Abstract
Various heterocyclic sulfur compounds such as naphtho[2,1-b]thiophene (NTH) and benzo[b]thiophene (BTH) derivatives can be detected in diesel oil, in addition to dibenzothiophene (DBT) derivatives. Mycobacterium phlei WU-0103 was newly isolated as a bacterial strain capable of growing in a medium with NTH as the sulfur source at 50°C. M. phlei WU-0103 could degrade various heterocyclic sulfur compounds, not only NTH and its derivatives but also DBT, BTH, and their derivatives at 45°C. When M. phlei WU-0103 was cultivated with the heterocyclic sulfur compounds such as NTH, NTH 3,3-dioxide, DBT, BTH, and 4,6-dialkylDBTs as sulfur sources, monohydroxy compounds and sulfone compounds corresponding to starting heterocyclic sulfur compounds were detected by gas chromatography–mass spectrometry analysis, suggesting the sulfur-specific desulfurization pathways for heterocyclic sulfur compounds. Moreover, total sulfur content in 12-fold-diluted crude straight-run light gas oil fraction was reduced from 1000 to 475 ppm S, with 52% reduction, by the biodesulfurization treatment at 45°C with growing cells of M. phlei WU-0103. Gas chromatography analysis with a flame photometric detector revealed that most of the resolvable peaks, such as those corresponding to alkylated derivatives of NTH, DBT, and BTH, disappeared after the biodesulfurization treatment. These results indicated that M. phlei WU-0103 may have a good potential as a biocatalyst for practical biodesulfurization of diesel oil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In petroleum, mainly in diesel oil, numerous organic sulfur compounds are present and, therefore, the combustion of diesel oil generates sulfur oxides leading to acid rain and air pollution. Today’s refineries remove organic sulfur compounds from crude oil by hydrodesulfurization (HDS) process. However, recalcitrant heterocyclic sulfur compounds such as alkyl-substituted derivatives of dibenzothiophene (DBT) cannot be completely removed by HDS process using chemical catalysts containing metals [4, 6]. Moreover, since HDS process is carried out under high temperature and pressure, increasing the amount of discharging CO2 becomes a serious problem for the environment. Therefore, the application of a biodesulfurization process under a mild condition using microorganisms to achieve a deeper desulfurization of diesel oil has attracted attention.
There are many reports focusing on the biodesulfurization of model compounds such as DBT [for review, see 2, 21, 23]. Among them, Rhodococcus strains, especially R. erythropolis IGTS8, and other bacteria such as Mycobacterium have been investigated in detail. Biodesulfurization by these strains resulted in removal of total sulfur between 30–70% for middle distillates [7, 11], 49–86% for HDS-treated diesel oil [3, 8, 12, 19, 20, 24] , and 24–33% for crude oil [3, 11, 20]. However, since these removal levels of biodesulfurization are insufficient to meet the required levels, for practical biodesulfurization, it is important to improve both the rate (desulfurizing activity) and extent (broad substrate specificity) of desulfurization to achieve extremely low-level sulfur in diesel oil and in other oil streams [21, 23]. For increasing desulfurizing activity, strain improvement and bioengineering have been carried out by many research groups [for review, see 2, 21, 23]. As for the strain improvement by genetic engineering, the gene shuffling and direct evolution techniques have been applied to create new DBT-desulfurizing enzymes with higher activity and broader substrate specificity for derivatives of R. erythropolis IGTS8 [1, 5]. The gene-dosage of DBT-desulfurizing genes by genetic engineering for R. erythropolis KA2-5-1 enhanced the desulfurizing activity toward light gas oil, but not the extent of desulfurization [12]. Recently, it has been confirmed that alkyl-substituted derivatives of naphtho[2,1-b]thiophene (NTH) and benzo[b]thiophene (BTH) can be detected in diesel oil, in addition to DBT derivatives [4]. However, at present the biodesulfurizing bacteria with broad specificity toward organic sulfur compounds are limited. On the other hand, biodesulfurization of high temperature around 40–50°C, i.e., thermophilic biodesulfurization, toward the straight-run and HDS-treated oil fraction could be integrated more easily to a refining stream because it would be unnecessary to cool the oil fraction to ambient temperature [2, 17, 23]. Moreover, since thermophilic biodesulfurization reduces the viscosity of crude oil under high-temperature conditions, it is considered to be effective for the development of the desulfurization process of crude oil [2]. Thus, for practical biodesulfurization, especially for application to crude oil, it may be useful to obtain a microorganism efficiently desulfurizing both types of heterocyclic sulfur compounds, symmetric ones such as DBT and asymmetric ones such as NTH and BTH, under high temperature around 40–50°C. There are many reports dealing with the biodesulfurization of HDS-treated diesel oil [3, 7, 8, 11, 12, 18–20, 24] and crude diesel oil [3, 11, 20] by DBT-desulfurizing bacteria, but only one report that deals with thermophilic NTH- and/or BTH-desulfurizing bacteria [8]. Moreover, there is no report dealing with the thermophilic biodesulfurization of crude diesel oil.
In this paper, we describe the thermophilic desulfurization by Mycobacterium phlei WU-0103, which was newly isolated as a bacterium desulfurizing NTH, as a source of sulfur at 50°C. Since this strain shows broad specificity toward heterocyclic sulfur compounds, to confirm whether or not M. phlei WU-0103 could desulfurize diesel oil, we performed biodesulfurization tests of crude straight-run light gas oil fraction at 45°C. By the biodesulfurization treatment with growing cells of M. phlei WU-0103, total sulfur contents in crude straight-run light gas oil fraction (12,000 ppm S) diluted 12-fold with n-tetradecane and HDS-treated light gas oil (50 ppm S) were reduced from 1000 ppm S to 475 ppm S and 1000 ppm S to 478 ppm S, with both at a 52% reduction, respectively.
Materials and Methods
Isolation and cultivation of NTH-desulfurizing microorganisms
Isolation and cultivation were performed using AN medium, containing 5.0 g glucose, 5.0 g sodium malate·1/2H2O, 1.0 g NH4Cl, 1.0 g KH2PO4, 8.0 g K2HPO4, 0.2 g MgCl2·6H2O, 0.05 g thiamine hydrochloride, 10 mL metal solution [15] , and 1.0 mL vitamin mixture [15] in 1000 mL distilled water (pH 7.5). The medium was supplemented with 1% (v/v) NTH solution (54 mM, in n-tetradecane) as a source of sulfur. Unless otherwise indicated, cultivation was performed at 50°C in test tubes (18 × 180 mm) containing 5 mL of the medium with NTH with reciprocal shaking at 240 strokes per minute. Single-colony isolation from turbid cultures was performed by plating appropriately diluted culture samples onto Luria-Bertani (LB) medium composed of 10 g Bacto Tryptone (Difco, Detroit, MI), 5 g yeast extract (Difco), and 10 g NaCl in 1000 mL distilled water (pH 7.0) supplemented with 15 g agar. Microorganisms isolated were stored in micro-tubes containing 10% (v/v) glycerol at −80°C.
Biodesulfurization of heterocyclic sulfur compounds
Biode- sulfurization of heterocyclic sulfur compounds was performed by growing cells of M. phlei WU-0103 in AN medium using one of the heterocyclic sulfur compounds, such as NTH, DBT, BTH, and their derivatives, dissolved in n-tetradecane as a source of sulfur. Cultivation was performed at 45°C in test tubes (18 × 180 mm) containing 5 mL of AN medium with 50 μL of substrate solution (54 mM, in n-tetradecane) with reciprocal shaking at 240 strokes per minute.
Biodesulfurization of diesel oils
Straight-run light gas oil (R-LGO) containing 1.2% (wt/wt) sulfur (12,000 ppm S) and HDS-treated light gas oil (H-LGO) containing 0.005% (wt/wt) sulfur (50 ppm S) were kindly supplied by the laboratory of the Japan Cooperation Center, Petroleum (JCCP, Shizuoka, Japan) and used in this study. R-LGO was diluted up to 0.1% (wt/wt) sulfur (1000 ppm S) with H-LGO or n-tetradecane and used for biodesulfurization by growing cells of M. phlei WU-0103. One milliliter of M. phlei WU-0103 cells grown with NTH was inoculated into 500-mL Erlenmeyer flasks containing 100 mL of AN medium with 10 mL of diluted R-LGO instead of NTH as the source of sulfur, and cultivation was carried out for 3 days at 45°C. In addition, the R-LGO after biodesulfurization was separated as an upper layer by centrifugation at 24,000g for 1 h at 20°C, and the biodesulfurization reaction by growing cells was performed by repeating the cultivation with the separated R-LGO as source of sulfur.
Analytical methods
Cell growth was turbidimetrically measured at 660 nm. NTH, DBT, BTH, their derivatives, and the metabolites in culture broth and reaction mixtures were measured by using gas chromatography (GC, type GC-2010; Shimadzu, Kyoto, Japan) equipped with a flame ionization detector (FID), a flame photometric detector (FPD), and a 30-m type DB-5 column (J&W Scientific, Folsom, CA). The mobile phase was helium gas and the flow rate was 0.57 mL/min. The culture broth or reaction mixture was acidified to pH 2.0 with 6 M HC1 and extracted with 3 mL of ethyl acetate including fluorene as an internal standard, respectively. The ethyl acetate layer was filtered through a 0.20-μm PTFE membrane filter (ADVANTEC Toyo, Tokyo, Japan), and the filtrate was used for GC analysis. The amounts of DBT and other compounds were calculated from standard calibration curves. The chemical structures of metabolites from NTH, NTH 3,3-dioxide (NTH sulfone), DBT, BTH, and 4,6-dialkylDBTs were determined by gas chromatography-mass spectrometry (GC-MS, type 5890II; Hewlett Packard, Mississauga, Ontario, Canada). Total sulfur contents of reaction mixtures and diesel oils were determined by total sulfur analyzer (ANTEK 7000V, Antek, TX).
Chemicals
DBT, 2-HBP and 2,8-dimethylDBT were purchased from Tokyo Kasei (Tokyo, Japan). DBT 5,5-dioxide (DBT sulfone) and benzo[b]naphtho[2,1-d]thiophene were purchased from Aldrich (Milwaukee, WI). NTH and the other heterocyclic sulfur compounds were kindly supplied by the laboratory of the JCCP. All other reagents were of analytical grade and commercially available.
Results
Identification of the NTH-desulfurizing bacterium WU-0103
To isolate thermophilic NTH-desulfurizing microorganisms, approximately 200 types of soil and seawater samples were transferred into test tubes containing 5 mL of AN medium with NTH as the source of sulfur and cultivated at 50°C. By means of subcultivations in liquid AN medium and single-colony isolation on LB medium agar plate, strains showing an optical density at 660 nm (OD660) of more than 1.0 in AN medium with NTH within 5 days at 50°C were selected. Among the strains, one bacterium, WU-0103, which showed stable growth and NTH-desulfurizing ability in AN medium with NTH as the source of sulfur at 50°C, was selected for further studies.
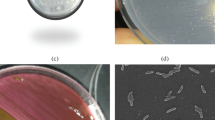
WU-0103 is a rod-shaped bacterium with dimensions 0.7 μm by 1.5–2.0 μm. This strain is Gram-positive, catalase-positive and oxidase-negative, and does not form spores. Further taxonomic identification of WU-0103 was performed by the National Collection of Industrial and Marine Bacteria Japan Ltd. (Shizuoka, Japan). The fatty acid pattern of WU-0103 was typical of the genus Mycobacterium, and the mycolic acid pattern of this strain was similar to the type strain of M. phlei. In addition, a partial sequence of the 16S rDNA of WU-0103 was found to show a similarity of 99.4% to that of the type strain of M. phlei. From these results, WU-0103 was tentatively identified as M. phlei.
NTH-desulfurization of M. phlei WU-0103
M. phlei WU-0103 grew at 50°C in AN medium with 0.54 mM NTH as the source of sulfur, and completely degraded NTH within 5 days. Two sulfur-free compounds, 2′-hydroxynaphthylethene (HNE) and naphtho[2,1-b]furan (NFU), were detected as metabolites of NTH and NTH sulfone by GC-MS analysis. Moreover, 2-hydroxybiphenyl (2-HBP) was detected as metabolites of DBT, and BTH sulfone and benzofuran (BFU) were detected as metabolites of BTH. Therefore, M. phlei WU-0103 desulfurized NTH via NTH sulfone to NFU or HNE through the sulfur-specific degradation pathways with selective cleavage of C—S bonds (Fig. 1), suggesting it to be identical to that of Rhodococcus sp. WU-K2R previously reported [16]. The effects of temperature on NTH degradation by growing cells of M. phlei WU-0103 were investigated. M. phlei WU-0103 showed NTH-degrading ability over a wide temperature range from 30°C to 50°C, and most efficiently at 45°C (data not shown).
Thermophilic biodesulfurization of heterocyclic sulfur compounds
Since many types of heterocyclic sulfur compounds are detected in diesel oil [4, 6], degradation of heterocyclic sulfur compounds by growing cells of M. phlei WU-0103 at 45°C was investigated in oil/water two-phase system. M. phlei WU-0103 was cultivated in AN medium with 0.54 mM concentration of each heterocyclic sulfur compound as the sulfur source at 45°C for 4 days. As shown in Tables 1, 2, and 3, M. phlei WU-0103 could degrade various heterocyclic sulfur compounds dissolved in n-tetradecane at high temperature. Moreover, M. phlei WU-0103 could desulfurize 4,6-dialkylDBTs such as 4,6-dimethylDBT, 4,6-diethylDBT, 4,6-dipropylDBT and 4,6-dibutylDBT, which are highly refractory to HDS process. When M. phlei WU-0103 was cultiva-ted with 4,6-dialkylDBTs, monohydroxy alkylated biphenyls, and heterocyclic sulfone compounds, which were metabolites through the postulated sulfur-specific desulfurization pathway as shown in Fig. 1, they were detected as their metabolites by GC-MS analysis (data not shown). On the other hand, it seems likely that degradation ratio tended to be low as molecular mass of heterocyclic sulfur compounds exceeded 300.
Thermophilic biodesulfurization of straight-run light gas oil
Since M. phlei WU-0103 exhibited desulfurizing ability toward various heterocyclic sulfur compounds, desulfurization of crude straight-run light gas oil fraction was also examined under high temperature, at 45°C. The straight-run light gas oil used, R-LGO, was not treated with HDS and contained 12,000 ppm S of sulfur. Since R-LGO contained a high concentration of sulfur compounds for biodesulfurization by M. phlei WU-0103, R-LGO was diluted up to 1000 ppm S with H-LGO containing 50 ppm S or n-tetradecane. When M. phlei WU-0103 grew in AN medium with R-LGO diluted with H-LGO as the source of sulfur, the sulfur content in R-LGO reduced form 1000 to 860 ppm S for 3 days at 45°C. Since after 3 days no further reduction of sulfur content occurred, the biodesulfurization by growing cells was performed by repeating the cultivation with the separated R-LGO as source of sulfur every 3 days. Through 10 consecutive reactions, the sulfur content in R-LGO diluted with H-LGO was reduced from 1000 to 478 ppm S, with 52% reduction (Fig. 2). In the case of R-LGO diluted with n-tetradecane, the sulfur content was also reduced from 1000 to 475 ppm S, with 52% reduction. As shown in Fig. 1, GC-FPD analysis for organic sulfur compounds revealed that most of the resolvable peaks of heterocyclic sulfur compounds such as alkylated derivatives of BTH, DBT, and NTH disappeared after the biodesulfurization treatment.
GC-FPD chromatograms of sulfur components in the straight-run light gas oil before (A) and after (B) thermophilic biodesulfurization at 50°C by growing cells of M. phlei WU-0103. The straight-run light gas oil, R-LGO, containing 12,000 ppm S was diluted up to 1000 ppm S with HDS-treated light gas oil, H-LGO, containing 50 ppm S, and was used for thermophilic biodesulfurization. Total sulfur content is 1000 ppm S for A and 478 ppm S for B, before and after the thermophilic biodesulfurization, respectively. A number in abbreviations such as C2-BTH, C1-DBT, and C1-NTH indicated the carbon number of the alkyl substituent group of BTH, DBT, and NTH derivatives. They were classified with standard substrates and the reference of previous reports [3,19].
At 45°C the growing cells of M. phlei WU-0103 desulfurized HDS-treated light gas oil, H-LGO, from 50 to 10 ppm S, with 80% reduction.
Discussion
In this report, we describe the isolation of the thermophilic NTH-desulfurizing bacterium M. phlei WU-0103 and the characterization of WU-0103 for desulfurization abilities toward various heterocyclic sulfur compounds and crude straight-run light gas oil fraction. Generally, thermophilic desulfurizing microorganisms are considered to be more advantageous than mesophilic ones for the application of biodesulfurization [2, 17, 23]. M. phlei WU-0103 could efficiently desulfurize various heterocyclic sulfur compounds under high temperature conditions. Total sulfur content in 12-fold-diluted crude straight-run light gas oil fraction was reduced from 1000 to 475 ppm S, with 52% reduction, by the biodesulfurization treatment at 45°C with growing cells of M. phlei WU-0103.
As for NTH-degradation, M. phlei WU-0103 completely degraded 0.54 mM NTH within 5 days at 50°C. Since mesophilic NTH-desulfurizing bacterium Rhodococcus sp. WU-K2R degraded 80% of 0.27 mM NTH within 5 days at 30°C [16] and thermophilic DBT-desulfurizing bacterium M. phlei WU-F1 degraded 39% of 0.27 mM NTH within 5 days at 50°C [9] , M. phlei WU-0103 showed a much higher NTH-desulfurizing activity than WU-K2R and WU-F1. As for biodesulfurization of heterocyclic sulfur compounds in oil/water two-phase system, M. phlei WU-0103 desulfurized 4,6-dipropylDBT and 4,6-dibutylDBT, which are highly refractory to HDS process, dissolved in n-tetradecane at 45°C. On the other hand, at 30°C DBT-desulfurizing bacterium Mycobacterium sp. G3 desulfurized 4,6-dipropylDBT and 4,6-dibutylDBT in water phase system, but not in oil/water two-phase system [13]. These results indicate that M. phlei WU-0103 has a high ability for substrate specificity and uptake on desulfurization at a high temperature, 45°C.
As for biodesulfurization of crude oils, several data were reported for mesophilic DBT-desulfurizing bacteria, as follows. Grossman et al. reported that the growing cells of Rhodococcus sp. ECRD-1 decreased sulfur content in a crude middle-distillate fraction diluted with decane (10-fold) from 2050 to 1440 ppm S, with 30% reduction, at 25°C [11]. Chang et al. reported that the growing cells of Nocardia sp. CYKS2 decreased sulfur content in light gas oil from 3000 to 2000 ppm S, with 33% reduction, at 30°C [3]. However, GC-SCD chromatogram for organic sulfur compounds showed many resolvable peaks of BTH and DBT derivatives in light gas oil after treatment using Nocardia sp. CYKS2, and the reduction of BTH derivatives was less than DBT ones. Maghsoudi et al. reported that the resting cells of Rhodococcus sp. P32C1 decreased sulfur content in straight-run light gas oil (16,200 ppm S) diluted with HDS-treated diesel oil (303 ppm S) from 1000 to 763 ppm S, with 24% reduction, at 30°C [20]. As for the present study, the desulfurization ability of M. phlei WU-0103 toward crude straight-run light gas oil fraction was comparable with those of the above-mentioned strains, and M. phlei WU-0103 showed a high ability for sulfur reduction ratio and desulfurization at a high temperature, 45°C.
The results shown in this report indicated that M. phlei WU-0103 may be a useful desulfurizing biocatalyst for the practical biodesulfurization process of diesel oil. We have reported the molecular analysis for DBT-desulfurization of B. subtilis WU-S2B, M. phlei WU-F1, and Paenibacillus sp. A11-2 [10, 14, 17]. At present, we are investigating the genetic analysis for desulfurization of M. phlei WU-0103, and the application of genetic engineering to WU-0103 by using genetic resources for DBT-desulfurization from B. subtilis WU-S2B, M. phlei WU-F1, and Paenibacillus sp. A11-2.
Literature Cited
JJ Arensdorf AK Loomis PM DiGrazia DJ Monticello PT Pienkos (2002) ArticleTitleChemostat approach for the direct evolution of biodesulfurization gain-of-function mutants Appl Environ Microbiol 68 691–698 Occurrence Handle10.1128/AEM.68.2.691-698.2002 Occurrence Handle1:CAS:528:DC%2BD38XhtV2nsrc%3D Occurrence Handle11823208
SL Borgne R Quintero (2003) ArticleTitleBiotechnological processes for the refining of petroleum Fuel Process Technol 81 155–169
JH Chang SK Rhee YK Chang HN Chang (1998) ArticleTitleDesulfurization of diesel oils by a newly isolated dibenzothiophene-degrading Nocardia sp strain CYKS2 Biotechnol Prog 14 851–855
J Chen Z Ring (2004) ArticleTitleHDS reactivities of dibenzothiophenic compounds in a LC-finer LGO and H2S/NH3 inhibition effect Fuel 83 305–313
WM Coco WE Levinson MJ Crist HJ Hektor A Darzins PT Pienkos CH Squires DJ Monticello (2001) ArticleTitleDNA shuffling method for generating highly recombined genes and evolved enzymes Nat Biotechnol 19 354–359
GA Depauw GF Froment (1997) ArticleTitleMolecular analysis of the sulphur components in a light cycle oil of a catalytic cracking unit by gas chromatography with mass spectrometric and atomic emission detection J Chromatogr A 761 231–247
BR Folsom DR Schieche PM DiGrazia J Werner S Palmer (1999) ArticleTitleMicrobial desulfurization of alkylated dibenzothiophenes from a hydrodesulfurized middle distillate by Rhodococcus erythropolis I-9 Appl Environ Microbiol 65 4967–4972
T Furuya Y Ishii K Noda K Kino K Kirimura (2003) ArticleTitleThermophilic biodesulfurization of hydrodesulfurized light gas oils by Mycobacterium phlei WU-F1 FEMS Microbiol Lett 221 137–142
T Furuya K Kirimura K Kino S Usami (2002) ArticleTitleThermophilic biodesulfurization of naphthothiophene and 2-ethylnaphthothiophene by a dibenzothiophene-desulfurizing bacterium, Mycobacterium phlei WU-F1 Appl Microbiol Biotechnol 58 237–240
T Furuya S Takahashi Y Ishii K Kino K Kirimura (2004) ArticleTitleCloning of a gene encoding flavin reductase coupling with dibenzothiophene monooxygenase through coexpression screening using indigo production as selective indication Biochem Biophys Res Commun 313 570–575
MJ Grossman MK Lee RC Prince KK Garrett GN George IJ Pickering (1999) ArticleTitleMicrobial desulfurization of crude oil middle-distillate fraction: analysis of the extent of sulfur removal and the effect of removal on remaining sulfur Appl Environ Microbiol 65 181–188 Occurrence Handle1:CAS:528:DyaK1MXjvVygsA%3D%3D Occurrence Handle9872778
K Hirasawa Y Ishii M Kobayashi K Koizumi K Maruhashi (2001) ArticleTitleImprovememt of desulfurization activity in Rhodococcus erythropolis KA2-5-1 by genetic engineering Biosci Biotechnol Biochem 65 239–246
H Okada N Nomura T Nakahara K Maruhashi (2002) ArticleTitleAnalysis of substrate specificity of the desulfurizing bacterium Mycobacteium sp G3. J Biosci Bioeng 93 228–233
Y Ishii J Konishi H Okada K Hirasawa T Onaka M Suzuki (2000) ArticleTitleOperon structure and functional analysis of the genes encoding thermophilic desulfurizing enzymes of Paenibacillus sp A11-2. Biochem Biophys Res Commun 270 81–88
K Kirimura T Furuya Y Nishii Y Ishii K Kino S Usami (2000) ArticleTitleBiodesulfurization of dibenzothiophene and its derivatives through selective cleavage of carbon-sulfur bonds by a moderately thermophilic bacterium Bacillus subtilis WU-S2B J Biosci Bioeng 91 262–266
K Kirimura T Furuya R Sato Y Ishii K Kino S Usami (2002) ArticleTitleBiodesulfurization of naphthothiophene and benzothiophene through selective cleavage of carbon-sulfur bonds by Rhodococcus sp. strain WU-K2R Appl Environ Microbiol 68 3867–3872
K Kirimura K Harada H Iwasawa T Tanaka Y Iwasaki T Furuya Y Ishii K Kino (2004) ArticleTitleIdentification and functional analysis of the genes encoding dibenzothiophene-desulfurizing enzymes from thermophilic bacteria Appl Microbiol Biotechnol 65 703–713
J Konishi Y Ishii T Onaka K Okumura M Suzuki (1997) ArticleTitleThermophilic carbon-sulfur-bond-targeted biodesulfurization Appl Environ Microbiol 63 3164–3169 Occurrence Handle1:CAS:528:DyaK2sXlt1Wisrc%3D
FL Li P Xu CQ Ma LL Luo XS Wang (2003) ArticleTitleDeep desulfurization of hydrodesulfurization-treated diesel oil by a facultative thermophilic bacterium Mycobacterium sp X7B. FEMS Microbiol Lett 223 301–307
S Maghsoudi M Vossoughi A Kheirolomoon E Tanaka S Katoh (2001) ArticleTitleBiodesulfurization of hydrocarbons and diesel fuels by Rhodococcus sp strain P32C1 Biochem Eng J 8 151–156
DJ Monticello (2000) ArticleTitleBiodesulfurization and the upgrading of petroleum distillates Curr Opin Biotechnol 11 540–546
SG Mossner MJL Alda LC Sander ML Lee SA Wise (1999) ArticleTitleGas chromatographic retention behavior of polycyclic aromatic sulfur heterocyclic compounds, (dibenzothiophene, naphtho[b]thiophenes, benzo[b]thiophenes and alkyl-substituted derivatives) on stationary phases of different selectivity J Chromatogr A 841 207–228
T Ohshiro Y Izumi (1999) ArticleTitleMicrobial desulfurization of organic sulfur compounds in petroleum Biosci Biotechnol Biochem 63 1–9
SK Rhee JH Chang YK Chang HN Chang (1998) ArticleTitleDesulfurization of dibenzothiophene and diesel oils by a newly isolated Gordona strain, CYKS1 Appl Environ Microbiol 64 2327–2331 Occurrence Handle1:CAS:528:DyaK1cXjslWhtLc%3D Occurrence Handle9603863
Acknowledgments
This study was supported in part by Industrial Technology Research Grant Program in ‘01B63002c (to Y. Ishii) from New Energy and Industrial Technology Development Organization (NEDO) subsidized by the Ministry of Economy, Trade and Industry of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ishii, Y., Kozaki, S., Furuya, T. et al. Thermophilic Biodesulfurization of Various Heterocyclic Sulfur Compounds and Crude Straight-Run Light Gas Oil Fraction by a Newly Isolated Strain Mycobacterium phlei WU-0103. Curr Microbiol 50, 63–70 (2005). https://doi.org/10.1007/s00284-004-4403-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-004-4403-7