Abstract
Purpose
Biodesulfurization (BDS) has the potential to desulfurize dibenzothiophene (DBT) and its alkylated derivatives, the compounds that are otherwise refractory to hydrodesulfurization (HDS). Thermophilic microorganisms are more appropriate to be used for BDS applications following HDS. The aim of the present study was to isolate a thermophilic microorganism and to explore its commercial relevance for BDS process.
Methods
The desulfurizing thermophilic strain was isolated and enriched from various soil and water samples using sulfur free medium (SFM) supplemented with DBT. Microbiological and genomic approach was used to characterize the strain. Desulfurization reactions were carried out using DBT and petroleum oils at 45°C followed by different analytical procedures.
Results
We report the isolation of a thermophilic bacterium Klebsiella sp. 13T from contaminated soils collected from petroleum refinery. HPLC analysis revealed that Klebsiella sp. 13T could desulfurize DBT to 2-hydroxybiphenyl (2-HBP) at 45°C through 4S pathway. In addition, adapted cells of Klebsiella sp. 13T were found to remove 22–53% of sulfur from different petroleum oils with highest sulfur removal from light crude oil.
Conclusion
Klebsiella sp. 13T is a potential candidate for BDS because of its thermophilic nature and capability to desulfurize petroleum oils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Combustion of petroleum derived fuels lead to the release of vast amount of sulfur dioxide (SO2) into the atmosphere, which is a principal source of acid rain and air pollution. Thus, most countries have imposed strict regulations to control these releases mainly by enforcing stringent restrictions on the levels of sulfur in transportation fuels. Further higher levels of sulfur in the present crude oil stocks have been suggested, indicating an additional problem to be faced by petroleum refineries worldwide (Bhatia and Sharma 2006).
Hydrodesulfurization (HDS) is the conventional process that has been routinely practised in the refineries for removing sulfur from petroleum products before their use by combustion. HDS is a high-pressure and high-temperature catalytic process and is plagued by the catalytic poisoning due to the presence of sulfur compounds in petroleum. Thiophenic sulfur compounds such as dibenzothiophene (DBT) and its alkylated derivatives are generally found to be resistant to HDS (Chen et al. 2009; Monticello 2000). To desulfurize these refractory compounds, HDS has to be carried out under extreme conditions using sophisticated and costly catalysts that make the process more energy intensive and expensive. Moreover, the used catalysts form a hazardous waste and its disposal poses problems. The recent years have seen the upcoming/development of biodesulfurization (BDS) technology that has potential to desulfurize refractory compounds such as DBT in a cost effective and environmentally benign manner. Furthermore, development of deep desulfurization (reducing the sulfur content from 500 to 50 ppm) using BDS process downstream to HDS, i.e., using BDS as a complementary technology to HDS, instead of deep HDS, seems to be the more convincing approach (Bhatia and Sharma 2006).
Various DBT desulfurizing microorganisms have been isolated to date, but most of them are mesophilic (reviewed by Mohebali and Ball 2008). Since HDS of petroleum oils is being carried out at elevated temperature, therefore thermophilic microorganisms are more appropriate to use for biorefining applications following HDS (Konishi et al. 1997, 2000). This will enable BDS reaction to be carried out at higher temperatures, and therefore there is no need to cool the HDS treated oil to ambient temperatures, which would be a more practical approach for a large0scale industrial process and could result in higher rates and low processing costs (Konishi et al. 1997; Kayser et al. 2002). In addition, higher temperature decreases oil viscosity and therefore contamination by undesirable bacteria which affect the BDS process could also be avoided (Kirimura et al. 2001; Gray et al. 2003; Soleimani et al. 2007). However, little research work has been conducted on searching for thermophiles for the BDS of petroleum and its products. It appears that only six thermophilic DBT desulfurizing microorganisms have been reported so far (Table 1). These are Paenibacillus sp. strain A11-2 (Konishi et al. 1997), Bacillus subtilis WU-S2B (Kirimura et al. 2001), Mycobacterium phlei GTIS10 (Kayser et al. 2002), Mycobacterium phlei WU-F1 (Furuya et al. 2001, 2003), Mycobacterium sp. X7B (Li et al. 2003) and Mycobacterium phlei WU-0103 (Ishii et al. 2005). Out of these, only Mycobacterium X7B has been extensively studied (Li et al. 2003, 2005, 2007).
Despite so many reports of DBT desulfurizing microorganisms, BDS remains far from commercialization. Most of the reports found in the literature are concerned with desulfurization of model compounds (reviewed by Mohebali and Ball 2008; Soleimani et al. 2007). To explore the commercial relevance of BDS process, the isolated biocatalysts must have the ability to remove sulfur from petroleum oils through “4S” pathway (Bhatia and Sharma 2010a). BDS through “4S” pathway involves the cleavage of C–S bond only in the petroleum oils and not the C–C bonds. Thus, the petroleum hydrocarbons are not degraded through this pathway. Therefore, this pathway is more efficient for the BDS of petroleum oils as this helps in the conservation of petroleum oil. In other pathways such as Kodama pathway of BDS, the costly petroleum oil is also degraded. The authors have recently reported the isolation of a mesophile Pantoea agglomerans which follows the 4S pathway for the BDS of petroleum oils (Bhatia and Sharma 2010b).
In this study, the isolation of a thermophilic microorganism, i.e., Klebsiella sp. 13T, that can degrade DBT in a sulfur-specific manner — i.e., through “4S” pathway — is described. Further, it is shown that Klebsiella sp. 13T can remove sulfur from different petroleum oils.
2 Materials and methods
2.1 Enrichment and cultivation of DBT desulfurizing microorganisms
The DBT desulfurizing thermophilic strain was isolated and enriched from soil and water samples collected from petroleum refinery in India as described previously (Bhatia and Sharma 2010b). Briefly, DBT (final concentration 100 ppm) dissolved in n-hexane was added to the sterilized sulfur free medium (SFM; 10 ml) and inoculated with different soil and water suspensions. Cultures were incubated on a rotary shaker at 180 rpm at 45°C for 5 days. Aliquotd (0.1 ml) of turbid cultures were transferred into fresh 10 ml SFM medium with DBT. Then, five such sub cultivations culture was appropriately diluted, plated onto LB agar plates and incubated at 45°C overnight. The individual colonies were picked up and isolates were further screened for their ability to grow and degrade DBT on DBT coated SFM agar plates, i.e., appearance of the zone of clearance.
2.2 Identification and characterization
Preliminary characterization was made by colony characteristics, microscopy and Gram’s staining. Genomic DNA of the isolate was extracted using a commercial kit as per the manufacturer’s instruction (Promega, USA). 16S rRNA gene was amplified as described (Rawlings 1995) and amplicon (~900 bp) was custom sequenced (Microsynth Pvt. Ltd., Switzerland). The 16S rDNA sequence was compared with others in a non-redundant sequence database at the NCBI by using the BLASTn program in order to find the genetic makeup of the isolate (Altschul et al. 1990). The presence of the genes responsible for the DBT degradation, i.e., dszB and dszC, were screened using polymerase chain reaction (PCR) as described (Duarte et al. 2001). The PCR reaction was performed four times, and the representative figure is shown.
2.3 Desulfurization reactions
For desulfurization of DBT, fresh overnight grown culture (late exponential phase) of the characterized isolate was inoculated in 20 ml SFM in 100-ml Erlenmeyer flasks (starting OD660 of 0.02) containing 100 ppm of DBT dissolved in n-hexane 10% (v/v) (Bhatia and Sharma 2010b). The flasks were incubated at 45°C and 200 rpm for 24–120 h. After the respective time point of cultivation, the culture supernatant was harvested and analyzed. For desulfurization of different petroleum oils, the isolate was first adapted on different petroleum oils. Earlier, it was observed that 10% of oil in desulfurizing reactions does not give a high level of toxicity and is appropriate for investigating the desulfurizing capacity of isolates (Bhatia and Sharma 2010a). SFM containing various petroleum oils (10% of oil) individually were inoculated with the fresh culture of adapted cells (starting OD660 of 0.02) and incubated at 45°C under shaking condition (200 rpm). After 120 h of incubation, the treated oil was analysed for sulfur content. Later, petroleum oils were used: light crude oil, heavy crude oil, diesel and HDS diesel.
2.4 Analytical procedures
Gibb’s assay was used to screen the conversion of DBT to 2-HBP phenolic compounds by the isolates (Oldfield et al. 1997). For this culture supernatant, 1 ml was taken and incubated with 50 μl of Gibb’s reagent (10 mM in ethanol) at 30°C. Positive reactions developed blue to purple color after 30 min of incubation at room temperature and was also monitored at OD610 against a blank containing no DBT. Gibb’s assay is employed as a qualitative method to determine the presence of 2-HBP in the medium, but this analytical method presents problems due to interferences; therefore, the HPLC technique was employed to be sure of the presence of the final compound of the 4S route (Bhatia and Sharma 2010b). After desulfurization reaction the flask with reaction mixture was removed from the shaking incubator and kept aside for 15 min. After that, the reaction mixture was slowly transferred to a separating funnel. The emulsion phase was centrifuged (15,000 rpm, 20 min) to obtain the organic/oil phase. This organic phase was acidified to pH 2.0 with 6N HCl and extracted with equal volume of ethyl acetate. The extract was then filtered and quantitative and qualitative estimation of the degradation of DBT was performed by HPLC (Waters 1525 binary HPLC pump connected with 2487 dual wavelength absorbance detector, Germany) using reverse phase chromatography and C-18 column. Elution was performed with 80/20 (v/v) acetonitrile/water as mobile phase at the flow rate of 1 ml/min and peaks of DBT and its metabolites were detected at 280 nm. To measure sulfur content in the bacteria treated with different petroleum oils, XRF spectrophotometry was performed as described previously (Bhatia and Sharma 2010b). Briefly, the treated oil was separated by centrifugation at 15,000×g for 10 min from the culture reaction and was analyzed for total sulfur content using twin-x XRF analyzer (Oxford instruments, UK) in accordance with the ASTM standard method D-4294. All data shown in this paper are the average values with standard deviation of the experiment conducted in triplicates. Statistical analysis was performed using one-way ANOVA and GraphPad Prism version 4.
3 Results and discussion
3.1 Enrichment and isolation of DBT degrading microorganisms
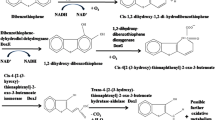
In order to isolate the thermophilic DBT desulfurizing microorganisms, 70 types of soil, water and sludge samples were collected as sources of microorganisms. The enrichment and cultivation was done in SFM supplemented with DBT at 45°C. After five rounds of subcultivation, three pure isolates, i.e., 13T, 17 + 18T and 23 + 24T, were selected based on their ability to produce zone of clearance of DBT on DBT-coated SFM agar plates. This was followed by induction experiments as described previously (Bhatia and Sharma 2010b), where it was observed that all three isolates have the intrinsic property of degrading DBT. Next, we investigated for the presence of sulfur specific “4S” pathway of DBT degradation in these isolates. In this pathway, DBT gets oxidized to DBTO (DBT sulfoxide), which is then transformed to DBT sulfone (DBTO2) with the help of monooxygenase. DBTO2 is further transformed to 2′-hydroxyphenyl benzene sulfinate (HBPSi) by monooxygenase, leading to cleavage of the thiophene ring. HBPSi is lastly desulfurized to 2-HBP by hydrolase enzyme leading to the subsequent release of sulfite or sulfate (Gallagher et al. 1993; Oldfield et al. 1997). The presence of this 2-HBP (a phenolic compound) in the culture broth of the three isolates was screened by Gibb’s assay as mentioned in Materials and methods. Out of three isolates, only 13T showed the positive Gibb’s reaction and was therefore selected for further characterization and BDS experiments.
3.2 Identification and characterization of DBT degrading thermophile
Routine microbiological and molecular biological techniques were performed for characterizing 13T isolate. Isolate 13T was found to be rod-shaped, Gram-negative bacteria. 16S rDNA sequencing was performed for identifying its genus/species. The 16S rDNA sequence was compared with others in a non-redundant sequence database at the NCBI by using the BLASTn program (Altschul et al. 1990). The highest score (99% homology) was obtained with the 16S rDNA sequence of Klebsiella sp. JT42. Based on this result, the isolate 13T was categorized as Klebsiella sp. 13T. The 16S rDNA sequence of Klebsiella sp. 13T has been submitted in the gene bank (Accession No. GU139546). Kobayashi et al. (2000) observed that amongst various Gibb’s assay positive isolates identified in their study one was Klebsiella planticola. However, the desulfurization activity of this microorganism was found to be unstable during repeated subculturing. Dudley and Frost (1994) reported that Klebsiella oxytoca KS3D desulfurizes aryl sulfonates into the corresponding phenols. The property of desulfurization found in Rhodococcus erythropolis IGTS8, and other microorganisms have been shown to be due to the presence of the desulfurization genes present on an operon, i.e., dszABC (Denis-Larose et al. 1997). This operon consists of three genes: dszA, dszB and dszC. DszC, DszA and DszB enzymes are sequentially required in 4S pathway. Therefore, we carried out PCR to have molecular evidence of the dszC (first enzyme) and dszB (last enzyme) genes in the genome of the isolate 13T. DNA of R. erythropolis IGTS8 and isolate D23W3, which was recently shown by us to have these genes (Bhatia and Sharma 2010b), was used as a positive control. DNA of isolate D27S that was shown by our group to have dszB, but not dszC, gene was used as additional control (Bhatia and Sharma 2010b). The presence of amplified product of 422 and 592 bp specific to dszB and dszC genes were observed in the genomic DNA of isolate 13T (Fig. 1, lanes 2 and 13) and D23W3 (Fig. 1, lanes 5 and 10). As expected the presence of dszB amplicon but not dszC amplicon was observed for isolate D27S (Fig. 1, lanes 3 and 11). Moreover, no amplification for dszC specific band was observed in the genomic DNA of isolate 17 + 18T, which was earlier shown to have negative Gibb’s reaction. Noteworthy, genes involved in DBT metabolism are present in almost all DBT degrading bacteria and have been shown to have almost 70% of the sequence homology (Monticello 2000).
PCR analysis of dszB and dszC genes in isolate 13T. PCR was performed on the genomic DNA of different isolates using primers specific for dszB (a) and dszC (b) genes. Amplified products were electrophoresed on 2% agarose gel. Lanes 1 and 7, Rhodococcus sp. IGTS8; lanes 2 and 13, isolate 13T; lanes 3 and 11, isolate D27S; lanes 4 and 9, 100 bp DNA marker; lanes 5 and 10, isolate D23W3; lanes 6 and 8, no genomic DNA control; lane 12, isolate 17 + 18T. The expected size of amplicons for dszB and dszC genes are indicated
3.3 DBT degradation by isolate 13T
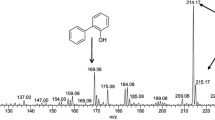
For identification of the DBT desulfurized metabolites, fresh overnight grown culture of isolate 13T was inoculated in SFM containing 100 ppm of DBT. The ethyl acetate extracts of culture broth at two different time points (1 and 5 days) was analyzed by HPLC. Chromatogram of the culture extract of the 1-day-old culture showed the presence of DBT-sulfone and unutilized DBT (Fig. 2a) whereas the peak of both DBT-sulfone and 2-HBP were observed in the 5-day-old culture (Fig. 2b). This was accompanied by the presence of very less DBT ~3% of the initial amount (Fig. 2b). The DBT/DBT-sulfone/2-HBP peak in these extracts were confirmed by spiking the extract with 100 ppm of DBT-sulfone/2-HBP and then performing HPLC as previously described (Bhatia and Sharma 2010b). The 4S pathway is a specific desulfurization pathway in which DBT is desulfurized and converted to 2-HBP. Through this pathway, the carbon skeleton of DBT is released intact and thus the calorific value of the fuel is not lost. The observation of 2-HBP in the culture broth of 13T isolate indicated that DBT degradation by 13T took place through 4S pathway with the selective cleavage of carbon–sulfur (C–S) bonds without reducing the energy content The presence of a high amount of DBT-sulfone (DBTO2) after 120 h in the culture broth of isolate 13T was unexpected since DBTO2 is a transition phase of the “4S” pathway of conversion of DBT to 2-HBP and supposed to be present for a very short time in the cultivation broth (Gupta et al. 2005). Conversion of DBTO2 to 2-HBP has been shown to be a rate-limiting step of the 4S pathway and that accumulation of 2-HBP leads to the inhibition of the activity of the microbe to degrade DBT (Chen et al. 2009). As the accumulation of 2-HBP was not observed initially in cultivation broth of 13T (in day 1 culture), this could eventually be beneficial for the degradation of DBT by 13T. Identification of 2-HBP (Fig. 2b) and presence of dszB and dszC genes (Fig. 1) confirmed that the degradation of DBT by Klebsiella sp. 13T was due to the existence of “4S” pathway.
DBT degradation by isolate 13T. HPLC was performed after DBT desulfurization reaction. HPLC spectrum of DBT desulfurization products for different time points by isolate 13T are shown: a after 24 h, b after 120 h. Retention times for DBT desulfurization products, i.e., DBT-sulfone (3.1), 2-HBP (3.6) and residual DBT (7.7) are indicated
3.4 Desulfurization of different petroleum oils by isolate 13T
Oil refineries usually separate crude oil into several fractions and then desulfurize them separately. Refineries can make substantial cost savings if most of the sulfur is removed from the crude oil before it is fractionated. Also, it has been suggested that due to the high content of water in crude oil, BDS of crude oil is more practicable compared to that of diesel oil and gasoline (Zhou and Zhang 2004). Therefore isolate Klebsiella sp. 13T was assessed for its ability to desulfurize light and heavy crude oil along with HDS diesel and diesel oil. Sulfur in each of the oil samples was estimated before and after the treatment (120 h, 45°C) by adapted cells of Klebsiella sp. 13T using XRF. The initial percentage of sulfur in HDS diesel, diesel, light crude and heavy crude oil was found to be 0.05%, 0.18%, 0.35% and 2.63% of sulfur, respectively. Upon treatment by adapted cells of Klebsiella sp. 13T, 22–53% of sulfur was found to be removed from different petroleum oils (Fig. 3). Percentage of sulfur removal from light crude oil, i.e., 53.21%, was found to be the highest. Despite the fact that the ultimate goal of desulfurizing microorganisms is to remove sulfur from petroleum oils, there are very few microorganisms — such as Gordona sp. (Chang et al. 2000), Rhodococcus sp. P32C1 (Maghsoudi et al. 2001), R. erythropolis XP (Yu et al. 2006), Mycobacterium phlei WU-0103 (Ishii et al. 2005), Mycobacterium sp. X7B (Li et al. 2007) and Pantoea agglomerans D23W3 (Bhatia and Sharma 2010b) — that have been shown to possess the capability of desulfurization of different petroleum oils. Among three thermophilic microorganisms that are shown to desulfurize different petroleum oils to date, Mycobacterium sp X7B seems to have the best ability to remove sulfur, at 86% and 59%, from diesel and crude oil after 24 and 72 h of reaction, respectively (Li et al. 2003, 2007). Further research work on the development of the process of BDS and biorefining of light and heavier crude oils and different petroleum products such as gasoline, diesel, vacuum residues, etc., may be extended to make this process more commercially attractive, as biorefining is a more environment-friendly technology.
4 Conclusion
To the best of our knowledge, this is the first report of the identification of thermophilic Klebsiella sp. as a sulfur removing bacteria. As the ultimate success of a biocatalyst isolated for the BDS process depends upon its capability to desulfurize petroleum oils through 4S pathway (i.e., by conserving the petroleum oil), Klebsiella sp. 13T offers a good potential for use in biocatalytic desulfurization of petroleum oils.
Abbreviations
- HDS:
-
Hydrodesulfurisation
- BDS:
-
Biocatalytic desulfurization
- DBT:
-
Dibenzothiophene
- SFM:
-
Sulfur free medium
- 2-HBP:
-
2-Hydroxybiphenyl
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Bhatia S, Sharma DK (2006) Emerging role of biorefining of heavier crude oils and integration of biorefining with petroleum refineries in future. Petrol Sci Technol 24:1125–1159
Bhatia S, Sharma DK (2010a) Mining of genomic databases to identify novel biodesulfurizing microorganisms. J Ind Microbiol Biotechnol 37:425–429
Bhatia S, Sharma DK (2010b) Biodesulfurization of dibenzothiophene, its alkylated derivatives and crude oil by a newly isolated strain Pantoea agglomerans D23W3. Biochem Eng J 50:104–109
Chang JH, Chang YK, Ryu HW, Chang HN (2000) Desulfurization of light gas oil in immobilized cell systems of Gordona sp. CYKS1 and Nocardia CYKS2. FEMS Microbiol Lett 182:309
Chen H, Cai YB, Zhang WJ, Li W (2009) Methoxylation pathway in biodesulfurization of model organosulfur compounds with Mycobacterium sp. Bioresour Technol 100:2085–2087
Denis-Larose C, Labbe D, Bergeron H, Jones AM, Greer CW, Al-Hawari J, Grossman MJ, Sankey BM, Lau PC (1997) Conservation of plasmid-encoded dibenzothiophene desulfurization genes in several rhodococci. Appl Environ Microbiol 63:2915–2919
Duarte GF, Rosado AS, Seldin L, de Araujo W, van Elsas JD (2001) Analysis of bacterial community structure in sulfurous-oil-containing soils and detection of species carrying dibenzothiophene desulfurization (dsz) genes. Appl Environ Microbiol 67:1052–1062
Dudley MW, Frost JW (1994) Biocatalytic desulfurization of arylsulfonates. Bioorg Med Chem 2:681
Furuya T, Kirimura K, Kino K, Usami S (2001) Thermophilic desulfurization of dibenzothiophene and its derivatives by Mycobacterium pheli WUF-1. FEMS Microbiol Lett 204:129–133
Furuya T, Ishii Y, Noda K, Kino K, Kirimura K (2003) Thermophilic biodesulfurization of hydrodesulfurized light gas oils by Mycobacterium phlei WU-F1. FEMS Microbiol Lett 221:137
Gallagher JR, Olson ES, Stanley DC (1993) Microbial desulphurisation of dibenzothiophene: a sulfur-specific pathway. FEMS Microbiol Lett 107:31–36
Gray KA, Mrachkoyz GT, Squiresy CH (2003) Biodesulfurization of fossil fuels. Curr Opin Microbiol 6:229–235
Gupta N, Roychoudhury PK, Deb JK (2005) Biotechnology of desulfurization of diesel: prospects and challenges. Appl Microbiol Biotechnol 66:356–366
Ishii Y, Kozaki S, Furuya T, Kino K, Kirimura K (2005) Thermophilic biodesulfurization of various heterocyclic sulfur compounds and crude straight-run light gas oil fraction by a newly isolated strain Mycobacterium phlei WU-0103. Curr Microbiol 50:63–70
Kayser KJ, Cleveland L, Park H, Kwak J, Kolhatkar A, Kilbane JJ (2002) Isolation and characterization of a moderate thermophile, Mycobacterium phlei GTIS10 capable of dibenzothiophene desulfurization. Appl Microbiol Biotechnol 59:737–746
Kirimura K, Furuya T, Nishii Y, Ishii Y, Kino K, Usami S (2001) Biodesulfurization of dibenzothiophene and its derivatives through the selective cleavage of carbon–sulfur bonds by a moderately theromophillic bacterium Bacillus subtilis WU-S2B. J Biosci Bioeng 91:262–266
Kobayashi M, Onaka T, Ishii Y, Konishi J, Takaki M, Okada H, Ohta Y, Koizumi K, Suzuki M (2000) Desulfurization of alkylated forms of both dibenzothiophene and benzothiophene by a single bacterial strain. FEMS Microbiol Lett 187:123–126
Konishi J, Ishii Y, Onaka T, Okumura K, Suzuki M (1997) Thermophilic carbon–sulfur-bond targeted biodesulfurization. Appl Environ Microbiol 63:3164
Konishi J, Onaka T, Ishii Y, Suzuki M (2000) Demonstration of the carbon–sulfur bond targeted desulfurization of benzothiophene by thermophilic Paenibacillus sp. strain A11-2 capable of desulfurizing dibenzothiophene. FEMS Microbiol Lett 187:151–154
Li FL, Xu P, Ma CQ, Luo LL, Wang XS (2003) Deep desulfurization of hydrodesulfurization-treated diesel oil by a facultative thermophilic bacterium Mycobacterium sp. X7B. FEMS Microbiol Lett 223:301–307
Li W, Zhang Y, Wang MD, Shi Y (2005) Biodesulfurization of dibenzothiophene and other organic sulfur compounds by a newly isolated Microbacterium strain ZD-M2. FEMS Microbiol Lett 247:45–50
Li F, Zhang Z, Feng J, Cai X, Xu P (2007) Biodesulfurization of DBT in tetradecane and crude oil by a facultative thermophilic bacterium Mycobacterium goodii X7B. J Biotechnol 127:222–228
Maghsoudi S, Vossoughi M, Kheirolomoom A, Tanaka E, Katoh S (2001) Biodesulfurization of hydrocarbons and diesel fuels by Rhodococcus sp. strain P32C1. Biochem Eng J 8:151
Mohebali G, Ball AS (2008) Biocatalytic desulfurization (BDS) of petrodiesel fuels. Microbiology 154:2169–2183
Monticello DJ (2000) Biodesulfurization and upgrading of petroleum distillates. Curr Opin Biotechnol 11:540–546
Oldfield C, Pogrebinsky O, Simmonds J, Olson ES, Kulpa CF (1997) Elucidation of the metabolic pathway for dibenzothiophene desulphurization by Rhodococcus sp. strain IGTS8 (ATCC 53968). Microbiology 143:2961–2973
Rawlings, DE (1995) Restriction enzyme analysis of 16S rRNA genes for the rapid identification of Thiobacillus ferrooxidans, Thiobacillus thiooxidans and Leptospirillum ferooxidans strains in leaching environments. In: Jerez V, Toledo W (eds) Biohydrometallurgical processing. Proceedings of the International Biohydrometallurgical Symposium (vol II), Chile, p 9
Soleimani M, Bassi A, Margaritis A (2007) Biodesulfurization of refractory organic sulfur compounds in fossil fuels. Biotechnol Adv 25:570–596
Yu B, Xu P, Shi Q, Ma C (2006) Deep desulfurization of diesel oil and crude oils by a newly isolated Rhodococcus erythropolis strain. Appl Environ Microbiol 72:54–58
Zhou ZY, Zhang K (2004) Development situation and prospect of oil fields in China. Petroleum Expl Dev 31:84–87
Acknowledgements
This work was supported by the grant from the Petroleum Conservation Research Association (PCRA), Ministry of Petroleum & Natural Gas, Government of India, India. The authors are thankful to Dr. H.K. Prasad for helping in genomic DNA isolation and PCR analysis. The gift of R. erythropolis IGTS8 from Dr. J. Kilbane is greatly acknowledged. We are also thankful to Panipat Refinery for carrying out XRF analysis.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Bhatia, S., Sharma, D.K. Thermophilic desulfurization of dibenzothiophene and different petroleum oils by Klebsiella sp. 13T. Environ Sci Pollut Res 19, 3491–3497 (2012). https://doi.org/10.1007/s11356-012-0884-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-0884-2