Abstract
During the process of domestic sewage treatment in the Subsurface Wastewater Infiltration System (SWIS), changes in the microbial populations (nitrifying and denitrifying bacteria) and enzyme activities (urease, nitrate reductase and nitrite reductase) involved in the nitrogen removal process were evaluated over a 2-year period. The results showed nitrifying bacteria number declined with depths increasing, while denitrifying bacteria increased, both of which increased nearer the inlet. The depth for nitrate reductase activity from high to low in sequence was 0.3, 0.5, 0.7, 0.9 and 1.1 m. For nitrite reductase, the sequence was 0.5, 0.3, 0.7, 0.9 and 1.1 m. Urease and nitrite reductase activities were in positive correlation with the total nitrogen removal efficiency, with correlation coefficients 0.8662 and 0.9140, respectively and could be alternative to monitor the nitrogen biodegradation process in SWIS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The idea of using Subsurface Wastewater Infiltration System (SWIS) for the treatment and improving of domestic wastewater emerged in the second half of the last century (Smiles 2006; Xu and Que-Hee 2007; Arienzo et al. 2009; Tunçsiper et al. 2009). Because of large demand of land area but no requirement of perfect sewage systems, SWIS has recently received considerable attention as low cost and efficient means of cleaning up domestic wastewater at secondary and tertiary levels (Arienzo et al. 2009). Up to now, over ninety million SWISs are in operation and benefit the people in North America, Europe and Asia (Belinda et al. 2007; Kim and Sansalone 2008; Li et al. 2011). In China, hundreds of SWIS projects are constructed in Guizhou, Yunnan, Shenzhen and Shenyang, etc. In the SWIS treatment, wastewater is firstly treated by conventional physico-chemical and/or biological treatment and then allowed to infiltrate through aerated unsaturated zone wherein it gets purified through processes such as filtration, adsorption, chemical reaction and biodegradation.
Despite relatively wide use of this environmentally friendly technology, relatively little is known about the changes of microbial populations and enzyme activities involved in nitrogen biotransformation and removal process in this system (Luanmanee et al. 2002; Parveen et al. 2006). The aim of the current study was to examine the microbial populations and enzyme activities involved in the nitrogen removal process in a SWIS, which was designed to improve the quality of wastewater at tertiary level. Also, the nitrogen removal efficiency of this SWIS was investigated during a 2-year experimental period.
Materials and Methods
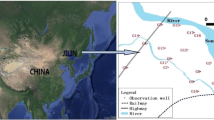
The SWIS system in this study is located in Shenyang, northeastern China, which covers 300 m2 (L × W = 20 m × 15 m) with effective depth (ED) of 1.5 m (Fig. 1). The pre-treated and settled wastewater flows under gravity action into the distributing pipes of the SWIS, which are 0.08 m in diameter, 0.5 m underneath, then the treated water is collected in the collecting pipe 1.5 m underneath with 0.1 m in diameter. The spacing interval between two distributing pipes is 1 m. In order to monitor the microbial populations and enzyme activities, five sampling points (labeled as A, B, C, D and E) are arranged vertical to the distributing pipes, with intervals of 0.3 m, as shown in Fig. 2.
The influent in this study was a combined wastewater from toilets, bathrooms, etc. The ranges of major water quality indices were pH 7.1–7.4, chemical oxygen demand (COD) 280–353 mg/L, biological oxygen demand (BOD5) 160–210 mg/L, suspended solid (SS) 150–200 mg/L, total nitrogen (TN) 30–45 mg/L, total phosphorus (TP) 3–4 mg/L, ammonia nitrogen (NH3–N) 20–30 mg/L, with an average ratio of 0.6 for BOD5/COD.
The matrix in the SWIS was composed of soil, coal slag and dewatered activated sludge mixed in volume ratio of 13:5:2. The soil used was meadow brown soil, sampled from the top 20 cm from Shenyang Ecological Station, with total organics 22.8 g/kg, TN 1.4 g/kg and TP 0.85 g/kg. The activated sludge was obtained from the aeration tanks in Shenyang Northern Municipal Sewage Treatment Plant, air dried after being centrifuged for 15 min at 1,500 rpm. Other materials (gravel and coal slag) were purchased from a local market (in diameter: gravel 10–25 mm and coal slag 4–8 mm). The infiltration rate, porosity and surface area of the matrix were 0.37 m3/m2·d, 59% and 5.21 m2/g, respectively. In the SWIS, the matrix was filled from the ground surface down to 1.4 m, followed by gravel.
The influent and effluent samples were collected once a week, stored at 4°C and analyzed within 24 h. The water samples were analyzed according to the Chinese Environmental Protection Agency standard methods (Chinese EPA 2002). Potassium dichromate method was used for COD determination; colorimetric method was used for NH3–N and NO3–N measurements. TN of soil was analyzed by Na2CO3 fusion method and TP was analyzed by Kjeldahl method.
The nitrifying and denitrifying bacteria in the soil samples were counted using the most probable number (MPN) calculation (Molle et al. 2006). The medium for the nitrifying bacteria contained per litre distilled water: 13.5 g Na2HPO4, 0.7 g KH2PO4, 0.1 g MgSO4·7H2O, 0.5 g NaHCO3, 2.5 g (NH4)2SO4, 14.4 mg FeCl3·6H2O and 18.4 mg CaCl2·7H2O, pH 8.0. The medium for the denitrifying bacteria contained per litre distilled water: 1.0 g KNO3, 0.1 g Na2HPO4, 2.0 g Na2S2O7, 0.1 g NaHCO3 and 0.1 g MgCl2, pH 8.0. The soil samples were taken from 0.3, 0.5, 0.7, 0.9 and 1.1 m depths, respectively. Aliquot (1 mL) of serial tenfold sterile distilled water dilutions of the soil samples were transferred to 96-cell microtiter plates containing each type of medium, then incubated at 28°C 14 d (for the nitrifying bacteria) and 15 d (for the denitrifying bacteria), respectively. Meanwhile, 10 g of soil samples were oven-dried at 105°C for 12 h to produce a constant weight. The amounts of the nitrifying and denitrifying bacteria were analyzed twice per month during the study. Urease, nitrate reductase (NAR) and nitrite reductase (NIR) activities were analyzed according to the method of Guan (1986) twice per month.
During the whole experimental period, intermittent operation mode was adopted as a passive method for oxygen transfer restoring in the SWIS (Hati et al. 2006; Kamitani and Kaneko 2007). Each cycle of the intermittent operation included a continuous flow period of 24 h (between 9:00 AM and 9:00 AM the next day) and a drying period of 24 h. Statistical analyses were carried out with MicroCal Origin 7.0 (OriginLab) and SPSS 17.0. The SWIS system was operated under hydraulic loading rate of 0.125 m3/(m2·d).
Results and Discussion
Purification processes in the SWIS were gradually established over 4 weeks. After which, soil samples were analyzed for the number of nitrifying and denitrifying bacteria at different depths and positions. As shown in Table 1, the amount of nitrifying bacteria declined with depths increasing. At the same time, the nearer to the distributing pipe, the higher amount. Meanwhile, the number of denitrifying bacteria increased with the depths increasing. Also, the more quantity of denitrifying bacteria achieved nearer the distributing area.
In the SWIS system, nitrification coupled with denitrification is generally thought to be the major method for nitrogen removal. For the fate of nitrification is significantly lower than that of the denitrification, so nitrification is a limiting step for nitrogen removal process. It was reported that for NH3–N with a concentration of 1 mg/L, nitrification will not occur successfully unless the DO concentration reaches 4.6 mg/L (Molle et al. 2006). Herein, benefiting from the oxygen transferring availability of the surface soil, the zone between 0.3 and 0.7 m depth was the most effective nitrifying reaction region in the SWIS. The denitrifying bacteria were more active between depths of 0.7–1.5 m. Meanwhile, the results implied that flow path of the wastewater in the SWIS was: firstly, the wastewater flew out of the distributing pipe, then went up to the 0.3 m underneath under capillary force, and then diffused to the intervals of the distributing pipes, finally flew under gravity to the collecting pipe. This flow path in the SWIS system was similar to the studies reported before (Head and Oleszkiewicz 2004; Hsu et al. 2006; Babatunde et al. 2008).
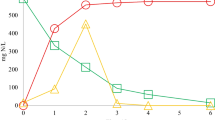
From April 2007 to March 2009, the activities of urease, NAR and NIR were investigated, as shown in Table 2.
From Table 2, it can be concluded that urease activity was in positive correlation with the temperature. The correlation equation was U = 0.3668T + 8.5679 (correlation coefficient 0.933), where U and T represented the urease activity and temperature, respectively. The reports before informed that once the temperature increased by 10°C, the urease activity will be 1–2 times higher (Head and Oleszkiewicz 2004; Kim and Sansalone 2008). The higher urease activity was achieved nearer the inlet, 0.5 m underneath. NAR activity was influenced by the soil depth. The depth for NAR activity from high to low in sequence was 0.3, 0.5, 0.7, 0.9 and 1.1 m. The sequence for NIR activity from high to low in depth was 0.5, 0.3, 0.7, 0.9 and 1.1 m.
At the same time, NH3–N and TN influent and effluent concentrations were analyzed and the removal efficiencies were calculated (Fig. 3). After 2 years operation, the average removal efficiencies were 88.7 ± 1.2% for NH3–N and 76.2 ± 1.5% for TN. Compared with the activated sludge method and membrane filtration technology (89.2 ± 2.1% and 78.5 ± 3.4% mean removal efficiencies for NH3–N and TN) (Luanmanee et al. 2002; Walid and Al-Qodah 2006), the SWIS had comparable nitrogen removal efficiency. In the inflow, NH3–N was the main form of TN, accounting for 83.3 ± 1.1%. NO3–N concentration in the influent was 0.2–0.3 mg/L, less than 1% of TN. In the outflow, NO3–N concentration increased to 2.0–2.5 mg/L, 29.0–30.5% accounting for TN. On the contrary, NH3–N concentration declined to 2.3–4.4 mg/L, accounting for 63.2–65.6% of TN. The NH3–N and TN removal efficiencies achieved and TN composition analytical results suggested that the nitrification–denitrification process worked well in the SWIS. Therefore, SWIS was proved to be an effective technique for sewage treatment in areas without adequate domestic treatment facilities.
SPSS analysis of the Fig. 3 results implied that the urease and NIR activities were in positive correlation with the TN removal. The correlation equations were U = 77.08R − 41.407for urease and N = 2.2R − 1.4467 for NIR, with correlation coefficients 0.8662 and 0.9140, respectively. In the equations, U, N and R represented the activities for urease and NIR, and TN removal efficiency. Therefore, the activities of urease and NIR could be the biological indexes during the nitrogen removal process in the SWIS.
References
Agency ChineseEnvironmentalProtection (2002) Methods for water and wastewater analysis. Environmental Science Publishing House of China, Beijing (in Chinese)
Arienzo M, Christen EW, Quayle W, Kumar A (2009) A review of the fate of potassium in the soil-plant system after land application of wastewaters. J Hazard Mater 164:415–422
Babatunde AO, Zhao YQ, O’Neill M, O’Sullivan B (2008) Constructed wetlands for environmental pollution control: a review of developments, research and practice in Ireland. Environ Int 34:116–126
Belinda EH, Tim DF, Ana D (2007) Treatment performance of gravel filter medium: implication for design and application of stormwater infiltration systems. Water Res 41:2513–2524
Guan SY (1986) Soil enzyme and the researching methods. Agriculture Publishing House of China, Beijing (in Chinese)
Hati KM, Mandal KG, Misra AK, Ghosh PK, Bandyopadhyay KK (2006) Effect of inorganic fertilizer and farmyard manure on soil physical properties, root distribution, and water-use efficiency of soybean in Vertisols of central India. Bioresour Technol 97:2182–2188
Head MA, Oleszkiewicz JA (2004) Bioaugmentation for nitrification at cold temperatures. Water Res 38:523–530
Hsu MJ, Selvaraj K, Agoramoorthy G (2006) Taiwan’s industrial heavy metal pollution threatens terrestrial biota. Environ Pollut 143:327–334
Kamitani T, Kaneko N (2007) Species-specific heavy metal accumulation patterns of earthworms on a floodplain in Japan. Ecotoxicol Environ Saf 66:82–91
Kim JY, Sansalone JJ (2008) Event-based size distributions of particulate matter transported during urban rainfall-runoff events. Water Res 42:2756–2768
Li YH, Li HB, Sun TH, Wang X (2011) Study on nitrogen removal enhanced by shunt distributing wastewater in a constructed subsurface infiltration system under intermittent operation mode. J Hazard Mater 189:336–341
Luanmanee S, Boonsook P, Attanandana T, Saitthiti B, Panichajakul C, Wakatsuki T (2002) Effect of intermittent aeration regulation of a multi-soil-layering system on domestic wastewater treatment in Thailand. Ecol Eng 24:415–428
Molle P, Liénard A, Grasmick A, Iwema A (2006) Effect of reeds and feeding operations on hydraulic behavior of vertical flow constructed wetlands under hydraulic overloads. Water Res 40:606–612
Parveen S, Nazif W, Ahmad MF, Khan A, Khattak IA (2006) Nutritional status of different orchards irrigated with wastewater in district Peshawar. J Agric Biol Sci 1:42–50
Smiles DE (2006) Sodium and potassium in soils of the Murray-Darling basin: a note. Aust J Soil Res 44:727–730
Tunçsiper B, Ayaz S, Akça L, Gunes K (2009) Performance of a pilot-scale, three-stage constructed wetland system for domestic wastewater treatment. Environ Technol 30:1187–1194
Walid KL, Al-Qodah Z (2006) Combined advanced oxidation and biological treatment processes for the removal of pesticides from aqueous solutions. J Hazard Mater B137:489–497
Xu W, Que-Hee SS (2007) Permeation of a straight oil metalworking fluid through disposable nitrile, chloroprene, vinyl, and latex gloves. J Hazard Mater B147:923–929
Acknowledgments
The authors would like to thank the National Key Technology R&D Program (no. 2011BAJ06B02), National Natural Science Foundation of China (no. 50908151 and no. 51008198), the Ministry of Science and Technology of the People’s Republic of China “Major projects on control and rectification of water body pollution, Research on Key Technology and its Demonstration of Liaohe River Estuary Land-source Pollution Control and Water Quality Improve” (no. 2008ZX07208-008-04) and the Program for Excellent Talents of Liaoning Province (LR201028) for the financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Li, H., Wang, X. et al. Changes in Microbial Populations and Enzyme Activities During Nitrogen Biodegradation of Domestic Sewage Treatment in the Subsurface Wastewater Infiltration System (SWIS). Bull Environ Contam Toxicol 87, 431–435 (2011). https://doi.org/10.1007/s00128-011-0359-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-011-0359-z