Abstract
Present study deals with responses of two cyanobacteria viz. Nostoc muscorum and Phormidium foveolarum against butachlor [2-chloro-2,6-diethyl-N-(butoxymethyl) acetanilide] (low dose; 5 µg mL−1 and high dose; 10 µg mL−1) and UV-B (7.2 kJ m−2) alone, and in combination. Butachlor and UV-B exposure, alone and in combination, suppressed growth of both the cyanobacteria. This was accompanied by inhibitory effect on whole cell oxygen evolution and photosynthetic electron transport activities. Both the stressors induced the oxidative stress as there was significant increase in superoxide radical (O2·−) and hydrogen peroxide (H2O2) contents resulting into increased lipid peroxidation and electrolyte leakage. In N. muscorum, low dose of butachlor and UV-B alone increased the activities of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD), while activity of all these enzymatic antioxidants declined significantly at treatments with high dose of butachlor alone, and with low and high doses of butachlor and UV-B in combination. In P. foveolarum, enhanced activity of SOD, CAT and POD (except POD at high dose of butachlor and UV-B combination) was noticed. Ascorbate level in N. muscorum declined progressively with increasing intensity of stress while in P. foveolarum varied response was noticed. Proline contents increased progressively under tested stress in both the organisms. Overall results suggest that N. muscorum was more sensitive than P. foveolarum against butachlor and UV-B stresses. Hence, P. foveolarum may be preferred in paddy field for sustainable agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen-fixing cyanobacteria are known to be a prominent component of the microbial population and as major biomass producers in wetlands, especially rice fields, contributing significantly to the fertility as a natural biofertilizer (Anand and Hopper 1987; Tiwari et al. 1991). In rice growing countries, certain cyanobacterial strains are being inoculated in fields to enhance the paddy production under algal technological programs (Anand and Hopper 1987; Tiwari et al. 1991; Vaishampayan et al. 2001). Being heterocystous cyanobacterium, Nostoc muscorum fixes molecular nitrogen under aerobic condition while Phormidium foveolarum, a non-heterocystous and a dominant cyanobacterium of paddy fields (Desikachary 1959), can also fix nitrogen under micro-aerobic condition as reported by Ohki et al. (1992) in Trichodesmium sp. Due to differential sensitivity of cyanobacteria, their tolerance and tolerance strategies against various stresses must be known (Fernandes et al. 1993; Ma et al. 2010; Singh et al. 2011; Wang et al. 2013a, b).
The contamination of aquatic ecosystems including paddy fields with herbicides adversely affecting physiological and biochemical processes of non-target cyanobacteria and algae is a serious global environmental concern (Ueji and Inao 2001; Ma et al. 2006; Singh and Datta 2006). Previous studies have shown that after application of butachlor a large portion accumulates in the top layer of soil (0–15 cm depth) where most of the microbiological activities occurs but impact of herbicides may get intensified many folds on soil microbial population including cyanobacteria by tillage an dirrigation (Das and Debnath 2006; Aktar et al. 2008; Chen et al. 2007, 2009).
Butachlor [2-chloro-2,6-diethyl-N-(butoxymethyl) acetanilide], having molecular formula C17H26ClNO2, belongs to the class of the chloroacetanilide herbicides and was introduced by Monsanto Company (USA) in 1968 for the pre-emergence control of undesirable annual grasses. The recommended application for butachlor ranges from 1 to 3 kg ha−1 for paddy field which is equivalent to 3.3–10 mg L−1 in fields containing 3 cm deep water (Martin and Worthing 1974). It is used to control certain broad-leaved weeds in both seeded and transplanted rice in India and other South Asian countries (Debnath et al. 2002; Chen et al. 2007, 2009; Kumari et al. 2009; He et al. 2013). Agro-ecosystem is facing potential treat due to Butachlor, which is a persistent pollutant in agriculture. Butachlor acts by inhibiting elongase enzyme, responsible for the elongation of very long-chain fatty acids and geranylgeranyl pyrophosphate cyclisation (Böger et al. 2000). Its half-life varies from 1.6 to 11.4 days depending on type of soil and micro-flora (Beestman and Deming 1974; Chen and Chen 1979; Yu et al. 2003). Further, the concentration of the residues of butachlor depends on soil organic matter and may reach even two times higher than applied concentrations with 2.82% of organic matter in the paddy field soil (Wang et al. 1996; Chen et al. 2007). Thus, the exceeding concentration of butachlor in soil may have an adverse impact on non-target beneficial microorganisms hence, results in greater loss to paddy productivity (Debnath et al. 2002; Das and Debnath 2006; Chen et al. 2009). Butachlor has also been shown to produce toxicity in nitrogen fixing cyanobacteria and green algae (Vaishampayan 1985; Ma et al. 2006; Singh and Datta 2006; Kumari et al. 2009, 2012; Dowidar et al. 2010; He et al. 2013).
In addition to herbicides, enhanced ultraviolet-B (UV-B) radiation (290–320 nm) is another threat that comes on earth surface due to the destruction of the ozone layer by man-made chlorofluorocarbons. The increase in UV-B at the earth surface poses serious threats to all living-beings, particularly photoautotrophs which have absolute dependency on solar radiation for their survival (He and Häder 2002; Flint and Caldwell 2003). The effect of individual stresses, either butachlor or UV-B radiation on cyanobacteria was demonstrated in several studies (Sinha et al. 1996; He and Häder 2002; Chen et al. 2007). However, in real field situation, the combined effect of multiple stresses on a specific organism could be additive, synergistic, antagonistic or equal to that expected from the sum of the effects of the individual chemical (Sheeba et al. 2011; Srivastava et al. 2012). Therefore, it becomes imperative to assess the impact of butachlor in combination with UV-B on non-target organisms like cyanobacteria. The impact of herbicide oxyfluorfen, insecticide chlorpyrifos and Cu stress on cyanobacteria is known in the presence of UV-B (Sheeba et al. 2011; Singh et al. 2012c; Srivastava et al. 2012), but butachlor induced impact on cyanobacteria in presence of UV-B radiation is not yet known. For better exploitation of cyanobacteria as biofertilizer we must know about their degree of tolerance and tolerance strategies to various stresses. Therefore, the present study is aimed at investigating the degree of tolerance and tolerance strategies of two paddy field cyanobacteria viz. N. muscorum and P. foveolarum against butachlor and UV-B by analyzing different physiological and biochemical parameters.
Materials and methods
Organisms and culture conditions
Axenic cultures of the two cyanobacteria Nostoc muscorum and Phormidium foveolarum were maintained at laboratory. Culture P. foveolarum was grown in BG 11 medium (Rippka et al. 1979) with supplemented nitrogen (NaNO3 as source), whereas N. muscorum was cultured in the same medium without nitrogen. Both cyanobacteria were grown at 25 ± 2 °C in culture room under the photosynthetically active radiation of 70 µmol photons m−2 s−1 with a 14:10 h light:dark period. All experiments were performed with 7 days old exponential phase of culture.
Butachlor and UV-B radiation treatments
Doses 5 (low) and 10 µg mL−1 (high) of butachlor [2-chloro-2,6-diethyl-N-(butoxymethyl) acetanilide] were selected within agronomically recommended range (Kumari et al. 2009; Martin and Worthing 1974). The 5 and 10 µg mL−1 of butachlor were LC15 (15% lethal concentration) and LC20 (20% lethal concentration) concentration for N. muscorum and LC5 and LC10 (5 and 10% lethal concentration) for P. foveolarum, respectively, which were determined by the plate colony count method (Prasad and Zeeshan 2005). The exponentially grown cyanobacterial cells were harvested by centrifugation at 4000×g for 5 min and washed twice with sterile distilled water and then the pellets were resuspended (1.5 µg chlorophyll a mL−1 culture) in BG 11 medium containing 0, 5 and 10 µg mL−1 of butachlor. Butachlor treated and untreated cultures were kept in open Petri dishes under sterilized condition for UV-B exposure, and were also illuminated from upper side with 70 µmol photons m−2 s−1 by fluorescent tubes (Osram L 40 W/25-1). Petri dishes were kept on a magnetic shaker (40 rpm) to reduce the cell aggregation and sedimentation. Then cultures were irradiated with UV-B on three consecutive days equivalent to 7.2 kJ m−2 as total energy which is biologically effective according Flint and Caldwell (2003). Ultraviolet-B radiation (maximum output at 313 nm) was provided by UV-lamp (TL 40 W/12 Philips, Holland). Study of Zheng and Ye (2001) clearly indicated that butachlor in aqueous solution absorbs light mainly below 280 nm (UV-C). Hence irradiation of samples with UV-B light in the present study may not produce UV-B-induced degradation on butachlor. Samples were also exposed with 70 μmol photons m−2 s−1 of PAR at the time of UV-B irradiation. Since there was considerable negative effect on the growth of tested cyanobacteria, particularly on N. muscorum after 72 h of experiment, therefore, after this time cells were harvested and different parameters were analyzed Cyanobacterial cells neither treated with butachlor and nor UV-B were regarded as control.
Estimation of growth and photosynthetic pigments
Growth was measured in terms of protein according to Lowry et al. (1951). Chlorophyll a (Chl a) and carotenoids were quantified by the method of Porra et al. (1989) and Goodwin (1954), respectively. For phycocyanin determination, cells of each sample were treated with toluene and extracted with 2.5 mM potassium phosphate buffer (pH 7.0) after repeated freezing and thawing, and the absorbance of the supernatant was recorded at 620 nm. The amount of phycocyanin was determined by the method of Blumwald and Tel-Or (1982). The absorbance of each sample was recorded with UV–Vis Spectrophotometer, Shimadzu, Japan.
Determination of photosynthesis and dark respiration
Photosynthetic activity and respiration of cyanobacterial cells were estimated by measuring O2-evolution and consumption, respectively, for 5 min with a Clark type O2-electrode (Rank Brothers, UK) in a temperature controlled airtight reaction vessel at 25 °C. The whole cell photosynthesis was measured under a of 440 μmol photons m−2 s−1 while respiration was recorded under darkness with the same instrument.
Determination of photosynthetic electron transport activities
Spheroplasts (3 µg Chl a mL−1) of N. muscorum and P. foveolarum were prepared according to the method of Spiller (1980) and re-suspended in the HEPES–NaOH buffer (pH 6.9) containing 0.5 M sucrose, 10 mM HEPES–NaOH, 5 mM K2HPO4, 10 mM MgCl2 and 2% (w:v) bovine serum albumin. The rates of photosystem I (PS I) and whole photosynthetic reaction chain were measured in terms of O2-consumption. The 6 mL reaction mixture consisted of HEPES–NaOH buffer (pH 6.9), 10 µM 3-(3,4-dichloro diphenyl)-1, 1, dimethyl urea), 1 mM sodium ascorbate, 50 µM 2, 6-dichlorophenol indophenol (DCPIP), 50 µM NaN3, 100 µM methyl viologen (MV) and spheroplasts (3 µg Chl a mL−1) was used for measuring PS I activity. For whole photosynthetic reaction chain the mixture consisted of HEPES–NaOH buffer, 50 µM NaN3, 100 µM MV and spheroplasts. Photosystem II (PS II) electron transport activity was measured in terms of O2-evolution. The 6 mL reaction mixture contained HEPES–NaOH buffer, p-benzoquinone (p-BQ; 1 mM) and spheroplasts (3 µg Chl a mL−1). Spectrophotometric assay of PS II activity as DCPIP photoreduction was also monitored by measuring changes in absorption at 600 nm in the presence and absence of exogenous electron donor i.e. diphenyl carbazide.
Determination of reactive oxygen species (ROS)
The level of superoxide radical (O2·−) cyanobacterial cells was estimated following the method of Elstner and Heupel (1976). This assay is based on formation of NO2− from hydroxylamine in the presence of O2·−. For estimation of hydrogen peroxide (H2O2), ferrithiocyanate method of Sagisaka (1976) was used.
Determination of lipid peroxidation and electrolyte leakage
Lipid peroxidation was determined following the method of Heath and Packer (1968) in terms of MDA (malondialdehyde)- a by-product of lipid peroxidation that reacts with thiobarbituric acid (TBA). The intactness of plasma membrane in cells of each sample was estimated in terms of electrolyte leakage by the method of Gong et al. (1998). The percentage of electrolyte leakage was calculated by using the formula: EC = (EC1/EC2) × 100%.
Determination of enzymatic antioxidants
Catalase (EC 1.11.1.6) activity was assayed by measuring O2 release from dissociation of H2O2 for 3 min in darkness, using the Clark type O2-electrode (Rank Brothers, UK) (Egashira et al. 1989). Superoxide dismutase activity (SOD; EC 1.15.1.1) was measured by monitoring the inhibition of photochemical reduction of nitroblue tetrazolium chloride (NBT) following the method of Giannopolitis and Ries (1977). Peroxidase activity (POD; EC 1.11.1.7) was determined according to Gahagen et al. (1968).
Determination of non-enzymatic antioxidants
Ascorbate level was measured according to the method of Oser (1979). Proline content was estimated according to the method of Bates et al. (1973).
Statistical analysis
The results presented are the means of six replicates. Since the results showed normal distribution, comparison between control and treatment’s means was carried out by using two-way ANOVA to test significance level (Duncan’s multiple range test, DMRT). Asterisk marks indicate significant level of different concentrations of butachlor, UV-B and their interactions with control at P < 0.005(*) and P < 0.001(**) level while ns represents ‘not significant’ with control. SPSS-10 software was used for DMRT. Prior to DMRT analysis, assumptions of homogeneity of variances were tested using a multivariate test (Anderson 1958). All data sets satisfied the assumptions of ANOVA based on homogeneity of variances, normality of errors, and independence of errors.
Results
Growth
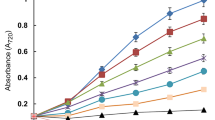
The growth behavior of both cyanobacyeria exposed to butachlor (low dose; 5 µg mL−1 and high dose; 10 µg mL−1) and UV-B radiation has been depicted in Fig. 1. Butachlor and UV-B, alone and together, declined the growth of N. muscorum and P. foveolarum significantly (P < 0.05) and the effects were more severe in N. muscorum. Butachlor at 5 and 10 µg mL−1 reduced the growth by 22 and 28% in N. muscorum and by 9% and 12% in P. foveolarum, respectively. The UV-B irradiation could also reduce the growth significantly (P < 0.05) in both the cyanobacteria i.e. by 19% in N. muscorum and 6% in P. foveolarum. Both the stresses together caused additive effects as reduction in growth was 44 and 53% in N. muscorum and 15 and 18% in P. foveolarum following the exposure of cells under low dose butachlor and UV-B, and high dose butachlor and UV-B combination, respectively.
Effect of butachlor and UV-B radiation, alone and in combination on protein content of N. muscorum and P. foveolarum after 72 h of experiment. Data are mean ± SE of six replicates. The control value of protein was to 62.0 ± 3.2 [µg (mL culture)−1] for N. muscorum and 70.0 ± 2.94[µg (mL culture)−1] for P. foveolarum. Asterisk marks indicate significant level of different concentrations of butachlor, UV-B and their interactions with control at P < 0.005(*) and P < 0.001(**) level with control
Photosynthetic pigments
Results pertaining to photosynthetic pigments i.e. chlorophyll a, carotenoids and phycocyanin of N. muscorum and P. foveolarum are depicted in Fig. 2. The control cells of N. muscorum contained 18.2 ± 0.75 µg Chl a mg−1 protein while in P. foveolarum it was 20.4 ± 0.57 µg Chl a mg−1 protein. Butachlor at low dose caused about 23% and 10% reduction (P < 0.05) in Chl a content while at high dose it was declined up to 35% and 15% in N. muscorum and P. foveolarum, respectively. The effect of herbicide on the carotenoids was the least among the pigments showing a reduction of (P < 0.05) 17 and 20% in N. muscorum and 10 and 13% in P. foveolarum after low and high dose of butachlor treatments, respectively. Among all the pigments estimated, UV-B alone caused minimum damaging effect on carotenoids showing reduction of 13 and 11% in N. muscorum and P. foveolarum, respectively. Simultaneous exposure of butachlor and UV-B further declined Chl a (52% with low dose and 61% with high dose in case of N. muscorum and 13% with low and 18% with high dose in case of P. foveolarum), carotenoids (30% with low dose and 48% with high dose in case of N. muscorum and 15% with low dose and 17% with high dose in case of P. foveolarum) and phycocyanin (74% with low dose and 79% with high dose in case of N. muscorum and 25% with low dose and 31% with high dose in case of P. foveolarum) contents in both the cyanobacteria showing more than additive effects in N. muscorum (7% with low dose and 5% with high dose for Chl a, 0.16% and 13% in case of Car, and 12 and 8% more than additive value in case of phycocyanin) while the damaging effect was less than their additive values in P. foveolarum (4% with low dose and 8% with high dose for Chl a, 7% and 13% in case of Car, and 3 and 18% less than additive value in case of phycocyanin). Compared to Chl a, butachlor at low as well as high dose produced greater effect on phycocyanin content as it was declined (P < 0.05) by 34 and 43% in N. muscorum and 20 and 27% in P. foveolarum, respectively. UV-B stress (alone) also declined (P < 0.005) the phycocyanin content by 28 and 8% in N. muscorum and P. foveolarum, respectively.
Effect of butachlor and UV-B radiation, alone and in combination on chlorophyll a (Chl a), carotenoids (Car) and phycocyanin (Phy) in N. muscorum and P. foveolarum after 72 h of experiment. The control value of Chl a was 18.2 ± 0.746 [μg (mg protein)−1] for N. muscorum and 20.4 ± 0.57 [μg (mg protein)−1] for P. foveolarum; the control value of Phy was 98.4 ± 2.8 [μg (mg protein)−1] for N. muscorum and 104 ± 2.7 [μg (mg protein)−1] for P. foveolarum and the control value of Car was 6.4 ± 0.166 [μg (mg protein)−1] for N. muscorum and 6.8 ± 0.272 [μg (mg protein)−1] for P. foveolarum. Asterisk marks indicate significant level of different concentrations of butachlor, UV-B and their interactions with control at P < 0.005(*) and P < 0.001(**) level with control
Photosynthesis and respiration
Results related to photosynthetic processes i.e. whole cell photosynthetic oxygen evolution and photosynthetic electron transport activities have been portrayed in Figs. 3 and 4 and Table 1. Results pointed out that photosynthetic processes were inhibited significantly (P < 0.005) after exposing both the cyanobacteria to butachlor and UV-B stress. Photosynthetic oxygen yield in untreated cells of N. muscorum cells was found to be 12.40 ± 0.85 [μmol O2 evolved (mg protein)−1 h−1] while in P. foveolarum it was 14.10 ± 0.54 [μmol O2 evolved (mg protein)−1 h−1]. Butachlor at low dose suppressed (P < 0.05) photosynthetic activity by 23% in N. muscorum and 14% in P. foveolarum (Fig. 3). Further, butachlor at high dose inhibited the activity by 38% in N. muscorum and 26% in P. foveolarum (Fig. 3). Ultraviolet -B irradiation alone suppressed (P < 0.05) oxygen yields significantly showing 32% in N. muscorum and 17% in P. foveolarum (Fig. 3). When both the stresses were combined together, more severe effect on oxygen evolution rate was observed, as it was declined (P < 0.05) by 41% with 5 µg mL−1 + UV-B and 67% with 10 µg mL−1 + UV-B in N. muscrorum and 21 with 5 µg mL−1 + UV-B and 31% with 10 µg mL−1 + UV-B in P. foveolarum (Fig. 3).
Effect of butachlor and UV-B radiation, alone and in combination on photosynthetic activity in N. muscorum and P. foveolarum after 72 h of experiment. Data are mean ± SE of six replicates. The control value of photosynthesis was 12.4 ± 0.852 [µmol O2 evolved (mg protein)−1 h−1] for N. muscorum and 14.1 ± 0.536 [µmol O2 evolved (mg protein)−1 h−1] for P. foveolarum. Asterisk marks indicate significant level of different concentrations of butachlor, UV-B and their interactions with control at P < 0.005(*) level with control
Restoration of photosystem II activity in presence and absence of exogenous electron donor in N. muscorum and P. foveolarum exposed to butachlor and UV-B, singly and in combination. The values for 100% DCPIP photoreduction were 100.0 ± 4.2 (without donor) and 122.0 ± 5.2 (with DPC) for N. muscorum, and 110.0 ± 6.4 (without donor) and 130.7 ± 7.4 (with DPC) μmol DCPIP photoreduced (mg Chl a)−1 h−1) for P. foveolarum. Data are mean ± SE of six replicates. Asterisk marks indicate significant level of different concentrations of butachlor, UV-B and their interactions with control at P < 0.005(*) and P < 0.001(**) level while ns represents ‘not significant’ with control
In order to elucidate the impact of both stresses on oxygen yield, photosynthetic electron transport activities were studied and results are shown in Table 1. Results indicated that butachlor and UV-B significantly (P < 0.05) inhibited the photosynthetic electron transport activities and the damaging effects were greater in N. muscorum as compared to P. foveolarum (except PS I activity at low butachlor dose alone in both organisms and UV-B alone for P. foveolarum). The inhibition induced by butachlor and UV-B stress on photosynthetic electron transport activity in both cyanobacteria showed the order: whole photosynthetic chain reaction > PSII > PSI, and the inhibitory effects were further enhanced under combined treatment of butachlor and UV-B (Table 1).
To understand whether butachlor and UV-B damaged the PSII mediated electron transport activity at oxidizing or reducing sides of PSII, photoreduction of DCPIP was measured using saturating concentration of DPC as an exogenous electron donor (Fig. 4). Results reveal that DPC restored PS II activity in both the cyanobacteria as there was insignificant (P < 0.05) inhibition i.e. 1–7% in N. muscorum and 2–5% in P. foveolarum in case of butachlor treatment while under UV-B stress the inhibition was only 4% in N. muscorum and 2% in P. foveolarum. Further, DPC restored the PS II activity showing insignificant inhibition in both the cyanobacteria previously exposed with low dose of butachlor together with UV-B. However, in case of high dose of butachlor and UV-B combination only partial restoration in PSII activity was recorded as N. muscorum and P. foveolarum still exhibited 12% and 15% inhibition (P < 0.05), respectively (Fig. 4).
The respiratory activity in terms of μmol O2 consumed (mg protein)−1 h−1 in treated and untreated cells of both cyanobacteria is depicted in Fig. 5. Nostoc muscorum exhibited an increase (P < 0.05) of 10 and 16% while P. foveolarum showed 8 and 14% rise in respiration rate following low and high dose of butachlor treatments, respectively. UV-B stress alone also enhanced (P < 0.05) respiration rate by 10% in N. muscorum and 8% in P. foveolarum. Both the stresses together further raised respiration rate in both the cyanobacteria, however, in case of high dose of butachlor and UV-B combination the rise in respiration was greater than their additive values.
Effect of butachlor and UV-B radiation, alone and in combination on respiratory activity in N. muscorum and P. foveolarum after 72 h of experiment. The control value of respiration was 1.5 ± 0.048 [µmol O2 consumed (mg protein)−1 h−1] for N. muscorum and 1.64 ± 0.077 [µmol O2 consumed (mg protein)−1 h−1] for P. foveolarum. Data are mean ± SE of six replicates. Asterisk marks indicate significant level of different concentrations of butachlor, UV-B and their interactions with control at P < 0.005(*) and P < 0.001(**) level with control
Reactive oxygen species, lipid peroxidation and electrolyte leakage
Results pertaining to reactive oxygen species (ROS): superoxide radical (O2·−)) and hydrogen peroxide (H2O2) are depicted in Fig. 6. The SOR content in untreated cells of N. muscorum and P. foveolarum was 9.67 ± 0.31 and 8.82 ± 0.32 nmol mg−1 protein, respectively. Treatment with low dose of butachlor caused 5 and 13% rise (P < 0.05) in O2·− content while with higher dose increased (P < 0.05) values were 13 and 26% in N. muscorum and P. foveolarum, respectively. UV-B dose alone also significantly (P < 0.05) enhanced O2·− content in both the organisms. Compared to O2·− content, the levels of H2O2 was appreciably high in both the cyanobacteria, and between two strains N. muscorum contained greater amount (25.3 ± 1.1 nmol mg−1 protein) of H2O2 than that of P. foveolarum (18.4 ± 0.66 nmol mg−1 protein). Both stresses alone enhanced (P < 0.05) H2O2 content in both cyanobacteria and the pattern was similar as recorded in case of O2·−. Butachlor and UV-B together raised the O2·− level further as the cells of N. muscorum and P. foveolarum exhibited 14–34% and 26–38% increase in SOR and 32–45% and 18–30% in H2O2 content, respectively (Fig. 6).
Effect of butachlor and UV-B radiation, alone and in combination on superoxide radical (O2·−) and hydrogen peroxide (H2O2) in N. muscorum and P. foveolarum after 72 h of experiment. The control value of O2·− was 9.67 ± 0.31 [nmol (mg protein)−1] for N. muscorum and 8.82 ± 0.327 [nmol (mg protein)−1] for P. foveolarum. The control value of H2O2 was 25.3 ± 1.09 [nmol (mg protein)−1] for N. muscorum and 18.4 ± 0.66 [nmol (mg protein)−1] for P. foveolarum. Data are mean ± SE of six replicates. Asterisk marks indicate significant level of different concentrations of butachlor, UV-B and their interactions with control at P < 0.005(*) and P < 0.001(**) level with control
Malondialdehyde content in untreated cells of N. muscorum was greater (2.45 ± 0.09; nmol MDA mg−1 protein) than that (1.65 ± 0.06; nmol MDA mg−1 protein) of P. foveolarum (Fig. 7). Nostoc muscorum exhibited an increase (P < 0.05) of MDA content from 68 to 96% under butachlor treatment and 26% under UV-B stress while in P. foveolarum cells it was enhanced (P < 0.05) by 7–18% and 18% under similar conditions, respectively. Nostoc muscorum cells exhibited greater electrolyte leakage than the cells of P. foveolarum (Fig. 7), however, the cells exposed with butachlor and UV-B either alone or in combination showed similar pattern in electrolyte leakage as noticed in case of MDA.
Effect of butachlor and UV-B radiation, alone and in combination on lipid peroxidation and electrolyte leakage in N. muscorum and P. foveolarum after 72 h of experiment. Data are mean ± SE of six replicates. The control value of MDA was 2.45 ± 0.088 [nmol MDA (mg protein)−1] for N. muscorum and 1.65 ± 0.058 [nmol MDA (mg protein)−1] for P. foveolarum. The control value of electrolyte leakage was 8.17 ± 0.392 for N. muscorum and 3.73 ± 0.187 for P. foveolarum. Asterisk marks indicate significant level of different concentrations of butachlor, UV-B and their interactions with control at P < 0.005(*) and P < 0.001(**) level with control
Activities of enzymatic antioxidants
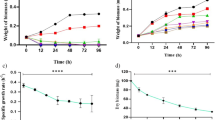
The activities of antioxidant enzymes in both the cyanobacteria treated with butachlor and UV-B alone or in combination have been presented in Table 2. The enzymatic antioxidants: SOD, CAT and POD showed differential responses in tested organisms when treated with butachlor and UV-B. In N. muscorum, SOD, CAT and POD activities were enhanced (P < 0.05) by 50, 15 and 30%, respectively following low dose of butachlor treatment while the activities were inhibited significantly (P < 0.05) under high dose of butachlor treatment. In contrast to N. muscorum, P. foveolarum exposed to both doses of butachlor exhibited significant (P < 0.05) rise in the activity of these enzymes. The UV-B exposure also raised (P < 0.05) the activities of these enzymes in both cyanobacteria. Both the stresses simultaneously further suppressed (P < 0.05) the activity of these enzymes in N. muscorum whereas stimulatory effects (except in case of POD at 10 µg mL−1 butachlor and UV-B combination) were noticed in P. foveolarum. Combined treatments of both the stresses in N. muscorum declined (P < 0.05) the activity of these enzymes.
Non-enzymatic antioxidants
Ascorbate and proline contents in both the cyanobacteria are shown in Table 3. In N. muscorum, ascorbate and proline contents were 466 ± 25.2 and 5.9 ± 0.27 nmol mg−1 dry mass, however, in P. foveolarum the contents were high showing 608 ± 28.0 and 6.6 ± 0.34 nmol mg−1 dry mass, respectively. Both the stresses either alone or in combination enhanced proline content in both the cyanobacteria, and the amount increased progressively with increasing degree of stress i.e. from low butachlor alone to high butachlor and UV-B combination. The response of both cyanobacteria with regard to ascorbate content differed under applied stress conditions. The amount of ascorbate in N. muscorum decreased with increasing degree of stress applied whereas in P. foveolarum it was significantly (P < 0.05) high under low dose of butachlor and UV-B either alone or in combination.
Discussion
The growth of N. muscorum and P. foveolarum declined significantly (P < 0.05) following butachlor and UV-B exposure and with their combined treatments the inhibitory effects on growth was enhanced (Fig. 1). The differential effects of both the stresses on growth suggested that P. foveolarum exhibited greater tolerance to tested stresses than N. muscorum. Our findings are in agreement with several other studies where butachlor induced diminished growth of Nostoc sp and cyanobacterium Aulosira fertilissima was reported (Chen et al. 2007; Kumari et al. 2009). Singh and Datta (2006) studied impact of butachlor (0–100 mg L−1) on five diazotrophic cyanobacteria dominant in rice field viz. Nostoc punctiforme, Nostoc calcicola, Anabaena variabilis, Gloeocapsa sp. and Aphanocapsa sp. and one standard laboratory strain Nostoc muscorum ISU and found that butachlor declined chlorophyll, photosynthetic oxygen evolution and nitrogen fixation in all the forms. However, in Anabaena variabilis was less affected. Recently, fluorescence measurements revealed that butachlor (80 mg L−1) declined growth of Nostoc sp. by inhibiting photosynthetic pigments synthesis (He et al. 2013). Dowidar et al. (2010) have reported that butachlor (8–20 mgL−1) declined the growth by inhibiting activities of glutamine synthetase, glutamic oxaloacetic acid transaminase and glutamic pyruvic transaminase in Nostoc muscorum and suppressed glutamine synthetase activity in wild type Anabaena vaiabilis (Singh et al. 2012a, b, c, d, e). In plants, the effect of butachlor on red kidney beans (Phaseolus vulgaris L.) was tested and lipid synthesis of isolated leaf cells along with glutathione was affected (Alla et al. 2007). Among all the plant parts, root was found to be more sensitive to butachlor followed by the shoot. Significant cell damage might be closely related to the hydrogen peroxide-induced oxidative stress than the superoxide-induced oxidative stress (Wang et al. 2013a, b). Chen et al. (2012) reported that UV-B radiation decreased growth and photosynthesis in bloom forming cyanobacteria viz. Anabaena sp. and Microcystis viridis by enhancing generation of ROS which caused serious damage to DNA. In Scytonema javanicum, it has been demonstrated that UV-B radiation directly affects photosynthetic apparatus, and enhanced formation of ROS which cause damage to DNA (Wang et al. 2012). Rastogi et al. (2011) have observed that UV-B radiation induces breaks in double stranded DNA and assigned it is one of the consequences of UV-B-mediated decline in growth of Anabaena variabilis PCC 7937. Prasad and Zeeshan (2004) and Singh et al. (2012a, b) have also demonstrated that UV-B suppressed the growth of cyanobacteria, Plectonema boryanum, Phormidium foveolarum and Nostoc muscorum by decreasing light harvesting pigments and photosynthesis due to enhanced generation of ROS, which cause severe damage to lipids and proteins. In agreement to these findings, reduction in growth of the tested cyanobacteria due to butachlor and UV-B exposure could be assigned to decreased light harvesting pigments and photosynthetic activities, and increased level of ROS which resulted into oxidative damage (Figs. 1, 2, 3, 4, 5, 67; Tables 1, 2, 3). Lesser sensitivity of P. foveolarum against UV-B stress may be explained on the basis of UV protective compounds such as mycosporine like amino acids (MAAs) which act as sunscreens and check penetration of UV-B inside the cell. Garcia-Pichel et al. (1993) have reported that Gloeocapsa sp. with high concentrations of MAAs are approximately 20% more resistant to UV radiation than those which have no or low concentrations, hence indicating their important role in imparting UV-B tolerance. Furthermore, Sinha et al. (2001) demonstrated that UV-B induces synthesis of MMAs (shinorine) in Anabaena sp., Nostoc commune and Scytonema sp. that help to protect them against damaging UV-B radiation. Therefore, relatively lesser or no induction of MMAs makes N. muscorum more prone towards UV-B induced damages.
Butachlor and UV-B alone and in combination declined (P < 0.05) photosynthetic pigments content in both cyanobacteria thereby affecting the growth; however, the effect was greater in N. muscroum than P. foveolarum (Fig. 2). Among photosynthetic pigments, there was greater damaging (P < 0.05) effect on phycocyanin followed by chlorophyll a, and carotenoids were least affected under butachlor and UV-B stress (Fig. 2). It has been reported that phycocyanin also possesses certain therapeutic properties, such as antioxidant (including the scavenging of peroxynitrite and peroxyl radicals), anti-inflammatory, and hepaprotective properties are involved in protecting membrane from damage. The localization of phycocyanin on exterior surface of thylakoid membrane and also the proteinacious nature of it could be one of the reasons for greater sensitivity to dual stress as compared to chlorophyll a and carotenoids which are integrated in the thylakoid membrane (Gantt and Conti 1966; Wilson et al. 2006). The essential function of carotenoids is to protect the photosynthetic system from photo-oxidative damage through the xanthophyll cycle, a process involved in dissipating excess excitation energy under stress conditions (Carletti et al. 2003). Though, the exact mechanism of chlorophyll degradation is not yet clear, however, this might have occurred due to the damage of thylakoid membrane by UV-B radiation (Renger et al. 1989). Besides the direct damaging effects on pigments, butachlor and UV-B stress might have caused degradation or inhibitory effect on biosynthetic processes of pigments as reported in earlier studies (Xie et al. 2009; Sheeba et al. 2011). The data related to lower reduction in carotenoids compared to other pigments in response to butachlor and UV-B stress can be interpreted in the light of their role in a general defense against photodynamic damage in photosynthetic apparatus of cyanobacteria under stress condition (Lemoine and Schoefs 2010; Zhu et al. 2010; Zhou et al. 2012).
Whole cell photosynthesis (O2-evolution) and photosynthetic electron transport activities (PS II, PSI and whole photosynthetic reaction chain) of N. muscorum and P. foveolarum were studied to explain the damaging effects on growth. Results pointed out that photosynthetic processes were inhibited significantly (P < 0.05) after exposing both the cyanobacteria to butachlor and UV-B stress (except PS I under 5 µg mL−1 butachlor in both cyanobacteria and under UV-B alone for P. foveolarum) (Fig. 3; Table 1). Photosynthetic activities of N. muscorum declined at faster rate as compared to the P. foveolarum. Studies reported decline of photosynthetic O2-evolution in the cyanobacterium Microcystis aeruginosa and diatom Thalassiosira pseudonana and several cynobacteria following UV-B exposure (Jiang and Qiu 2011; Singh and Datta 2006; Singh et al. 2012a, b, c, d, e). Photosynthetic O2-evolution and whole photosynthetic reaction chain activity in both the cyanobacteria was inhibited more than PS II and the least effect was noticed in PS I activity under butachlor and UV-B stress (Table 1). Renger et al. (1989) have observed that UV-B affects photosynthetic electron transport activities by interrupting thylakoid membrane integrity leading to the perturbations in the electron transport across the membrane. Photosystem II being more sensitive becomes further labile to UV-B due to association with oxygen evolving complex (OEC) which is known as electron as well as oxygen generator (Kamiya and Shen 2003; Prasad and Zeeshan 2004). UV-B radiation can damage the reaction center proteins D1 and D2 of PS II (Olsson et al. 2000). It has been reported that UV-B affects growth of Anabaena sp., Microcystis viridis and Scytonema javanicum by affecting either photosynthesis (electron transport chain) or by ROS-mediated process (oxidative stress) (Chen et al. 2012; Wang et al. 2012). Like UV-B, butachlor [150 µM (equivalent to 46.6 µg mL−1)] has been reported to significantly inhibit PS II activity and also the maximum quantum yield (Fv/Fm) of PS II (Chen et al. 2007; He et al. 2013). Similarly, Kumari et al. (2009) have reported that butachlor [65 µM (equivalent to 20 µg mL−1)] inhibited PS II, PS I and whole chain electron transport activities, and 14CO2-fixation which resulted into decline of growth in Aulosira fertilissima. Like butachlor, metolachlor, another chloroacetanilide herbicide has also been shown to adversely affect growth, pigments and PS II activity in Dunaliella tertiolecta and Aureococcus anophagefferens (Thakkar et al. 2013). Butachlor must be inhibiting photosystems of both cyanobacteria as butachlor has been shown to inhibit lipid biosynthesis (Gotz and Böger 2004; Ma et al. 2006) and thylakoid membrane encompasses PS II and PS I is mainly composed of lipids.
In order to locate possible sites of damage due to butachlor and UV-B on PS II activity, the PS II mediated DCPIP photoreduction was determined in the presence of exogenous electron donor- diphenyl carbazide (DPC) (Fig. 4). PS II together with oxygen evolving complex (OEC) was severely damaged by butachlor and UV-B stress therefore, oxidation side of PSII. Since DPC releases electrons in between oxygen evolving complex (OEC) and PS II. So in the case of high stress condition i.e. 10 µg mL−1of butachlor and UV-B combination, partial improvement in PSII activity by DPC symbolizes that this amount of stress must have damaged the reaction center and reducing side of PSII.
Respiration rate shows increased (P < 0.05) values in both the cyanobacteria under butachlor and UV-B treatments (Fig. 5). Our results are in agreement with earlier findings where butachlor and UV-B enhanced respiratory activity in cyanobacteria (Kumari et al. 2009; Singh et al. 2012b). Our results indicated that butachlor and UV-B considerably inhibited the photosynthetic electron transport activities (Table 1), hence reduced the supply of ATP for normal physiological activity of treated cells. Thus, under butachlor and UV-B stress the increased respiratory rate was probably needed to partially restore the supply of ATP to carry out the basal metabolism of treated cyanobacterial cells.
The damaging effect due to butachlor and UV-B was further analyzed by assessing oxidative stress induced by both stressors in test cyanobacteria. Under stress, the levels of superoxide radical and hydrogen peroxide (H2O2) increased over the values of untreated controls and their contents were further enhanced significantly (P < 0.05) under combined exposure of both the stressors (Fig. 6). Herbicides may generate reactive oxygen species (ROS) by inhibiting photosynthetic pathway (Galhano et al. 2011b; Sheeba et al. 2011; He et al. 2013; Wang et al. 2013a, b). In the present study, the decrease in photosynthetic contents and growth rates with increased level of ROS can be related with butachlor and UV-B induced inhibition in photosynthetic electron transport activities (Fig. 6; Table 1), and under this condition enhanced generation of ROS may occur due to spilling of electrons from over reduced photosynthetic electron transport chain to O2 as reported in earlier studies (Sheeba et al. 2011; Singh et al. 2012a, c). Studies by He and Häder (2002), Singh et al. (2012a, c), Wang et al. (2012) and He et al. (2013) have revealed that if there were excess ROS formed inside the cells, impairment of several metabolic processes like photosynthesis and chlorophyll biosynthesis could be expected that would eventually lead to cell death. Reactive oxygen species at higher amounts may cause severe damage, since they attack on lipids, proteins/enzymes and DNA, hence membrane damage, protein modification and DNA damage take place (He and Häder 2002; Kumari et al. 2009; Galhano et al. 2011a; Rastogi et al. 2011). The increased rate of lipid peroxidation i.e. MDA formation, and electrolyte leakage were resulted due to excess ROS formation under butachlor and UV-B treatments (Figs. 6, 7). Compared to P. foveolarum, the greater susceptibility of N. muscorum to both stresses was further indicated by higher rate of ROS formation, lipid peroxidation and electrolyte leakage.
The ability of organisms to metabolize O2·− and H2O2 against stress conditions largely depends on the coordination of the interrelated antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) (Chen et al. 2012; He et al. 2013; Wang et al. 2013a, b). The H2O2 formed by SOD is removed by CAT and POD (Bernroitner et al. 2009; Galhano et al. 2010, 2011a). Significant (P < 0.05) inhibition in the activity of SOD, CAT and POD is due to high dose of butachlor as well as under the combined treatment of UV-B and butachlor (low and high doses). Unlike the response noticed in N. muscorum, P. foveolarum under alone and combined treatment of butachlor and UV-B showed increase (P < 0.05) in the activity of tested enzymes (except in case of POD at high dose of butachlor + UV-B) compared to the controls. Decrease in POD activity at high dose of butachlor and UV-B combination might be due to its inactivation as a result of increased oxidative stress (Zhao et al. 2012). Under stress conditions e.g. herbicides and UV-B, differential responses of SOD, CAT and POD have also been reported (Galhano et al. 2010, 2011a; Chen et al. 2012; Singh et al. 2012a, c; Wang et al. 2012; He et al. 2013; Singh et al. 2013), and increase and/or decrease in antioxidant enzyme(s) have been related with the enhanced or suppressed stress tolerance ability of stressed organisms. Better antioxidant capacity (SOD, CAT and POD activity) in P. foveolarum leads us to conclude that this organism is well equipped with enzymatic antioxidants to counteract the adverse effects of ROS and thus, providing greater tolerance against tested stressors as compared to N. muscorum.
The non-enzymatic antioxidants ascorbate and proline were also analyzed in both the cyanobacteria exposed to butachlor and UV-B alone and in combination. Ascorbate is one of the most powerful antioxidants, directly scavenges hydroxyl radicals (·OH) and reduce H2O2 via ascorbate peroxidase reaction (Noctor and Foyer 1998). Ascorbate regenerates tocopherol from tocopheroxyl radical hence provides membrane protection (Noctor and Foyer 1998). The results reveal that the level of ascorbate continuously decreased (P < 0.05) in N. muscorum (Table 3). However, in P. foveolarum ascorbate content increased by butachlor and UV-B treatments either alone or in combination (except at high dose of butachlor alone and in combination with UV-B) (Table 3). Studies show that UV-B and other herbicide– oxyfluorfen, declined ascorbate content in cyanobacteria and algae (Estevez et al. 2001; Singh et al. 2012c). UV-B is known to cause significant decline in growth of the cyanobacterium Scytonema javanicum and cyanobacterium Anabaena torulosa by damaging DNA, however, exogenous addition of ascorbate protected the organism against UV-B damage (Singh et al. 2012a, b, c, d, e; Wang et al. 2012). The importance of ascorbate in protecting against oxidative stress has been confirmed in Arabidopsis ascorbate-deficient (vtc1) (Müller-Moulé et al. 2003). Taking into account the importance of ascorbate in mitigating oxidative stress, higher ascorbate level and further rise in its level under applied stress can be correlated with greater tolerance ability of P. foveolarum.
The accumulation of compounds such as proline in cells in response to environmental stress is well documented (Galhano et al. 2010, 2011b; Sheeba et al. 2011). Proline provides less than 5% of the total pool of free amino acids under stress free condition, whereas the concentration increases up to 80% of the amino acid pool during stress (Matysik et al. 2002). The function of proline in stressed cyanobacteria is often explained by its property as a protectant, hence able to quench excess ROS (Galhano et al. 2010; Sheeba et al. 2011; Galhano et al. 2011b). Therefore, under butachlor and UV-B stress, increased accumulation of proline in both cyanobacteria compared to control, with maximum increase in P. foveolarum, suggesting its protective role during stress.
Conclusion
Herbicides and UV-B are notorious for causing oxidative stress, which is also evident from our study which shows the inhibitory effect of butachlor and UV-B on both the cyanobacteria and effect of one stress is aggravated by other stress and vice versa. Damaging effects on pigments and photosynthetic activity caused significant reduction in the biomass of the tested cyanobacteria. Enhanced lipid peroxidation and electrolytes leakage was due to increased superoxide radical and hydrogen peroxide and one of the reasons for butachlor and UV-B-induced damage to photosynthetic process which leads reduced biomass accumulation in both the cyanobacteria. Phormidium foveolarum exhibited comparatively higher resistance against butachlor and UV-B than Nostoc muscorum probably due to its strong antioxidant defence system. Thus our study establishes that P. foveolarum may be preferred in paddy field under sustainable agriculture programs. However, further research is required at molecular level to gain deeper insight in understanding the interaction of the twin stresses–butachlor and UV-B.
References
Aktar MW, Sengupta D, Purkait S, Chowdhury A (2008) Vertical migration of some herbicides through undisturbed and homogenized soil columns. Interdiscip Toxicol 1:231–235
Alla MMN, Badawi AHM, Hassan NM, El-Bastawisy ZM, Badran EG (2007) Induction of glutathione and glutathione-associated enzymes in butachlor-tolerant plant species. Am J Plant Physiol 2:195–205
Anand N, Hopper RSSK (1987) Blue-green algae from rice fields in Kerala State, India. Hydrobiologia 144:223–232
Anderson TW (1958) An introduction to multivariate analysis. Wiley, New York
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Beestman GB, Deming JM (1974) Dissipation of acetanilide herbicides from soils. Agron J 66:308–311
Bernroitner M, Zamocky M, Furtmüller PG, Peschek GA, Obinger C (2009) Occurrence, phylogeny, structure, and function of catalases and peroxidases in cyanobacteria. J Exp Bot 60:423–440
Blumwald E, Tel-Or E (1982) Structural aspects of the adaptation of the Nostoc muscorum to salt. Arch Microbiol 132:163–167
Böger P, Matthes B, Schmalfuß J (2000) Towards the primary target of chloroacetamides: new findings pave the way. Pest Manag Sci 56:497–508
Carletti P, Masi A, Wonisch A, Grill D, Tausz M, Ferretti M (2003) Changes in antioxidants and pigment pool dimensions in UV-B irradiated maize seedlings. Environ Exp Bot 50:149–157
Chen YL, Chen CC (1979) Degradation and dissipation of herbicide butachlor. J Pestic Sci 4:431–438
Chen Z, Jauneau P, Qiu B (2007) Effects of three pesticides on the growth, photosynthesis and photoinhibition of the edible cyanobacterium Ge-Xian-Mi (Nostoc). Aquat Toxicol 81:256–265
Chen WC, Yen JH, Chang CS, Wang YS (2009) Effects of herbicide butachlor on soil microorganisms and on nitrogen-fixing abilities in paddy soil. Ecotoxicol Environ Saf 72:120–127
Chen L, Xie M, Bi Y, Wang G, Deng S, Liu Y (2012) The combined effects of UV-B radiation and herbicides on photosynthesis, antioxidant enzymes and DNA damage in two bloom-forming cyanobacteria. Ecotoxicol Environ Saf 80:224–230
Das AC, Debnath A (2006) Effect of systemic herbicides on N2-fixing and phosphate solubilizing microorganisms in relation to availability of nitrogen and phosphorus in paddy soils of West Bengal. Chemosphere 65:1082–1086
Debnath A, Das AC, Mukherjee D (2002) Persistence and effect of butachlor and basalin on the activities of phosphate solubilizing microorganisms in wetland rice soil. Bull Environ Contam Toxicol 68:766–770
Desikachary TV (1959) Cyanophyta. Indian Council of Agricultural Research, New Delhi
Dowidar SMA, Osman MEH, El-Naggar AH, Khalefa AE (2010) Effect of butachlor and thiobencarb herbicides on protein content and profile and some enzyme activities of Nostoc muscorum. J Gen Eng Biotechnol 8:89–95
Egashira T, Takahama U, Nakamura K (1989) A reduced activity of catalase as a basis for light dependent methionine sensitivity of a Chlamydomonas reinhardtii mutant. Plant Cell Physiol 30:1171–1175
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxyl ammonium chloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
Estevez MS, Malanga G, Puntarulo S (2001) UV-B effects on Antarctic Chlorella sp cells. J Photochem Photobiol B Biol 62:19–25
Fernandes TA, Iyer V, Apte SK (1993) Differential responses of nitrogen-fixing cyanobacteria to salinity and osmotic stresses. Appl Environ Microbiol 59:899–904
Flint SD, Caldwell MM (2003) A biological spectral weighting function for ozone depletion research with higher plants. Physiol Plant 117:137–144
Gahagen HE, Halm RE, Abeles FB (1968) Effect of ethylene on peroxidase activity. Physiol Plant 21:1270–1279
Galhano V, Peixoto F, Gomes-Laranjo J (2010) Bentazon triggers the promotion of oxidative damage in the Portuguese rice field cyanobacterium Anabaena cylindrica: response of the antioxidant system. Environ Toxicol 25:517–526
Galhano V, Gomes-Laranjo J, Peixoto F (2011a) Exposure of the cyanobacterium Nostoc muscorum from Portuguese rice fields to molinate (Ordram®): effects on the antioxidant system and fatty acid profile. Aquat Toxicol 101:367–376
Galhano V, Santos H, Oliveira MM, Gomes-Laranjo J, Peixotoc F (2011b) Changes in fatty acid profile and antioxidant systems in a Nostoc muscorum strain exposed to the herbicide bentazon. Process Biochem 46:2152–2162
Gantt E, Conti SF (1966) Granules associated with the chloroplast lamellae of Porphyridium cruentum. J Cell Biol 29:423–434
Garcia-Pichel F, Wingard CE, Castenholz RW (1993) Evidence regarding the UV sunscreen role of a mycosporine-like compound in the cyanobacterium Gloeocapsa sp. Appl Environ Microbiol 59:170–176
Giannopolitis CN, Ries SK (1977) Superoxide dismutase occurrence in higher plants. Plant Physiol 59:309–314
Gong M, Li YJ, Chen SZ (1998) Abscisic acid-induced thermo-tolerance in maize seedlings is mediated by calcium and associated with antioxidant system. J Plant Physiol 153:488–496
Goodwin TW (1954) Carotenoids. In: Paech K, Tracey MV (eds) Handbook of plant analysis, vol 3. Springer, Berlin, pp 272–311
Gotz T, Böger P (2004) The very-long-chain fatty acid synthase is inhibited by chloroacetamides. Z Naturforsch 59:549–553
He Y, Häder DP (2002) Reactive oxygen species and UV-B effects on cyanobacteria. Photochem Photobiol Sci 1:729–736
He H, Li Y, Chen T, Huang X, Guo Q, Li S, Yu T, Li H (2013) Butachlor induces some physiological and biochemical changes in a rice field biofertilizer cyanobacterium. Pestic Biochem Physiol 105:224–230
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Jiang H, Qiu B (2011) Inhibition of photosynthesis by UV-B exposure and its repair in the bloom-forming cyanobacterium Microcystis aeruginosa. J Appl Phycol 23:691–696
Kamiya N, Shen JR (2003) Crystal structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus at 3.7-Å resolution. Proc Natl Acad Sci USA 100:98–103
Kumari N, Narayan OP, Rai LC (2009) Understanding butachlor toxicity in Aulosira fertilissima using physiological, biochemical and proteomic approaches. Chemosphere 77:1501–1507
Kumari N, Narayan OP, Rai LC (2012) Cyanobacterial diversity shifts induced by butachlor in selected Indian rice fields in Eastern Uttar Pradesh and Western Bihar analyzed with PCR and DGGE. J Microbiol Biotechnol 22:1–12
Lemoine Y, Schoefs B (2010) Secondary ketocarotenoid astaxanthin biosynthesis in algae: a multifunctional response to stress. Photosynth Res 106:155–177
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Ma J, Wang S, Wang P, Ma L, Chen X, Xu R (2006) Toxicity assessment of 40 herbicides to the green alga Raphidocelis subcapitata. Ecotoxicol Environ Saf 63:456–462
Ma J, Tong S, Wang P, Chen J (2010) Toxicity of seven herbicides to the three cyanobacteria Anabaena flos-aquae, Microcystis flos-aquae and Mirocystis aeruginosa. Int J Environ Res 4:347–352
Martin H, Worthing CR (1974) Pesticide manual, 4th edn. British Crop Protection Council, Farnham
Matysik J, Alia BB, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82:525–532
Müller-Moulé P, Havaux M, Niyogi KK (2003) Zeaxanthin deficiency enhances the high light sensitivity of an ascorbate-deficient mutant of Arabidopsis. Plant Physiol 133:748–760
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Ohki K, Zehr PJ, Fujita Y (1992) Regulation of nitrogenase activity in relation to the light dark regime in the filamentous non-heterocystous cyanobacterium Trichodesmium sp. NIBB 1067. J Gen Appl Microbiol 138:2679–2685
Olsson LC, Fraysse L, Bornman JF (2000) Influence of high light and UV-B radiation on photosynthesis and D1 turnover in atrazine-tolerant and-sensitive cultivars of Brassica napus. J Exp Bot 51:265–274
Oser BL (1979) Hawks physiological chemistry. McGraw Hill, New York, pp 702–705
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents; verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Prasad SM, Zeeshan M (2004) Effect of UV-B and monocrotophos, singly and in combination, on photosynthetic activivity and growth of non-heterocystous cyanobacterium Plectonema boryanum. Environ Exp Bot 52:175–184
Prasad SM, Zeeshan M (2005) UV-B radiation and cadmium induced changes in growth, photosynthesis, and antioxidant enzymes of cyanobacterium Plectonema boryanum. Biol Plant 49:229–236
Rastogi RP, Singh SP, Hader DP, Sinha RP (2011) Ultraviolet-B-induced DNA damage and photorepair in the cyanobacterium Anabaena variabilis PCC 7937. Environ Exp Bot 74:280–288
Renger G, Volker M, Eckert HJ, Frome R, Hohm-Veit S, Graber P (1989) On the mechanism of photosystem II deterioration by UV-B irradiation. Photochem Photobiol 49:97–105
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Sagisaka S (1976) The occurrence of peroxide in a perennial plant Populas gelrica. Plant Physiol 57:308–309
Sheeba SV, Srivastava PK, Prasad SM (2011) Differential physiological and biochemical responses of two cyanobacteria Nostoc muscorum and Phormidium foveolarum against oxyfluorfen and UV-B radiation. Ecotoxicol Environ Saf 74:1981–1993
Singh S, Datta P (2006) Screening and selection of most potent diazotrophic cyanobacterial isolate exhibiting natural tolerance to rice field herbicides for exploitation as biofertilizer. J Basic Microbiol 46(3):191–225
Singh S, Datta P, Tirkey A (2011) Response of multiple herbicide resistant strain of diazotrophic cyanobacterium Anabaena variabilis exposed to atrazine and DCMU. Ind J Exp Biol 49:298–303
Singh DP, Khattar JS, Kaur K, Sandhu BS, Singh Y (2012a) Toxicological impact of anilofos on some physiological processes of a rice field cyanobacterium Anabaena torulosa. Toxicol Environ Chem 94:1304–1318
Singh S, Datta P, Tirkey A (2012b) Isolation and characterization of a multiple herbicide resistant strain [Av(MHR)Ar, Al, B, D] of diazotrophic cyanobacterium Anabaena variabilis. Ind J Biotechnol 11:77–85
Singh VP, Srivastava PK, Prasad SM (2012c) Impact of low and high fluence rates of UV-B radiation on growth and oxidative stress in Phormidium foveolarum and Nostoc muscorum under copper toxicity: differential display of antioxidants system. Acta Physiol Plant 34:2225–2239
Singh VP, Srivastava PK, Prasad SM (2012d) Differential effects of UV-B radiation fluence rates on growth, photosynthesis and phosphate metabolism in two cyanobacteria under copper toxicity. Toxicol Environ Chem 94:1511–1535
Singh VP, Srivastava PK, Prasad SM (2012e) Differential effect of UV-B radiation on growth, oxidative stress and ascorbate-glutathione cycle in two cyanobacteria under copper toxicity. Plant Physiol Biochem 61:61–70
Singh DP, Khattar JIS, Kaur M, Kaur G, Gupta M, Singh Y (2013) Anilofos tolerance and its mineralization by the cyanobacterium Synechocystis sp. strain PUPCCC 64. PLoS ONE 8(1):e53445. https://doi.org/10.1371/journal.pone.0053445
Sinha RP, Singh N, Kumar A, Kumar AD, Häder H, Häder DP (1996) Effects of UV irradiation on certain physiological and biochemical process in cyanobacteria. Photochem Photobiol 32:311–314
Sinha RP, Klisch M, Helbling EW, Häder D (2001) Induction of mycosporine-like amino acids (MAAs) in cyanobacteria by solar ultraviolet-B radiation. J Photochem Photobiol, B 60:129–135
Spiller H (1980) Photophosphorylation capacity of stable spheroplasts preparations of Anabaena. Plant Physiol 66:446–450
Srivastava PK, Singh VP, Prasad SM (2012) Compatibility of ascorbate-glutathione cycle enzymes in cyanobacteria against low and high UV-B exposures, simultaneously exposed to low and high doses of chlorpyrifos. Ecotoxicol Environ Saf 83:79–88
Thakkar M, Randhawa V, Wei L (2013) Comparative responses of two species of marine phytoplankton to metolachlor exposure. Aquat Toxicol 126:198–206
Tiwari DN, Kumar A, Mishra AK (1991) Use of cyanobacterial diazotrophic technology in rice agriculture. Appl Biochem Biotechnol 28–29:387–396
Ueji M, Inao K (2001) Rice paddy field herbicides and their effects on the environment and ecosystems. Weed Biol Manag 1:71–79
Vaishampayan A (1985) Mutagenic activity of alachlor, butachlor and carbaryl to a N2-fixing cyanobacterium Nostoc muscorum. J Agric Sci 104:571–576
Vaishampayan A, Sinha RP, Hader DP, Dey T, Gupta AK, Bhan U, Rao AL (2001) Cyanobacterial biofertilizers in rice agriculture. Bot Rev 67:453–516
Wang YR, Liu CW, Niu CY, Cai LB, Li ZX, Zhu CS, He SJ, Wang QS (1996) Phototransformation of butachlor in aquatic system and its fate in rice fields. Acta Sci Circ 16:475–481
Wang G, Deng S, Li C, Liu Y, Chen L, Hu C (2012) Damage to DNA caused by UV-B radiation in the desert cyanobacterium Scytonema javanicum and the effects of exogenous chemicals on the process. Chemosphere 88:413–417
Wang J, Wu G, Chen L, Zhang W (2013a) Cross-species transcriptional network analysis reveals conservation and variation in response to metal stress in cyanobacteria. BMC Genom 14:112. https://doi.org/10.1186/1471-2164-14-112
Wang S, Li H, Lin C (2013b) Physiological, biochemical and growth responses of Italian rye grass to butachlor exposure. Pestic Biochem Physiol 106:21–27
Wilson A, Ajlani G, Verbavatz JM, Vassc I, Kerfeldd CA, Kirilovsky D (2006) A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell 18:992–1007
Xie Z, Wang Y, Liu Y, Liu Y (2009) Ultraviolet-B exposure induces photo-oxidative damage and subsequent repair strategies in a desert cyanobacterium Microcoleus vaginatus Gom. Eur J Soil Biol 45:377–382
Yu YL, Chen YX, Luo YM, Pan XD, He YF, Wong MH (2003) Rapid degradation of butachlor in wheat rhizosphere soil. Chemosphere 50:771–774
Zhao S, Pan WB, Ma C (2012) Stimulation and inhibition effects of algae-lytic products from Bacillus cereus strain L7 on Anabaena flos-aquae. J Appl Phycol 24:1015–1021
Zheng HH, Ye CM (2001) Identification of UV photoproducts and hydrolysis products of butachlor by mass spectrometry. Environ Sci Technol 35:2889–2895
Zhou Z, Yang Z, Li Y (2012) Effect of CO2 increase in atmosphere and UV-B intensification on Nostoc commune photosynthetic pigment and UV-B masking materials. Wuhan Univ J Nat Sci 17:255–260
Zhu Y, Graham JE, Ludwig M, Xiong W, Alvey RM, Shen G, Bryant DA (2010) Roles of xanthophyll carotenoids in protection against photoinhibition and oxidative stress in the cyanobacterium Synechococcus sp. strain PCC 7002. Arch Biochem Biophys 504:86–99
Acknowledgements
The authors are thankful to The Head, Department of Botany, University of Allahabad, Allahabad for providing necessary laboratory facilities. The authors are also thankful to University Grants Commission, New Delhi for providing financial support to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sheeba, Ruhil, K. & Prasad, S.M. Nostoc muscorum and Phormidium foveolarum differentially respond to butachlor and UV-B stress. Physiol Mol Biol Plants 26, 841–856 (2020). https://doi.org/10.1007/s12298-019-00754-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-019-00754-5