbiosis indirectly by reducing plant growth and available organic matter or by direct effects on the infection process and/or nodule function (Singleton 1983; Zahran 2001; Dulormne et al. 2010). It is evident that the enhancement of the leguminous productivity in areas irrigated with water contaminated by cyanobacterial toxins requires the involvement of cyanotoxins tolerant symbiotic bacteria and tolerant leguminous plants.
In the present paper, we have investigated the effects of cyanotoxins (MC), introduced via the water irrigation contaminated with a toxic cyanobacterial extract. The experiments were carried out with three rhizobial strains nodulating faba bean plants. The prospected effects aim to identify the eventual impact of the MC exposure on the faba bean seeds germination and seedlings growth, biomass production and nitrogen (N) assimilation balance of faba bean cultivars inoculated separately by three selected rhizobial strains. This way, we examined the ability of these strains to nodulate and to uptake N in symbiosis with faba bean knowing that, the research of rhizobia and legumes tolerant to extreme environmental conditions constitutes an important role in sustainable agricultural production (Zahran 2001; Khan et al. 2010). Our experimental approach could supplement scientific information related to MC tolerant rhizobia-V. faba symbioses.
Materials and methods
Cyanobacterial aqueous crude extracts preparation (cyanobacteria collection and microcystins quantification)
In Morocco, especially in Marrakesh region, Lalla Takerkoust reservoir is an aquatic ecosystem with frequent and massif proliferations of the toxic cyanobacterium M. aearuginosa during summer and autumn. The bloom toxicity, diversity of MC variants and MC cell quota are monitored since 1994 (Oudra et al. 2001, Oudra et al. 2002). A bloom sample was collected in September 2005 from Takerkoust reservoir with a 27 μm phytoplankton net. The sample was freeze–dried and the lyophilized material was stored at −20°C for MC extraction and HPLC analysis. The methodology of extraction and toxins HPLC quantification were previously described in El Khalloufi et al. (2011). For plant treatment, the frozen bloom material was thawed, sonicated (3 Hz × 10 min) and centrifuged (4000×g × 12 min).
In this report, the aqueous extract of natural cyanobacterial bloom was used in order to mimic natural conditions during bloom event when cyanobacteria cells lyses and toxins liberation occurred. The same experimental approach has been already used in our previous published works (Saqrane et al. 2007, 2008, 2009; El Khalloufi et al. 2011).
MC allelopathic effects on rhizobia strains
Three strains of rhizobia were isolated from root nodules of V. faba plants collected from Marrakesh region. Nodules of V. faba plants were previously disinfected with sodium hypochlorite (4°) and washed several times with sterile physiological water. The nodule was crushed in a sterile tube. The suspension was seeded on Petri dishes containing YEM medium agar with Congo Red. After incubation for 48 h at 28°C, colonies of rhizobia, characterized by a gluey aspect and without absorption of Congo red, were isolated and purified on YEM medium (Vincent 1970). The strains were stored at −25°C in glycerol 30%.
Flasks containing 100 ml of YEM broth medium were inoculated with each isolated rhizobial strain. Four parallel exposures to MC were performed with three replicates each (0, 10, 50 and 100 μg MC/l); in the control flasks (0 μg/l) we added 1 ml of sterile distilled water. Flasks were incubated at 28°C, in darkness and under continuous agitation (250 rpm).
The growth of rhizobia was estimated by counting colonies forming units per ml (CFU/ml) on YEM medium agar.
Effects of MC extracts on Faba bean seeds germination
In order to evaluate the effect of MC on the germination process, the seeds of V. faba were sterilized with sodium hypochlorite 6° for 10 min, followed by rinsing them several times with sterile distilled water. The seeds were placed on wet filter papers in the Petri dishes (10 seeds in each Petri dish). For the test, the seeds were exposed (every 24 h for 7 days) to 3 ml of the aqueous extract containing MC at various concentrations (10, 50 and 100 μg/l MC) or sterile distilled water for the control treatment. Six replicates for each treatment were prepared. The germination boxes were placed into the incubator at 25°C in the dark under sterile conditions, and the rate of germination was determined.
Plant culture and MC extracts exposure experiments
A commercial cultivar of V. faba L. (variety with violet seeds) was used to perform the MC exposure experiments. Seedlings of V. faba were raised from pre-germinated seeds in pots with 15 cm of sand (three seedlings in each pot). The sand, used as a neutral substrate, was previously washed with distilled water and sterilized at 200°C for 4 h in a muffle furnace. The experiment was carried out in a glasshouse under natural conditions during April–May 2010.
Three treatment groups were designed with the three different rhizobia strains. Each group of seedling was inoculated with 2 ml of liquid inoculant containing approximately 109 CFU/ml of each RhOF4, RhOF6 and RhOF21 strains. Four parallel MC treatment exposures (0, 10, 50 and 100 μg/l MC) of seedling were performed with three replicates (three pots). The exposure to MC began 15 days after inoculation and lasted for 6 weeks.
The aqueous extract of MC was supplemented with N-free nutrient solution. At the end of the experiment, plants were harvested, washed out of the sand; nodules were detached from their root and counted. After desiccation at 70°C during 72 h, the dry matter of the different parts of the plants (shoot, root and nodules) was quantified. Nitrogen content in shoots and roots was dosed by the Kjeldahl procedure. For total N determination, 0.5 g dry weight was mixed with 1 g of a catalyst mixture (K2SO4, CuSO4·5H2O and Se) and digested with 10 ml of sulfuric acid (98%). After mineralization, the volume was adjusted to 100 ml with distilled water; 40 ml of the solution was transferred to Kjeldahl bottles containing few drops of NaOH (8N), and the resulting distillated. The distillate was titrated with 0.05N sulfuric acid.
Statistical analysis
The experimental design was a randomized complete block. The growth values, the parameters related to nodulation were means of four replicates per treatment per rhizobia strain. Moreover, three faba bean plants were put in each pot. Data were analyzed by variance analysis (ANOVA), and the mean separation was achieved by LSD test by the COSTAT software. All numeric differences in the data were considered significantly different at the probability level of P ≤ 0.05.
Results
The results of HPLC–PDA obtained from the extract of the M. aeruginosa bloom, revealed a mixture of five variants of MC: DMC-LR (4.20%); MC-(H4)-YR (4.32%); MCLY (8.32%); C-FR (9.45%) and MC-LR (73.71%). The total MC content of the bloom extract was 22.24 μg/ml. The detail of these results was previously described in El Khalloufi et al. (2011). According to these results, we assume that the global MC effect will be connected to the effect of MC-LR which is the dominant MC variant in the extract (around 74%).
The growth of the strains RhOF4, RhOF6 and RhOF21 depended on both, strain and cyanotoxin concentration (Table 1). The exposure of rhizobia to MC showed that the toxins had a negative effect on the rhizobial growth. A significant decrease in CFU/ml compared to the control was registered (P < 0.05). From the 1st day of exposure, the negative effect of MC appeared especially at the highest concentrations of 50 and 100 μg/l (Table 1).
At the end of experiment, the bacterial abundances at 100 μg MC/l were 122 × 1011, 100 × 108 and 61 × 1010 CFU/ml, respectively for the strains RhOF4, RhOF6 and RhOF21. These abundances were significantly lower (P < 0.05) as compared to the controls without MC (Table 1).
The behavior of the studied rhizobia was different with RhOF4 growing faster than RhOF21 and RhOF6, in both microcosms with and without cyanotoxins. Indeed, after only 2 days of growth at 100 μg MC/l, the abundances of RhOF4 were 100 × 109 CFU/ml which was significantly higher than those of RhOF21 (135 × 108 CFU/ml) and RhOF6 (127 × 106 CFU/ml).
After 7 days of exposure to MC crude extract, a negative effect of MC on seed germination rate of V. faba seeds was registered, being this effect significantly concentration dependent. MC did not have any effect at the concentration of 10 μg MC/l as compared to the control (100%). However at the highest toxin levels of 50 and 100 μg/l, we registered germination rates of respectively 75 and 68.75% (Table 2).
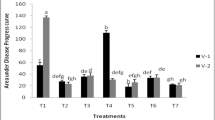
The data in Fig. 1 reveal that dry matter yield of both shoots and roots decreased significantly (P < 0.05), but only at the highest concentration of cyanotoxins (100 μg MC/l).
Only faba bean plants inoculated with RhOF6 showed a significant dry matter decrease also at the concentration of 50 μg MC/l as compared to the control. Whereas, the plants inoculated with RhOF21 showed no significant difference at the tested toxins levels in their shoots and roots dry weight. There was a significant negative correlation (P < 0.05) between concentrations of cyanotoxins and the dry matter of faba bean plants inoculated by RhOF4, RhOF6 or RhOF21.
The coefficients correlation were respectively r = −0.96, −0.64 and −0.98 for shoots and r = −0.99, −0.92 and −0.83, respectively for roots.
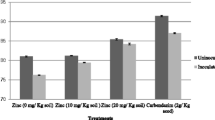
In the presence of cyanotoxins at 100 μg MC/l, the dry matter of the nodules significantly (P < 0.05) decreased by 46.57 and 43.64% in the plants inoculated with RhOF21 and RhOF4 respectively, while at 10 and 50 μg MC/l, cyanotoxins had no significant effect (Fig. 2). However in the case of plants inoculated with the RhOF6 strain, cyanotoxins caused a significant dry matter decrease at 50 μg MC/l (Fig. 2).
The effect of MC is stronger against nodulation. After 5 weeks of treatment, the obtained results showed that in the case of plants inoculated with RhOF21, RhOF6 and RhOF4, 100 μg MC/l inhibited nodulation by about 42, 32 and 37%, respectively. Thus MC inhibited not only the nodule growth, but also the nodulation process. Cyanotoxins induced a significant negative effect (P < 0.05) on nodules development of faba bean inoculated with RhOF4, RhOF6 and RhOF21 (Fig. 2); the coefficients correlation were respectively r = −0.7, −0.67 and −0.88 for nodule dry weight and r = −0.75, −0.71 and −0.81, respectively for nodule number.
In the plants nodulated by RhOF6 or RhOF21, MC decreased significantly (P < 0.05) the total N content of both shoots and roots of V. faba plants (Tables 3, 4). The effect of cyanotoxins was more obvious for the highest toxins levels (50 and 100 μg MC/l). On the contrary, MC did not significantly modify the N content in shoots and roots of plants inoculated by RhOF4. The assimilation of N under cyanotoxins effects was different according to the strain and the concentration of MC (Tables 3, 4).
Discussion
The crude extract of cyanotoxins used in this study had an inhibitory effect on the growth of the tested rhizobial strains nodulated faba bean plants. According to our knowledge, few data are available on the effects of cyanotoxins on bacteria and especially on rhizobia. El Khalloufi et al. (2011) have demonstrated that MC-LR had a negative effect on the growth of rhizobia nodulating M. sativa. Some studies have reported that microcystins affect cell wall permeability (Dixon et al. 2004). Even at low concentrations, it was also indicated that cyanobacteria can produce different types of secondary metabolites with fungicidal and/or antibacterial effects, such as cyanotoxins (Sivonen and Jones 1999; Dixon et al. 2004).
Otherwise, Valdor and Aboal (2007) studied the effects of cyanobacterial extracts and pure microcystins on growth and ultrastructure of microalgae and bacteria, and demonstrated the inhibitory effect of both cyanobacterial extracts and pure microcystins on the growth of microalgae and bacteria. This inhibitory effect was more persistent in pure microcystins than in the extracts, which lost their properties 8 days after exposure. In addition, the effects on bacteria were longer lasting than those on microalgae. Similarly, Vassilakaki and Pflugmacher (2007) have reported that exposure to both cyanobacterial crude extracts and pure MC induced an oxidative stress response of Synechocystis sp. and had negative effects on the growth of microalgae and bacteria.
The seeds germination is an important process in plant development. It represents the earlier stage of seedlings growth. So, the possible effect of microcystins on this process may affect the crop productivity. The inhibition of seeds germination was observed in leguminous plants, after exposure of alfalfa (M. sativa) (Pflugmacher et al. 2006; El Khalloufi et al. 2011) and the seeds of P. sativum (Saqrane et al. 2008) to cyanobacterial toxins and cyanobacteria crude extracts. Our study confirms this finding; the concentrations of equivalent microcystins MC-LR at 50 and 100 μg/l, induced a significant reduction of germination rate of V. faba seeds. No significant difference was observed at the concentration of 10 μg MC/l as compared to the control.
Adding to the inhibitory effect on seeds germination, cyanobacteria toxins induce a decrease of dry matter in a range of plants such as the leguminous; P. sativum (Saqrane et al. 2008) and other terrestrial plants L. esculenta, Z. mays, T. durum and, Rye grass, Clover, Rape, and Lettuce (Crush et al. 2008; Saqrane et al. 2008). The data obtained in our study revealed comparable results; the dry matter of both shoots and roots decreased generally at the highest concentration of cyanotoxins (100 μg MC/l) (P < 0.05).
Measured nodulation, as nodule number, is usually considered an indication of successful infections of legume plants (Zahran 1991). The possible effect of spry irrigation practices with water containing microcystins on nodule number was previously investigated by El Khalloufi et al. (2011). The authors demonstrated that cyanotoxins decreased nodule number during the symbiosis rhizobia-M. sativa, the reduction rate was concentration-dependent. The present study revealed that the development of root systems and nodulation was strongly affected by cyanotoxins. The total nodule number per plant was affected, especially at the highest toxin levels (50 and 100 μg MC/l). Cyanotoxins also reduced nodule dry weight of faba bean. Velagaleti and Marsh (1989) have demonstrated that the reduction of nodule weight resulted from the reduction of carbohydrate translocation towards nodules that follows the shoot growth inhibition and the decrease of intrinsic photosynthetic capacity.
The symbiotic relationship between rhizobia and legumes allows the fixation of atmospheric N which makes it in available form for plants. We have evaluated the effects of cyanotoxins stress on this process by the measurement of the total N assimilated by faba bean plants. However RhOF4 strain formed 90% of nodule compared to RhOF21, these nodules appeared to be more effective for N assimilation. In our experiment, we have found that MC did not significantly modify N content in shoots and roots of plants infected by RhOF4 strain. In contrast, the total N content decreased significantly in both shoots and roots of faba bean inoculated by RhOF6 or RhOF21 and exposed to MC. According to our knowledge, the effect of cyanotoxins on N assimilation have not previously been reported, but the decline of N uptake by bean plants under other environmental stresses, has been reported in several studies (Hunt and Layzell 1993; Sadiki and Rabih 2001; Zahran 2001; Garg and Singla 2004; Abdelly et al. 2005; Tejera et al. 2006; Krouma 2009) and they could be due to: (i) a preferential degradation of the leghemoglobin (Delgado et al. 1993), (ii)a decrease in the activity of enzymes involved in tissue protection against reactive oxygen species (Sheokand and Dhandi 1995), and (iii) an inhibition of ammonium assimilation pathways, particularly as a consequence of a decrease in glutamine synthetase activity (Cordovilla et al. 1994). Results of other investigators attribute the decline of nitrogenase activity to the limitation of oxygen diffusion in the nodules.
References
Abdelly C, Krouma A, Drevon JJ (2005) Nitrogen fixation and yield of Chickpea in saline Mediterranean zones. Grain legumes 42:16–17
Asada K (1992) Ascorbate peroxidise: a hydrogen peroxide scavenging enzyme in plants. Physiol Plant 85:235–241
Chen J, Song L, Dai J, Gan N, Liu Z (2004) Effects of microcystins on the growth and the activity of superoxide dismutase and peroxidase of Rape (Brassica napus L.) and Rice (Oryza sativa L.). Toxicon 43:393–400
Cordovilla MP, Ligero F, Lluch C (1994) The effect of salinity on N fixation and assimilation in Vicia faba. J Exp Bot 45:1483–1488
Crush JR, Briggs LR, Sprosen JM, Nichols SN (2008) Effect of irrigation with lake water containing microcystins on microcystin content and growth of ryegrass, clover, rape, and lettuce. Environ Toxicol 23:246–252
Delgado MJ, Ligero F, Lluch C (1993) Effects of salt stress on growth and N2 fixation by pea, faba bean, common bean, and soybean plants. Soil Biol Biochem 26:371–376
Dixon RA, Al-Nazawi M, Alderson G (2004) Permeabilising effects of sub-inhibitory concentrations of microcystin on the growth of Escherichia coli. FEMS Microbiol Lett 230:167–170
Dulormne M, Musseau O, Muller F, Toribio A, Bâ A (2010) Effects of NaCl on growth, water status, N2 fixation, and ion distribution in Pterocarpus officinalis seedlings. Plant Soil 327:23–34
El Khalloufi F, Oufdou K, Lahrouni M, El Ghazali I, Saqrane S, Vasconcelos V, Oudra B (2011) Allelopatic effects of cyanobacteria extracts containing microcystins on Medicago sativa-rhizobia symbiosis. Ecotoxicol Environ Saf 74:431–438
Garg N, Singla R (2004) Growth, photosynthesis, nodule nitrogen and carbon fixation in the chickpea cultivars under salt stress. Braz J Plant Physiol 16:137–146
Haider S, Naithani V, Viswanathan PN, Kakkar P (2003) Cyanobacterial toxins: a growing environmental concern. Chemosphere 52:1–21
Hunt S, Layzell DB (1993) Gas exchange of legume nodules and the regulation of nitrogenase activity. Ann Rev Plant Physiol Mol Biol 44:483–511
Khan HR, Paull JG, Siddique KHM, Stoddard FL (2010) Faba bean breeding for drought-affected environments: a physiological and agronomic perspective. Field Crops Res 115:279–286
Ko′s P, Gorzo G, Suranyi G, Borbely G (1995) Simple and efficient method for isolation and measurement of cyanobacterial hepatotoxins by plant tests (Sinapis alba L.). Anal Biochem 225:49–53
Krouma A (2009) Physiological and nutritional response of chickpea (Cicer arietinum L.) to salinity. Turk J Agric 33:503–512
Kurki-Helasmo K, Meriluoto J (1998) Microcystin uptake inhibits growth and protein phosphatase activity in Mustard (Sinapis alba L.) seedlings. Toxicon 36:1921–1926
McElhiney J, Lawton LA, Leifert C (2001) Investigations into the inhibitory effects of microcystins on plant growth, and the toxicity of plant tissues following exposure. Toxicon 39:1411–1420
Oudra B, Loudiki M, Sbiyyaa B, Martins R, Vasconcelos V, Namikoshi M (2001) Isolation, characterization and quantification of microcystins (heptapeptides hepatotoxins) in Microcystis aeruginosa dominated bloom of Lalla Takerkoust lake reservoir (Morocco). Toxicon 39:1375–1381
Oudra B, Loudiki M, Sbiyyaa B, Sabour B, Amorim A, Martins R, Vasconcelos V (2002) Detection and variation of microcystin contents of Microcystis blooms in eutrophic Lalla Takerkoust Lake (Morocco). Lakes Reservoirs Res Manag 7:35–44
Peuthert A, Chakrabarti S, Pflugmacher S (2007) Uptake of microcystins-LR and–LF (cyanobacterial toxins) in seedlings of several important agricultural plant species and the correlation with cellular damage (lipid peroxidation). Environ Toxicol 22:436–442
Pflugmacher S (2002) Possible allelopathic effects of cyanotoxins, with reference to microcystin-LR, in aquatic ecosystems. Environ Toxicol 17:407–413
Pflugmacher S, Jung K, Lundvall L, Neumann S, Peuthert A (2006) Effects of cyanobacterial toxins and cyanobacterial cell-free crude extract on germination of Alfalfa (Medicago sativa) and induction of oxidative stress. Environ Toxicol Chem 25:2381–2387
Polle A (2001) Dissecting the superoxide dismutase-ascorbate-glutathionepathway in chloroplasts by metabolic modelling. Computer simulations as a step towards flux analysis. Plant Physiol 126:445–462
Sadiki M, Rabih K (2001) Selection of chickpea (Cicer arietinum) for yield and symbiotic nitrogen fixation ability under salt stress. Agronomy 21:659–666
Saqrane S, Oudra B (2009) CyanoHAB occurrence and water irrigation cyanotoxin contamination: ecological impacts and potential health risks. Toxins 1:113–122
Saqrane S, El ghazali I, Ouahid Y, El hassani M, El Hadrami I, Oudra B, Bouarab L, Del Campo FF, Vasconnelos V (2007) Phytotoxic effects of cyanobacteria extract on the aquatic plant Lemna gibba: microcystin accumulation, detoxication and oxidative stress induction. Aquat Toxicol 83:284–294
Saqrane S, El Ghazali I, Oudra B, Bouarab L, Vasconcelos V (2008) Effects of cyanobacteria producing microcystins on seed germination and seedling growth of several agricultural plants. J Environ Sci Health B 43:443–451
Saqrane S, Ouahid Y, El Ghazali I, Oudra B, Bouarab L, del Campo FF (2009) Physiological changes in Triticum durum, Zea mays, Pisum sativum and Lens esculenta Cultivars, caused by irrigation with water contaminated with microcystins: a laboratory experimental approach. Toxicon 53:786–796
Sheokand S, Dhandi S (1995) Studies on nodule functioning and hydrogen peroxide scavenging enzymes under salt stress in chickpea nodules. Plant Physiol 33:561–566
Singleton PW (1983) Asplit-root growth system for evaluating the effect of salinity on the components of the soybean Rhizobium japonicum symbiosis. Crop Sci 23:259–262
Sivonen K, Jones G (1999) Cyanobacterial toxins. In: Toxic cyanobacteria in water: a guide to public health significance, monitoring, management. In: Chorus I, Bertram J (eds) The world health organization. ISBN 0–419–23930–8. E & FN Spon, London, pp 41–111
Tejera NA, Soussi M, Lluch C (2006) Physiological and nutritional indicators of tolerance to salinity in chickpea plants growing under symbiotic conditions. Environ Exp Bot 58:17–24
Valdor R, Aboal M (2007) Effects of living cyanobacteria, cyanobacterial extracts and pure microcystins on growth and ultrastructure of microalgae and bacteria. Toxicon 49:769–779
Vassilakaki M, Pflugmacher S (2007) Oxidative stress response of Synechocystis sp. (PCC 6803) due to exposure to microcystin-LR and cell-free cyanobacterial crude extract containing microcystin-LR. J Appl Phycol 20:219–225
Velagaleti RR, Marsh S (1989) Influence of host cultivars and Bradyrhizobium strain on the growth and symbiotic performance of soybean under salt stress. Plant Soil 119:133–138
Vincent JM (1970) The cultivation, isolation and maintenance of rhizobia. In: Vincent JM (ed) A manual for the practical study of root-nodule. Blackwell, Oxford, pp 1–13
Zahran HH (1991) Conditions for successful Rhizobium legume symbiosis in saline environments. Biol Fertil Soils 12:73–80
Zahran HH (2001) Rhizobia from wild legumes: diversity, taxonomy, ecology, nitrogen fixation and biotechnology. J Biotechnol 91:143–153
Acknowledgments
This study was financially supported by the International Foundation for Sciences (ifs project No F/2826-3F). This work is also carried out within the framework of the cooperation Morocco–Portuguese collaboration (convention of cooperation CNRST-Morocco/GRICES or FCT-Portugal; Prof. Brahim Oudra/Prof. V. M. Vasconcelos) and the Morocco–Spanish collaboration (AECID projects n°A/018163/08 and n°A/025374/09; Prof. Khalid Oufdou/Prof. Alvaro Peix). Authors thank F. F. Del Campo and Y. Ouahid from the UAM-Spain for help and HPLC technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lahrouni, M., Oufdou, K., Faghire, M. et al. Cyanobacterial extracts containing microcystins affect the growth, nodulation process and nitrogen uptake of faba bean (Vicia faba L., Fabaceae). Ecotoxicology 21, 681–687 (2012). https://doi.org/10.1007/s10646-011-0826-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0826-7