Abstract
The present study was undertaken to determine the toxic effect of a lethal concentration of six different commercially used textile dyes on the 46th stage of Xenopus laevis tadpoles. The tadpoles were exposed to Astrazon Red FBL, Astrazon Blue FGRL, Remazol Red RR, Remazol Turquoise Blue G-A, Cibacron Red FN-3G, and Cibacron Blue FN-R for 168 h in static test conditions, and thus, 168-h median lethal concentrations (LC50s) of each dye were determined to be 0.35, 0.13, 112, 7, 359, and 15.8 mg/L, respectively. Also, to evaluate the sublethal effects of each dye, tadpoles were exposed to different concentrations of dyes (with respect to 168-h LC50s) for 24 h. The alteration of selected enzyme activities was tested. For this aim, glutathione S-transferase (GST), carboxylesterase, and lactate dehydrogenase (LDH) were assayed. After dye exposure, the GST induction or inhibition and LDH induction indicated some possible mechanisms of oxidative stress and deterioration in aerobic respiration processes induced by the tested dyes. Findings of the study suggest that selected biomarker enzymes are useful in understanding the toxic mechanisms of these dyes in X. laevis tadpoles as early warning indicators. Therefore, these selected biomarkers may evaluate the effect of environmental factors, such as textile dye effluents and other industrial pollutants, on amphibians in biomonitoring studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The textile industry is one of the most common and essential sectors in the world (Verma 2008) and also plays an important economical role in Turkey. The industry in Turkey has grown significantly over the last few decades, and it was reported that a total of 143 × 103 kg of textile dye stuffs has been used in Turkey in 1997 (Wang et al. 2002). The world consumption for dyes and organic pigments was about $10.6 billion in 2008 (Doshi 2006).
The textile industries consume large volumes of water and chemicals for wet processing of textiles, and approximately 10–15 % of the dyes are released into the environment during the dyeing process (Marungrueng and Pavasant 2006; Verma 2011). The presence of even very low concentrations of dyes in effluent is highly visible and undesirable (Robinson et al. 2002). In addition to color, a high concentration of organic compounds, high pH, chemical oxygen demand (COD) values, and substantial levels of heavy metals are characterized in textile dyes and wastewaters (Cing et al. 2003; Yesilada et al. 2003). Apart from their negative esthetic effects on the receiving aquatic ecosystem, recent studies showed that some textile dyes and their wastewater were significantly toxic to aquatic organisms (Alves de Lima et al. 2007; Gholami-Borujeni et al. 2011; Rajaguru et al. 2001; Sharma et al. 2009; Sumathi et al. 2001; Sun et al. 2007; Suryavathi et al. 2005; Ulson de Souza et al. 2007).
The six widely used dyes in the textile industry that belong to different chemical groups have been tested in this study. According to chemical structure, five of these dyes belong to the azo group. Azo dyes make up approximately 70 % of all dyestuffs used worldwide by weight, making them the largest group of synthetic colorants and the most common synthetic dyes released into the environment (Saratale et al. 2011). The textile azo dyes, with synthetic intermediates as contaminants and their products, have undoubtedly attracted attention with regard to their potential to form toxic aromatic products with carcinogenic and mutagenic properties (Osugi et al. 2006). The carcinogenicity of some products is caused by reductive splitting from azo dyes (e.g., certain aromatic amines), which split from dyestuffs or dyed textiles (Pielesz et al. 2002).
The experiments on the toxicity of different industrial effluents, such as textile dye wastewaters, revealed that a conventional approach of using chemical analysis alone cannot provide sufficient information on the potentially harmful effects of chemicals on the aquatic environment (Sponza 2006). Estimation of pollution results and prediction of toxic effects on aquatic organisms, especially in anurans, is important for environmental monitoring. On the other hand, because anurans are in decline in the recent years due to environmental pollution around the world (Blaustein and Dobson 2006), it is important to understand the toxicity of dyestuff and/or its wastewaters in amphibians, especially in the early stage of their life, which may be exposed to toxicants via different routes (Gutleb et al. 1999). Xenopus laevis, the South African clawed frog, is one of the most useful amphibian vertebrates, with well-characterized developmental stages and toxicology tests to evaluate the poisoning capacity of xenobiotics, often performed in live embryos of X. laevis (Peng et al. 2004).
Assessment of the biomarkers as an early warning of adverse changes and damage has been shown to be a suitable tool in amphibians (Buryskova et al. 2006; Güngördü et al. 2010). Determination of enzyme activities that have different functions is important for assessing different ways of toxicity. Glutathione S-transferases (GSTs) are a family of enzymes with important roles in the biotransformation of both xenobiotics and endogenous substances, and induction of GST has been used as a biomarker of exposure to different xenobiotics (Osten et al. 2005). Carboxylesterases (CaEs) are ubiquitous proteins identified in species ranging from bacteria to man; hydrolytic metabolism of xenobiotics, such as pesticides, phathalate ester plasticizers, and oil-spill dispersants, was shown in aquatic animals (Al-Ghais et al. 2000). Lactate dehydrogenase (LDH) catalyzes the interconversion of pyruvate and lactate with concomitant interconversion of NADH and NAD+ in a glycolytic way so that it may diagnose cell, tissue, and organ damage due to toxicity (Diamantino et al. 2001).
This study was designed to validate our previous work on X. laevis embryos, but in this case, the 46th developmental stage of tadpoles of X. laevis were used to determine adverse effects of six commonly used textile dyes on these organisms. Metabolic responses to textile dye toxicity were determined by using three enzyme activities. For this aim, from the variety of available biomarkers, the activity of detoxification enzymes (GST; CaE) and a metabolic enzyme (LDH) was assayed as biomarkers. Thus, whether or not these biomarkers could be used to determine the toxic effects of textile dyes on X. laevis tadpoles at short periods of exposure was assessed.
Material and methods
Chemicals and reagents
The textile dyes were provided from a textile dyeing industry. Astrazon Red FBL (AR-FBL), Astrazon Blue FGRL (AB-FGRL), Remazol Red RR (RR-RR), Remazol Turquoise Blue G-A (RB-GA), Cibacron Red FN-3G (CR-FN3G), and Cibacron Blue FN-R (CB-FNR) were selected as test materials (Table 1).
Reagents for enzymatic reactions, 1-chloro-2,4-dinitrobenzene (CDNB), p-nitrophenyl acetate (PNPA), reduced glutathione (GSH), bovine serum albumin (BSA), and LDH diagnostic kits were obtained from Sigma (MO, USA). Human chorionic gonadotropin (hCG; Pregnyl, 5,000 IU) was provided by Organon (Istanbul, Turkey).
Test organism and treatment
A colony of X. laevis was maintained in the Laboratory of Environmental Toxicology at Inonu University, Biology Department, Turkey. The breeding of X. laevis adults and the collection of embryos were performed as described in ASTM-E1439-98 (ASTM 2003). Two pairs of adults were used to produce embryos for the toxicity test enzymatic assays. Male and female adults were maintained in separate aquariums for 48 h before pairing. Males were injected with 200 IU hCG at 0 h and a second dose of 300 IU hCG at 36 h. Also, females were injected with a single dose of 600 IU hCG before being transferred into the breeding tanks. All injections were performed at midnight, and the deposition of eggs occurred in the early morning. The fertilized eggs from two females were selected under a dissecting microscope and were mixed together. Normal developing embryos were maintained in well-aerated Frog Embryo Teratogenesis Assay–Xenopus (FETAX) solution until the embryos reached the tadpole stage (stage 46) according to Nieuwkoop and Faber (1994), and these tadpoles were used for experiments. Control groups of tadpoles were maintained in standard FETAX solution (ASTM 2003). All dye dilutions were prepared daily in FETAX solution, and a static renewal test system was used. The tadpoles were exposed to different concentrations of dye solutions at 23 °C (±1 °C) in a 12:12-h light/dark photoperiod.
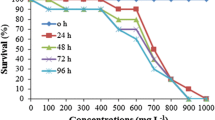
For the toxicity test, four groups with 15 tadpoles in each group (60 tadpoles in total) were randomly placed in covered polycarbonate dishes containing 50 ml of varying concentrations of dye solution. Test solutions were changed every 24 h during the 7-day (168 h) test period; any dead tadpoles were removed, and the incidence of tadpole deaths was recorded. At the end of the experiment, the median lethal concentrations (LC50) of the dyes were calculated for the 72- and 168-h exposure periods separately, as mentioned in the “Statistical analysis” section.
For the enzymatic assays, six groups with 30 tadpoles in each group (180 tadpoles in total) were randomly placed into covered polycarbonate dishes containing 50 ml of varying concentrations of the tested dye solutions. The tadpoles were exposed to the dye solutions for 24 h. Following exposure, live tadpoles were collected and were placed in microcentrifuge vials. Collected tadpoles in vials were chilled on ice and stored immediately at −80 °C until the enzymatic procedures.
Preparation of enzymatic samples
The X. laevis tadpoles were thawed on ice, weighed, and homogenized on ice in 0.1 M K-phosphate homogenization buffer. Four volumes of ice-cold homogenization buffer (0.15 M KCl, 1 mM EDTA, 1 mM DTT, pH7.4) were used per gram of tadpoles. The homogenates were centrifuged at 16,000 × g for 20 min at 4 °C, and the supernatants were transferred into clean microcentrifuge tubes. The cytosolic enzyme activities present in the supernatants was determined as soon as possible following the centrifugation procedure.
Determination of enzyme activities
Selected enzyme activities were determined on cytosolic samples spectrophotometrically at appropriate wavelengths using a microplate reader (VersaMax, Molecular Devices Corp., USA) at 25 °C and were expressed as specific activity. Samples were assayed in triplicate.
GST activity was measured according to Habig et al. (1974). The reaction mixture contained 210-μl 0.1 M K-phosphate buffer (pH 6.5), 1 mM GSH, 1 mM CDNB, and 10 μl of cytosolic sample. The change in absorbance was monitored at 344 nm, and the activity was calculated using the extinction coefficient for CDNB (ε = 9,600 M−1 cm−1).
CaE activity was assayed using a procedure described by Santhoshkumar and Shivanandappa (1999) that was modified for a microplate reader, with PNPA serving as the substrate. A reaction solution containing 5 μl of sample and 250 μl of 0.1 mM Trizma buffer (pH 7.4) was incubated for 3 min at 25 °C. The reaction was initiated by the addition of 5 μl of the substrate (final concentration, 0.5 mM) to the reaction solution. The liberated p-nitrophenol was measured at 405 nm for 2 min in the microplate reader. The enzyme activities were calculated from the extinction coefficient of p-nitrophenol (ε = 1,830 M−1 cm−1).
The LDH assay was conducted with a microplate reader at appropriate wavelengths using appropriate commercial test kits according to procedures described in the kit manuals by the manufacturer (Sigma, USA). Ten microliters of supernatant was dispensed into each well and were mixed with 200 μl of the reaction solution A. After 1 min of incubation at room temperature, 10 μl of reaction solution B was added to the reaction mixture. The change in absorbance was monitored at 340 nm for 2 min, and the activity was calculated according to the methods described in the manufacturers’ manuals.
Total protein concentrations of the supernatants were determined according to the method of Lowry et al. (1951), using BSA standard at 750 nm. The protein concentration was calculated from the calibration curve constructed from measurements of the following BSA standard solutions (0–300 μg BSA/ml). The calculated protein values were used to calculate specific activity values of each assayed enzyme.
Statistical analysis
The 72- and 168-h LC50 values were determined following the elaboration of the mortality by the probit analysis using version 1.5 of the EPA probit analysis program. Statistical analyses of biochemical markers were performed using statistical software (SPSS Inc., USA). Multiple variant analysis (ANOVA) was used to compare changes in enzyme activity. The significance level was set to p < 0.05 or p < 0.005. Mann–Whitney U test was performed if there was any significance detected within groups.
Results
Calculated LC50 values of tested dyes for 72- and 168-h exposure periods in stage 46 tadpoles are shown in Table 2. Due to seven-day toxicity tests, the 168 h LC50 values were determined as 0.35 mg/L for AR-FBL, 0.13 mg/L for AB-FGRL, 112 mg/L for RR-RR, 7 mg/L for RB-GA, 359 mg/L for CR-FN3G, and 15.8 mg/L for CB-FNR. Also, 72-h test results for LC50 were parallel to the 168-h tests—that AB-FGRL was found to be the most toxic dye on tadpoles, while CR-FN3G was moderately toxic.
On the other hand, biochemical assay of selected biomarkers showed that GST, CaE, and LDH activities were induced by AR-FBL exposure, and the enzyme activities were increased on tadpoles at a rate of 274, 147, and 137 %, respectively, compared to the control tadpoles after the highest exposed dose (7.14 mg/L) of dye (Table 3). However, relatively important changes in biochemical markers were recorded after exposure of X. laevis tadpoles to AR-FBL, but no significant or clear concentration-dependent effects were considered for this dye.
The GST activity was generally increased due to increased dye concentrations; there was a linear dose–response relationship for RR-RR exposure (r 2: 0.88). Highest dose of RR-RR (2,190 mg/L) significantly increased the GST activity at a rate of 208 % compared to control tadpoles. However, highest induction of activity of CaE and LDH was determined after being exposed to the 38-mg/L dye concentration (Table 3). Similar to RR-RR exposure, CaE and LDH activities showed similar changes after RB-GA exposure, but these changes did not represent a good correlation with the tested concentrations.
In contradiction to astrazon and remazol dyes, both tested cibacrons significantly inhibited GST activity on tadpoles. GST activity was determined as 201.4 nmol/min/mg protein in control group tadpoles, while the enzyme activities were determined as 141.5 and 132.3 nmol/min/mg protein in tadpoles that were exposed to the highest concentrations of CR-FN3G (1,297.2 mg/L) and CB-FNR (648.1 mg/L), respectively.
Discussion
Dyes pollute the aquatic environment through wastewaters released at various steps of the dyeing and printing of textile products (Sharma et al. 2009). Some of the dyes are very recalcitrant to biodegradation, causing esthetic, acute, and chronic toxicity problems in receiving waters (Kunz et al. 2002). The presence of these dyes in aquatic environments, even at very low concentrations, is highly visible and undesirable. Therefore, color is the first contaminant to be recognized, and environmental regulations in most of the countries have made it mandatory to decolorize the dye wastewater prior to discharge (Robinson et al. 2002; Marungrueng and Pavasant 2006). Thus, studies are primarily focused on chemical and biological color removal methodologies (Akyol et al. 2004; Cing et al. 2003; Guerra et al. 2008; Kunz et al. 2002; Marungrueng and Pavasant 2006; Ozmen 2008; Sedighi et al. 2009; Sun et al. 2009). However, color alone does not reflect the degree to which effluent is polluted; dyes with similar spectra may behave in a biochemically different manner (O’Neill et al. 1999). Not only the chemical composition of textile dye affect environmental pollution, but also high total dissolved solids, biological oxygen demand, COD, pH, color, and hardness may affect the pollution and toxicity in aquatic environments (Zaharia and Suteu 2012).
After the textile dyeing and finishing processes, a predicted environmental concentration of a dye in the receiving water can be estimated based on different factors such as daily dye usage, dye fixation degree on the substrate, dye removal degree into the effluent treatment process, and dilution factors in the receiving water (Zaharia and Suteu 2012). On the other hand, inadequate-treated dyes may remain in an aquatic environment for a long period. For instance, the half-life of hydrolyzed reactive blue-19 is about 46 years at pH 7 and 25 °C (Hao et al. 2000). Dye concentrations in watercourses higher than 1 mg/L caused by the direct discharges of textile effluents, treated or not, can give rise to public compliant. High concentrations of textile dyes in water bodies stop the reoxygenation capacity of the receiving water and cutoff sunlight, which may affect the toxicity of dyes (Zaharia and Suteu 2012). Chemical pollutants such as textile dyes may affect organisms due to molecular, cellular, biochemical, and physiological failure. Therefore, these biomarkers may significantly reflect the toxicity of environmental pollutants on non-target organisms. The biological activities of dyes also differ greatly, despite similarities in their structure; so, their toxicological properties cannot be generalized (Al-Sabti 2000). Moreover, the toxicity of textile dyes may be increased after hydrolysis and decolorization processes (Gottlieb et al. 2003). For example, anaerobic transformation of azo dyes begins with reductive fission of the azo linkage, resulting in the formation and accumulation of colorless aromatic amines, which can be highly toxic and carcinogenic (Bhunia et al. 2001). The teratogenicity of p-chloroaniline, which is a metabolite of the azo dye, was shown in X. laevis tadpoles previously (Øllgaard et al. 1998).
Teratogenicity and toxicity of dyes, also tested in this study, have been revealed at developmental stage 8 of X. laevis embryos previously (Birhanli and Ozmen 2005). When we compared our study results with these previous findings, it appeared that the stage 46 tadpoles were more sensitive to lethal effects than stage 8 embryos after a 72-h exposure period. It may be attributed to embryonic developmental characteristics—that embryos proceed to tadpole stages after stage 44 according to Nieuwkoop and Faber (1994)—and that postembryonic skeletal and organ development starts at this stage. In fact, very little information is reported in the literature on the sensitivity of different stages of amphibians, differences in the routes of exposure, uptake, and what may affect the lethality. The protective effect of the presence of protective coat of the vitelline membrane and cortex in early developmental stages (8th and 13th stages) may also cause more resistance to toxicants. However, the increases in toxicity in higher development stages due to the gradual increase in the external surface of embryo and the development of gills were shown in cadmium-exposed X. laevis tadpoles (Herkovits and Helguero 1998); similar effects could be attributed to dye exposure. The stage 46 tadpoles were especially found to be more sensitive to AR-FBL, AB-FGRL, RB-GA, and CB-FNR exposure. The 72-h LC50 values of AR-FBL and AB-FGRL for stage 46 tadpoles were about three to six times lower than 96-h LC50s of stage 8 embryos, respectively. Our results and a previous study showed that both AR-FBL and AB-FGRL dyes were more toxic than other tested dyes, while RR-RR and CR-FN3G were the moderately toxic compounds, based on lethality. These toxicity differences may also be attributed to the structure of the tested dyes in this study. On the other hand, the 168-h LC50 values (0.13–0.35 mg/L) on tadpoles represent a high-risk potential, especially for tested astrazon dyes. Therefore, we assume that the tested astrazon dyes may be hazardous for other amphibian species in their embryonic development in aquatic environments.
As far as what is known, there is no information in the literature on the effect of sublethal concentrations of textile dyes on the biomarker enzymes in amphibians. Selected potential biomarkers were assayed for the first time for the tested xenobiotics in the present study. The GST activities in X. laevis tadpoles were clearly increased after a 24-h exposure to AR-FBL, RR-RR, and RB-GA compared to control (p < 0.05). These changes indicated the possible mechanism of oxidative stress induced by these dyes. GST is a multicomponent enzyme involved in the detoxification of many xenobiotics (Sun et al. 2006). A critical role for GST is obviously defense against oxidative damage and peroxidative products of DNA and lipids. The increased GST activities were probably a metabolic adaptation to the exposure to higher levels of these dyes and were obviously a defense against oxidative damage. Similar GST inductions were determined in goldfish (Carassius auratus) that were exposed to HC Orange No. 1 dye (Sun et al. 2006, 2007). Also, the exposure to the other textile dye, metanil yellow, led to a significant increase in GST activities in hepatic and intestinal tissues (Ramchandani et al. 1997). Moreover, results of some previous studies showed that other organic contaminants, such as oil, PAHs, and PCBs, may modulate increases of GST activity in X. laevis (Buryskova et al. 2006; Gillardin et al. 2009) and in fish (Lu et al. 2009; Tridico et al. 2010) for protection from the cytotoxic effects of biologically reactive molecules.
On the other hand, both CR-FN3G and CB-FNR dyes caused GST inhibition. In fact, a variety of chemicals or chemical mixtures are known to inhibit the GST activity due to a general impairment of the chemical metabolism, interfering with mechanisms involved in GST induction (Stephensen et al. 2002; Tlili et al. 2010). According to Birhanli and Ozmen (2005), the malformation rate of live tadpoles after AB-FGRL, CR-FN3G, and CB-FNR (39.07, 40.12, and 49.5 %) exposure was higher than other dyes that caused GST induction (malformation rates: 22.97 % for AR-FBL, 16.12 % for RR-RR, and 26 % for RB-GA). GST plays an important role in the detoxification and excretion of xenobiotics (Frasco and Guilhermino 2002). The inhibition or lower induction of GST by these dyes or their derivatives may prevent protective effects of GST against the genotoxic effects of radical oxygen species as a result of the metabolism of dyes. ROS production may cause higher malformation rates in tadpoles exposed to AB-FGRL, CR-FN3G, and CB-FNR. Also, another previous study suggested that the early expression of GST genes in Bufo arenarum embryos after insecticide exposure provides protection against the risk of tumor formation. These results show that GST may be a potential good biomarker of dye toxicity in Xenopus tadpoles.
The CaE activity assay, which is an important phase I biotransformation enzyme, was also evaluated in this study. Previous results show that CaE may be a good bioindicator for assessment of the toxicity of some xenobiotics in frog (Leite et al. 2010), fish (Tridico et al. 2010), and mussel (Escartin and Porte 1997) in aquatic biomonitoring studies. The CaEs are a class of serine-dependent esterases that hydrolyze a wide range of xenobiotic substrates (Bonacci et al. 2004). These are ubiquitous enzymes that are found in both prokaryotes and eukaryotes. However, there is no information in the literature on whether or not CaE activity may be used as a potential biomarker of dye toxicity in amphibians. Dyes contain organic ester bonds, and therefore, they may cause CaE inductions due to dye exposure. The results showed that CaE activity was increased expectedly, and one or more concentrations of AR-FBL, RR-RR, CR-FN3G, and CB-FNR significantly increased the enzyme activity compared to control tadpoles. Exposure to 7.14 mg/L of AR-FBL, 38 mg/L of RR-RR, 1,297 mg/L of CR-FN3G, and 128 mg/L of CB-FNR caused increases in CaE activity by 148, 117, 100, and 148 %, respectively, after a 24-h period (Table 3). CaEs have wide specificity and can therefore be used in removing or discharging a wide variety of disperse dyes. In RR-RR exposure, low concentrations increased CaE activities, while higher concentrations caused inhibition of its activities. The decreases in CaE activities at higher exposure doses may be a result of free radical increases due to exposure to higher RR-RR concentrations. The CaE inhibition was reported in tadpoles of Scinax fuscovarius (Anura, Hylidae) after exposure to organophosphate pesticides (Leite et al. 2010).
Several studies showed that changes in energy metabolism may occur to overcome toxic stress. Organic compounds that interfere with the aerobic metabolic pathway may alter the mitochondrial structure and may cause a disturbance in enzymatic activities (Frasco and Guilhermino 2002). Moreover, the activity of several regulatory enzymes may be altered in order to meet the required energy demands under toxic stress (Frasco and Guilhermino 2002). In hypoxia or under chemical stress, animals might need additional energy in a short period of time, increasing the use of the anaerobic pathway for energy production, which can be detected by an increase in activity of LDH, which catalyzes the interconversion of pyruvate to lactate in glycolysis (Cunha et al. 2007). In this study, short-term exposure to sublethal concentrations of five of the six dyes (except CB-FNR) caused a two- or three-fold increase of LDH activity at one or more concentrations compared with the control group. Especially, to AR-FBL exposure, LDH activity was increased 2.4 times, while GST and CaE activities reached 3.7 and 2.5 times of the control at higher exposure concentrations. These results indicate that AR-FBL interferes with energy production, detoxification, and/or anti-stress oxidative defenses in this species. Moreover, simultaneous increases in the activity of three enzymes may show simultaneous increases of metabolites due to biotransformation, and metabolites may also lead to impaired levels of energetic metabolism (Frasco and Guilhermino 2002). A similar induction of GST and LDH activities was reported as a consequence of zinc exposure in fish (Elumalai et al. 2007). On the other hand, exposure to some RB-GA concentrations caused increases in both enzyme activities. The inhibitions of these enzymes may show deterioration in anaerobic respiration processes in tadpole metabolism.
In conclusion, previous studies have demonstrated the abilities of a number of xenobiotics to induce oxidative stress and the positive correlations between enzyme responses. Results of this study show that toxicity mechanisms of the studied dyes were different from each other. The tested dyes affected biomarker enzymes in different levels and in different concentrations in X. laevis tadpoles. From the results above, AR-FBL, AB-FGRL, and RB-GA could be metabolized, which can cause a high level of oxidative stress. Also, the biomarker measured, such as GST, CaE, and LDH, clearly revealed metabolic changes after dye exposure. The possible toxicity mechanism of tadpoles can be explained with related oxidative stress mechanism, in which tested dyes were transformed by GST and oxidative stress injury to the aerobic respiration. Furthermore, results of this study provided some new information for the literature on the effects of sublethal textile dye exposure on biomarker enzymes. Also, this information can provide the answer to whether the induction of these parameters is useful as early biomarkers of tested textile dyes in a practical environment of amphibians in environmental monitoring studies.
References
Akyol A, Yatmaz H, Bayramoglu M (2004) Photocatalytic decolorization of remazol red RR in aqueous ZnO suspensions. Appl Catal B Environ 54:19–24
Al-Ghais S, Ahmad S, Ali B (2000) Differential inhibition of xenobiotic-metabolizing carboxylesterases by organotins in marine fish. Ecotoxicol Environ Saf 46:258–264
Al-Sabti K (2000) Chlorotriazine Reactive Azo Red 120 textile dye induces micronuclei in fish. Ecotoxicol Environ Saf 47:149–155
Alves de Lima RO, Bazo AP, Salvadori DM, Rech CM, de Palma Oliveira D, de Aragão Umbuzeiro G (2007) Mutagenic and carcinogenic potential of a textile azo dye processing plant effluent that impacts a drinking water source. Mutat Res 626:53–60
American Society for Testing Materials (ASTM) (2003) Standard guide for conducting the Frog Embryo Teratogenesis Assay-Xenopus (FETAX). E1439-98. In: Annual book of ASTM standards, vol. 11.05. Philadelphia, pp 447–457
Bhunia A, Durani S, Wangikar P (2001) Horseradish peroxidase catalyzed degradation of industrially important dyes. Biotechnol Bioeng 72:562–567
Birhanli A, Ozmen M (2005) Evaluation of the toxicity and teratogenity of six commercial textile dyes using the frog embryo teratogenesis assay-Xenopus. Drug Chem Toxicol 28:51–65
Blaustein A, Dobson A (2006) Extinctions: a message from the frogs. Nature 439:143–144
Bonacci S, Browne M, Dissanayake A, Hagger J, Corsi I, Focardi S, Galloway TS (2004) Esterase activities in the bivalve mollusc Adamussium colbecki as a biomarker for pollution monitoring in the Antarctic marine environment. Mar Pollut Bull 49:445–455
Buryskova B, Hilscherova K, Babica P, Vrskova D, Marsalek B, Blaha L (2006) Toxicity of complex cyanobacterial samples and their fractions in Xenopus laevis embryos and the role of microcystins. Aquat Toxicol 80:346–354
Cing S, Asma D, Apohan E, Yesilada O (2003) Decolorization of textile dyeing wastewater by Phanerochaete chrysosporium. Folia Microbiol 48:639–642
Cunha I, Neuparth T, Caeiro S, Costa M, Guilhermino L (2007) Toxicity ranking of estuarine sediments on the basis of Sparus aurata biomarkers. Environ Toxicol Chem 26:444–453
Diamantino TC, Almeida E, Soares AMVM, Guilhermino L (2001) Lactate dehydrogenase activity as an effect criterion in toxicity tests with Daphnia magna straus. Chemosphere 45:553–560
Doshi G (2006) Dyestuff industry in India and China. http://EzineArticles.com/370606. Accessed 04 February 2012
Elumalai M, Antunes C, Guilhermino L (2007) Enzymatic biomarkers in the crab Carcinus maenas from the Minho River estuary (NW Portugal) exposed to zinc and mercury. Chemosphere 66:1249–1255
Escartin E, Porte C (1997) The use of cholinesterase and carboxylesterase activities from Mytilus galloprovincialis in pollution monitoring. Environ Toxicol Chem 16:2090–2095
Frasco M, Guilhermino L (2002) Effects of dimethoate and beta-naphthoflavone on selected biomarkers of Poecilia reticulata. Fish Physiol Biochem 26:149–156
Gholami-Borujeni F, Mahvi AH, Nasseri S, Faramarzi MA, Nabizadeh R, Alimohammadi M (2011) Enzymatic treatment and detoxification of acid orange 7 from textile wastewater. Appl Biochem Biotechnol 165:1274–1284
Gillardin V, Silvestre F, Divoy C, Thome J, Kestemont P (2009) Effects of Aroclor 1254 on oxidative stress in developing Xenopus laevis tadpoles. Ecotoxicol Environ Saf 72:546–551
Gottlieb A, Shaw C, Smith A, Wheatley A, Forsythe S (2003) The toxicity of textile reactive azo dyes after hydrolysis and decolourisation. J Biotechnol 101:49–56
Guerra G, Domínguez O, Ramos-Leal M, Manzano AM, Sánchez MI, Hernández I, Palacios J, Arguelles J (2008) Production of laccase and manganese peroxidase by white-rot fungi from sugarcane bagasse in solid bed: use for dyes decolourisation. Sugar Tech 10:260–264
Güngördü A, Birhanli A, Ozmen M (2010) Assessment of embryotoxic effects of cadmium, lead and copper on Xenopus laevis. Fresenius Environ Bull 19:2528–2535
Gutleb A, Appelman J, Bronkhorst M, van den Berg J, Spenkelink A, Brouwer A, Murk AJ (1999) Delayed effects of pre- and early-life time exposure to polychlorinated biphenyls on tadpoles of two amphibian species (Xenopus laevis and Rana temporaria). Environ Toxicol Pharmacol 8:1–14
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hao OJ, Kim H, Chang PC (2000) Decolorization of wastewater. Crit Rev Environ Sci Technol 30:449–505
Herkovits J, Helguero LA (1998) Copper toxicity and copper–zinc interactions in amphibian embryos. Sci Total Environ 221:1–10
Kunz A, Mansilla H, Duran N (2002) A degradation and toxicity study of three textile reactive dyes by ozone. Environ Technol 23:911–918
Leite P, Margarido T, de Lima D, Rossa-Feres D, de Almeida E (2010) Esterase inhibition in tadpoles of Scinax fuscovarius (Anura, Hylidae) as a biomarker for exposure to organophosphate pesticides. Environ Sci Pollut Res 17:1411–1421
Lowry OH, Rosenbrough NJ, Farr AL, Randal RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Lu G, Wang C, Zhu Z (2009) The dose–response relationships for EROD and GST induced by polyaromatic hydrocarbons in Carassius auratus. Bull Environ Contam Toxicol 82:194–199
Marungrueng K, Pavasant P (2006) Removal of basic dye (Astrazon Blue FGRL) using macroalga Caulerpa lentillifera. J Environ Manage 8:268–274
Nieuwkoop PD, Faber J (1994) Normal table of Xenopus laevis (Daudin). Garland Publishing, New York
Øllgaard H, Frost L, Galster J, Hasen OC (1998) Survey of azocolorants in Denmark: consumption, use, health and environmental aspects. Ministry of Environment and Energy, Denmark
O’Neill C, Hawkes F, Hawkes D, Lourenco N, Pinheiro H, Delee W (1999) Colour in textile effluents—sources, measurement, discharge consents and simulation: a review. J Chem Technol Biotechnol 74:1009–1018
Osten R-V, Ortiz-Arana A, Guilhermino L, Soares A (2005) In vivo evaluation of three biomarkers in the mosquitofish (Gambusia yucatana) exposed to pesticides. Chemosphere 58:627–636
Osugi ME, Umbuzeiro GA, De Castro FJ, Zanoni MV (2006) Photoelectrocatalytic oxidation of remazol turquoise blue and toxicological assessment of its oxidation products. J Hazard Mater 137:871–877
Ozmen N (2008) The use of laccase containing culture filtrates for the decolorization and detoxification of some textile dyes. PhD Thesis. Graduate School of Natural and Applied Sciences, Inonu University, Turkey
Peng Y, Yang PH, Ng SSM, Lum CT, Kung HF, Lin MC (2004) Protection of Xenopus laevis embryos against alcohol-induced delayed gut maturation and growth retardation by peroxiredoxin 5 and catalase. J Mol Biol 340:819–827
Pielesz A, Baranowska I, Rybak A, Wlochowicz A (2002) Detection and determination of aromatic amines as products of reductive splitting from selected azo dyes. Ecotoxicol Environ Saf 53:42–47
Rajaguru P, Kalpana R, Hema A, Suba S, Baskarasethupathi B, Kumar P, Kalaiselvi K (2001) Genotoxicity of some sulfur dyes on tadpoles (Rana hexadactyla) measured using the comet assay. Environ Mol Mutagen 38:316–322
Ramchandani S, Das M, Joshi A, Khanna SK (1997) Effect of oral and parenteral administration of metanil yellow on some hepatic and intestinal biochemical parameters. J Appl Toxicol 17:85–91
Robinson T, Chandran B, Nigam P (2002) Removal of dyes from a synthetic textile dye effluent by biosorption on apple pomace and wheat straw. Water Res 36:2824–2830
Santhoshkumar P, Shivanandappa T (1999) In vitro sequestration of two organophosphorus homologs by the rat liver. Chem Biol Interact 120:277–282
Saratale R, Saratale G, Chang J, Govindwar S (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Inst Chem Eng 42:138–157
Sedighi M, Karimi A, Vahabzadeh F (2009) Involvement of ligninolytic enzymes of Phanerochaete chrysosporium in treating the textile effluent containing Astrazon Red FBL in a packed-bed bioreactor. J Hazard Mater 169:88–93
Sharma S, Sharma S, Singh P, Swami R, Sharma K (2009) Exploring fish bioassay of textile dye wastewaters and their selected constituents in terms of mortality and erythrocyte disorders. Bull Environ Contam Toxicol 83:29–34
Sponza D (2006) Evaluation of aquatic toxicity in wastewater of a dye-producing factory in Turkey. Fresenius Environ Bull 15:1115–1121
Stephensen E, Sturve J, Förlin L (2002) Effects of redox cycling compounds on glutathione content and activity of glutathione-related enzymes in rainbow trout liver. Comp Biochem Physiol C Toxicol Pharmacol 133:435–442
Sumathi M, Kalaiselvi K, Palanivel M, Rajaguru P (2001) Genotoxicity of textile dye effluent on fish (Cyprinus carpio) measured using the comet assay. Bull Environ Contam Toxicol 66:407–414
Sun Y, Yu H, Zhang J, Yin Y, Shen H, Liu H, Wang X (2006) Bioaccumulation and antioxidant responses in goldfish Carassius auratus under HC orange no. 1 exposure. Ecotoxicol Environ Saf 63:430–437
Sun Y, Yin Y, Zhang J, Yu H, Wang X (2007) Bioaccumulation and ROS generation in liver of freshwater fish, goldfish Carassius auratus under HC orange no. 1 exposure. Environ Toxicol 22:256–263
Sun QY, Hong YZ, Xiao YZ, Fang W, Fang J (2009) Decolorization of textile reactive dyes by the crude laccase produced from solid-state fermentation of agro-byproducts. World J Microbiol Biotechnol 25:1153–1160
Suryavathi V, Sharma S, Saxena P, Pandey S, Grover R, Kumar S, Sharma KP (2005) Acute toxicity of textile dye wastewaters (untreated and treated) of Sanganer on male reproductive systems of albino rats and mice. Reprod Toxicol 19:547–556
Tlili S, Jebali J, Banni M, Haouas Z, Mlayah A, Helal AN, Boussetta H (2010) Multimarker approach analysis in common carp Cyprinus carpio sampled from three freshwater sites. Environ Monit Assess 168:285–298
Tridico C, Rodrigues A, Nogueira L, da Silva D, Moreira A, de Almeida E (2010) Biochemical biomarkers in Oreochromis niloticus exposed to mixtures of benzo[a]pyrene and diazinon. Ecotoxicol Environ Saf 73:858–863
Ulson de Souza SM, Forgiarini E, Ulson de Souza AA (2007) Toxicity of textile dyes and their degradation by the enzyme horseradish peroxidase (HRP). J Hazard Mater 147:1073–1078
Verma Y (2008) Acute toxicity assessment of textile dyes and textile and dye industrial effluents using Daphnia magna bioassay. Toxicol Ind Health 24:491–500
Verma Y (2011) Toxicity assessment of dye containing industrial effluents by acute toxicity test using Daphnia magna. Toxicol Ind Health 27:41–49
Wang C, Yediler A, Lienert D, Wang Z, Kettrup A (2002) Toxicity evaluation of reactive dyestuffs, auxiliaries and selected effluents in textile finishing industry to luminescent bacteria Vibrio fischeri. Chemosphere 46:339–344
Yesilada O, Asma D, Cing S (2003) Decolorization of textile dyes by fungal pellets. Process Biochem 38:933–938
Zaharia C, Suteu D (2012) Textile organic dyes—characteristics, polluting effects and separation/elimination procedures from industrial effluents—a critical overview. In: Puzyn T, Mostrag-Szlichtyng A (eds) Organic pollutants ten years after the Stockholm Convention—environmental and analytical update, 1st edn. InTech, Croatia, pp 55–86
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Henner Hollert
Rights and permissions
About this article
Cite this article
Güngördü, A., Birhanli, A. & Ozmen, M. Biochemical response to exposure to six textile dyes in early developmental stages of Xenopus laevis . Environ Sci Pollut Res 20, 452–460 (2013). https://doi.org/10.1007/s11356-012-1063-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1063-1