Abstract
Purpose
Non-resective pharyngoplasty techniques have been shown to be effective to treat oropharyngeal collapse in patients affected by obstructive sleep apnea-hypopnea syndrome (OSAHS). The aim of our study is to evaluate outcome predictors in a cohort of patients affected by OSAHS and treated with non-resective pharyngoplasty, including variation of pharyngeal measures at the end of the surgical procedure.
Methods
A cohort of patients affected by OSAHS, with palatal or lateral pharyngeal wall collapse, who underwent non-resective pharyngoplasty, were enrolled between 2014 and 2017. Surgical procedures encompassed non-resective pharyngoplasty by expansion sphincter pharyngoplasty (ESP) or barbed antero-lateral pharyngoplasty with barbed reposition pharyngoplasty (BRP) or barbed suspension pharyngoplasty (BSP) techniques, eventually associated with nasal surgery. Pharyngeal measures were recorded intraoperatively and their variation at the end of the procedure was considered. Surgical success was evaluated at least 6 months after surgery with respiratory polygraphy and ESS questionnaire. Outcome predictors were examined by multivariable logistic regression and ROC curve analysis.
Results
Seventy patients met the study inclusion criteria. ESP, BRP, and BSP in a uni-/multilevel setting led to significant improvement of all respiratory polygraphic parameters and daily sleepiness (p < 0.0001). Outcome analysis showed that greater variation of antero-posterior pharyngeal measure was associated with success (p = 0.01), with an optimal cutoff value of 8.5 mm; low AHIpre, high ESSpre, and antero-lateral pharyngoplasty with barbed sutures were associated with a higher rate of cure (p < 0.05).
Conclusions
Non-resective pharyngoplasty is effective in treating OSAHS patients affected by palatal or lateral pharyngeal wall collapse, and intraoperative variation of antero-posterior width may be a useful tool to predict surgical success.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a common sleep breathing disorder whose prevalence is increasing throughout the world. In the general population, the prevalence of OSA ranges from 9 to 25% [1] and in bariatric surgical patients may be more than 70% [2]. OSA is characterized by repeated episodes of significant reduction or complete cessation in breathing during sleep caused by narrowing or obstruction of the upper airway.
Untreated OSAHS may contribute to pathophysiological mechanisms underlying the origin and/or development of hypertension, cardiac ischemia, myocardial infarction, congestive heart failure, and stroke [3]. Therefore, prompt diagnosis and optimal treatment for OSAHS is important to prevent complications and improve the patient’s health. There are a variety of therapeutic options for OSAHS such as positive airway pressure (PAP), weight loss, surgery, positional therapy, and oral appliance (OA) [4]. In general, the optimal treatment is determined according to the patient’s anatomical structures, respiratory polygraphic and endoscopic results, and personal preferences [5].
The critical pathophysiologic feature of OSAHS is sleep-related collapse of the upper airway mainly at the level of the pharynx [6]. Obstructive apneas and hypopneas occur, respectively, because of intermittent partial or complete collapse of the pharynx (from the retropalatal area lower to the hypopharynx) or of the larynx during sleeping; pharyngeal collapse can occur at the end of expiration or at the beginning of inspiration [7].
An obstructive event, lasting more than 10 s, is defined as hypopnea with a 50% or more reduction in respiratory flow or less reduction associated with a 3% desaturation or arousal, while apnea is characterized by complete airflow stop.
According to the American Academy of Sleep Medicine Task Force recommendations, obstructive sleep apnea-hypopnea syndrome (OSAHS) is defined as an apnea-hypopnea index (AHI) > 5, measured during nocturnal respiratory polygraphy, along with excessive daytime somnolence. In contrast, a habitual snorer is considered a subject who always snores at night, with an AHI < 5. OSAHS is further classified into mild (AHI = 5–14), moderate (AHI = 15–30), and severe (AHI > 30) [7].
Continuous PAP therapy (CPAP) is the gold standard for treatment of patients affected by moderate to severe OSAHS, and in patients who are not compliant to CPAP, surgery has also been shown to be effective. One of the most popular surgical procedures proposed for treatment of OSAHS patients who are not suitable for CPAP is uvulopalatopharyngoplasty (UPPP); however, as shown by Tang et al., this procedure may be associated with postoperative side effects such as foreign body sensation (38.6%) and velopharyngeal insufficiency (10%) [8].
Since 2003, newer palatal techniques have been introduced, especially after the evolution of palatal surgeries focused on lateral pharyngeal wall collapse [9] or antero-posterior collapse [10]. Some palatal surgical techniques that have been recently described in the literature are expansion sphincter pharyngoplasty (ESP) [11, 12], Roman blind technique (RBT) [13], barbed reposition pharyngoplasty (BRP) [14], and barbed suspension pharyngoplasty (BSP) [15]. Barbed procedures allow surgeons to achieve widening and stiffening of the retropalatal space without tissue sacrifice by means of a bidirectional barbed suture that is inserted through the fibro-muscular tissues of the soft palate and posterior tonsillar pillars, and tightened around three steady holds: the posterior nasal spine and the two pterygoid hamuli lateral to the pterygomandibular raphe [13,14,15,16,17].
During the last decades, criteria for surgical success have evolved using significant variation of some respiratory polygraphic parameters first, such as the apnea index (AI) or apnea-hypopnea index (AHI) [18] and later introducing criteria such as those suggested by Sher [19], i.e., reduction of respiratory polygraphic measures and improvement of quality of life parameters like the ESS [20, 21].
The newer non-resective techniques have been shown to be as effective as the older resective ones (uvulopalatopharyngoplasty (UPPP)) but with less invasiveness, especially if a barbed suture technique is used [16, 22]. The passage from resective to non-resective surgery has led to three-dimensional surgical planning, but little is known about the morphological oropharyngeal modifications that predict surgical success.
The aim of this study is to evaluate the outcome of a cohort of patients treated by non-resective pharyngoplasty, analyzing variations in palatal measures and searching for predictors.
Methods
Study design and population
An observational retrospective study was carried out, analyzing the records of a cohort of 70 consecutive patients with OSAHS who refused or failed to tolerate CPAP and who underwent non-resective pharyngoplasty between January 2014 and October 2017 at the Department of Otorhinolaryngology—Head and Neck Surgery of the University of Genoa, Italy. Ethical review and approval was not required for this study in accordance with national and institutional requirements. However, each patient preoperatively signed a consent form for disclosure of privacy in managing personal data for scientific purposes.
Preoperative assessment included respiratory polygraphy, endoscopic evaluation, and drug-induced sedation endoscopy (DISE). All patients underwent respiratory polygraphic exam at least 6 months postoperatively. Inclusive criteria were as follows: diagnosis of mild to severe OSAHS (AHI) ≥ 5, with the main site of obstruction at the oropharyngeal level; as palatal or lateral pharyngeal wall collapse, according to the NOHL classification [23]; failure to tolerate or comply with CPAP or mandibular advancement device (MAD); and with body mass index (BMI) ≤ 35. We excluded patients with complete base-tongue, hypopharyngeal, or laryngeal collapse or significant craniofacial anomalies affecting the airway, severe comorbidities, contraindications for surgery, or incomplete clinical data.
Clinical evaluation
Clinical evaluation included complete head and neck examination and nasofibrolaryngoscopy (NFL) with a flexible video nasopharyngoscope (diameter 3.5 mm, Olympus Medical Systems Corporation, Tokyo, Japan) to determine the extent of retropalatal (Muller RP) and retrolingual (Muller RL) obstruction. The BMI was calculated. Moreover, all patients were evaluated by comprehensive history that covered sleep habits and occurrence of sleep disturbances. Excessive daytime sleepiness was estimated by the Epworth sleepiness scale (ESS) [24, 25].
Respiratory polygraphic study
The sleep study was performed with a cardiorespiratory monitor (Vital night) to record the following variables simultaneously: nocturnal snoring sound, arterial oxygen saturation measured by finger oxymetry, body position, nasal and mouth airflow, thoracic and abdominal respiratory movements recorded by inductive plethysmography, and heart rate. To determine the severity of sleep apnea, we considered AHI, oxygen desaturation index (ODI), and t < 90% (percent of the total time with oxygen saturation level lower than 90%).
Drug-induced sedation endoscopy
Drug-induced sedation endoscopy (DISE) was performed in a supine position without neck extension with transnasal flexible endoscopy using an ENF-VH videoendoscope connected to an Evis Exera II CLV-180B light source (Olympus Medical Systems Corporation, Tokyo, Japan). The entire exam was recorded. No local anesthesia was used in the nasal cavity in agreement with the European position statement on DISE [26].
Sedation was induced by the synergy between two drugs, midazolam and propofol, in order to exploit the muscle relaxant and the hypnotic effects when used at low dose together. Midazolam is administered with intravenous repeated bolus in a range of 1–3 mg, while propofol is administered intravenously via TCI (target-controlled infusion) with a target between 0.8 and 1.8 μg/ml according to the Schnider model. This is an advanced pharmacokinetic model that establishes the effect-site concentration of drugs to achieve a desired clinical effect. At the end of the procedure, we normally use flumazenil to antagonize the effects of midazolam. The use of low dose of midazolam and propofol combined together allows sedation to be as physiologic as possible, with snoring, apneas, controlled desaturations, and rapid recovery.
The degree of obstruction was evaluated at the level of the nasal cavity, nasopharynx, oropharynx, hypopharynx, and larynx using the NOHL classification [23].
Surgical procedure and intraoperative measures
All patients underwent non-resective pharyngoplasty with either the expansion sphincter pharyngoplasty (ESP) technique [12, 17] or with antero-lateral pharyngoplasty with barbed sutures (BP) with BRP [14] or BSP technique [15]. If tonsils were still present, tonsillectomy was performed and a simultaneous multilevel procedure was chosen if nasal obstruction was significant, also performing turbinoplasty and/or septoplasty. Patients treated up to May 2015 underwent ESP; since June 2015, we started treating patients with barbed pharyngoplasty: till December 2016, we adopted the BRP technique and since January 2017 the BSP.
Oropharyngeal exposure was achieved with a Boyle-Davis mouth gag
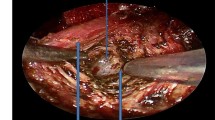
The following linear measures were obtained, under direct vision by the surgeon, during the general anesthesia, just before and after the surgical procedure, with the Boyle-Davis mouth gag kept open for the correct exposure of the surgical field and using a regular surgical ruler with 1 mm as the unit of measure (Figs. 1a–c and 2a–d):
-
Uvula length (Uvula): the distance between the posterior nasal spine to the apex of the uvula (Figs. 1a and 2a)
-
Arch length (Arch): the distance between the palatal arch and the posterior end of the hard palate (Figs. 1a and 2b)
-
Lateral width (Lateral): the distance from both posterior pillars measured on an axial plane at the level of the apex of the uvula (Figs. 1a and 2c)
-
Antero-posterior width (A-P): distance from the posterior pharyngeal wall to the soft palate at the level of the uvula (Figs. 1b and 2d)
The retropalatal area was virtually estimated, being modeled as a hemi-ellipse having semi-axes A-P width and Lateral/2 width, so that its value is defined by \( \frac{1}{2}\ast \pi \ast AP\ast \frac{\mathrm{Lateral}}{2} \) (Fig. 1b). For each measure, the actual difference value (∆) was recorded (∆ = postoperative value−preoperative value).
Outcome evaluations
Surgical success was evaluated at least 6 months after surgery, performing respiratory polygraphy and repeating the ESS questionnaire [25]. Definition of outcomes, in agreement with Montevecchi et al. [21], are reported in Table 1.
Statistical analysis
Categorical variables were described as absolute and relative frequencies; standard descriptive statistics were used expressing means, standard deviations, quartiles, and CV of quantitative variables. The Shapiro-Wilk normality test was applied and Wilcoxon signed-rank test for matched samples was chosen to compare the pre- and posttreatment values of variables of interest.
Two binomial logistic regressions were performed to ascertain the effects of age, BMI, ESSpre, AHIpre, type of pharyngoplasty, and ∆ values of intraoperative measures on the likelihood that patients obtain curative treatment (outcome = cure) or at least a successful treatment (Outcome = Cure or Success). The model was estimated with a stepwise selection procedure. The optimal cutoffs for predictors were defined by receiver operating characteristic (ROC) curve analysis using the Euclidean distance method [27].
GraphPad Prism Version 6.0 (San Diego, CA, USA), IBM SPSS Statistics version 24.0, and Cutoff Finder version 2.1 [27] were used for statistical analysis. For all tests, a two-tailed p value < 0.05 was considered significant.
Results
Patients and treatments
Seventy patients, 60 males and 10 females, met study inclusion criteria and were included in the analysis. The mean age was 53 ± 12 years, ranging from 24 to 76, and the group was composed primarily of men (86%). The mean preoperative AHI, ODI, and t90 were 32.1 ± 18.1 events/h, 24.7 ± 11.4 events/h, and 7.8 ± 7.0%, respectively; 10 patients (14%) were affected by mild OSAHS, 34 (49%) by moderate, and 26 (37%) by severe OSAHS. Forty-two (60%) patients underwent antero-lateral pharyngoplasty with barbed sutures (22 treated with BRP and 20 with BSP technique) and 28 (40%) ESP; due to the presence of nasal obstruction, a multilevel surgical procedure was chosen for 60 patients (85%) by performing turbinoplasty in 15 (21%) and both septoplasty and turbinoplasty in 45 (64%). Categorical data and summary statistics of quantitative variables are reported in Table 2 and Suppl. Table 1.
Improvement in respiratory polygraphic parameters
Non-resective pharyngoplasty in a uni-/multilevel setting led to significant improvement of all respiratory polygraphic parameters (p < 0.0001, Table 3 and Suppl. Table 1) and daily sleepiness assessed by the ESS questionnaire (ESSpre 9.4 ± 5.0, ESSpost 1.1 ± 1.4, p < 0.0001; Fig. 3a). In particular, the surgical procedure achieved a mean AHI reduction of 77.2% (Fig. 3b), a mean ODI reduction of 85.8% (Fig. 3c), and mean t90% reduction of 85.4% (Fig. 3d). Considering posttreatment AHI, measured by the respiratory polygraphy, in 14 patients (20%) the exam was normal, 54 patients (77%) showed a mild grade OSAHS, in 2 (3%) a moderate grade OSAHS, and no patient was affected by severe grade OSAHS (Table 2).
According to criteria explained in Table 1, a successful treatment was achieved in 62 patients (89%) with a cure rate of 20%; 8 patients (11%) experienced a failure, but only 2 had an AHIpost ≥ 20 events/h (both with AHIpost of 20 events/h) and with no patient having an ESSpost value ≥ 10.
Oropharyngeal measures and postsurgical modifications
Surgical procedure led to a significant variance of all linear measures and of the retropalatal area (Table 3) and was associated with a decrease in Uvula length (Uvulapre 40 ± 7 mm, Uvulapost 31 ± 5 mm, p < 0.0001; Fig. 4a) and Arch length (Archpre 31 ± 5 mm, Archpost 24 ± 4 mm, p < 0.0001; Fig. 4b) given by the elevation of the soft palate. The lateral measure significantly increased (Lateralpre 27 ± 6 mm, Lateralpost 34 ± 5 mm, p < 0.001; Fig. 4c) vs. A-P diameter (A-Ppre 5 ± 4 mm, A-Ppost 18 ± 4 mm, p < 0.0001; Fig. 4d), and consequently the retropalatal area augmented by a mean of 357%, increased from 2.08 ± 2.13 to 9.51 ± 3.28 cm2 (p < 0.0001; Fig. 4e).
Dot plots and mean values (bars) of preoperative and postoperative measures: Uvula (a), Arch (b), Lateral (c), A-P diameter (d), and retropalatal area (e). p values are estimated by Wilcoxon signed-rank test. Receiver operating characteristics (ROC) curves for ∆A-P and at least success as outcome (f) and for AHIpre and cure outcome (g)
Outcome predictors
In evaluating outcomes, as proposed by Montevecchi et al. [21], taking both AHI and ESS into account, two binomial logistic regression models were built.
The first model considered the cure (AHIpost < 5 and ESSpost < 10 and reduction of both of them > 50%) as outcome and was statistically significant, χ2(3) = 43.9, p < 0.001, and explained 73.7% (Nagelkerke R2) of the variance of curative treatment and correctly classified 90.0% of cases. Preoperative AHI (O.R. 0.672; p = 0.002), ESS (O.R. 1.542; p = 0.029), and type of pharyngoplasty (BP O.R. 66.303; p = 0.026) significantly influenced the outcome with lower AHI, higher ESS, and BP as good predictors of curative treatment (Table 4).
Considering at least a success as outcome (AHIpost < 20 and ESSpost < 10 and reduction of both of them > 50%), the logistic regression model obtained was statistically significant χ2(4) = 33.73, p < 0.001, and explained 75.2% (Nagelkerke R2) of the variance for successful treatment, correctly classifying 97.1% of cases. The only variable that lasted in the model at the end of the stepwise selection was the ∆A-P, whose higher values were associated with successful treatment (O.R. 4.60, p = 0.01; Table 4).
Optimal cutoff values for continuous variables, which were significant in multivariate analysis (AHI, ESS, and ∆A-P), were searched with ROC curve analysis. A variation of A-P measure greater than 8.5 mm best predicted successful treatment (AUC = 0.95, sensitivity = 93.5%, specificity = 87.5%; p < 0.001, Fig. 4f); AHIpre higher than 24.5 was significantly associated with an outcome that was not more than successful (AUC = 0.88, sensitivity = 78.6%, specificity = 75%; p < 0.001, although a significant cutoff value for ESSpre variable was not identified (Fig. 4g).
Discussion
Although the exact pathophysiology leading to pharyngeal collapse in OSAHS is not completely understood, subjective anatomy, pharyngeal dilatator muscle dysfunction during sleep, and ventilator control instability are all factors contributing to the pathogenesis of OSAHS [28].
Resective palatal surgery technique first (as UPPP) and then non-resective techniques later (ESP or BRP) have both demonstrated that soft palate modifications and pharyngeal tensions, aimed to improve the retrovelar space, are the key factors to obtain improvement of respiratory polygraphic parameters [16]. However, little is known about the parameters that can predict surgical success or failure, and the lack of standardized criteria for reporting surgical outcomes represents a difficulty in comparing surgical outcomes [19].
To the best of our knowledge, this is the first study measuring pharyngeal diameters, palatal measures, and correlating their postsurgical variation with outcomes.
As proposed by Sher [19] and applied by Montevecchi et al. [21], we considered either a respiratory polygraphic parameter (AHI) and the ESS questionnaire in defining an outcome as cure, success, or failure, as reported in Table 1. In this way, multivariate analysis identified the parameters that best predict the outcome as curative or, at least, successful.
Multivariate analysis showed that variation of A-P width was the only variable independently significant for prediction of successful treatment with a O.R. 4.6 (CI 1.4–14.7) for each millimeter increase of ∆A-P. For ∆A-P, the cutoff value of 8.5 mm found by ROC curve analysis can be a useful tool to predict the success at the end of the surgical procedure, with a sensitivity of 93.5% and specificity of 87.5% (p < 0.001).
Considering cure as the positive outcome, multivariate analysis showed that higher ESSpre and AHIpre values, and a BP technique (BRP or BSP) were all significantly associated with higher rates of cure (p = 0.029, p = 0.002, p = 0.026 respectively). Looking for cutoff values of continuous variables (ESSpre and AHIpre) by ROC curve analysis, an AHIpre > 24.5 can predict a not more than successful outcome with a sensitivity of 78.6% and specificity of 75% (p < 0.001). Previously, in patients who underwent pharyngeal surgery for OSAS, higher AHIpre values have been correlated with poorer outcome [29], in agreement with our results.
Our success rate was 89% with a cure rate of 20%, but it is clear that the criteria chosen influence the success rate, since by applying Sher criteria (AHIpost < 20 events/h and AHI reduction ≥ 50%), we would have had a success rate of 93%. In our series, 8 patients (11%) experienced a failure, even if no patient had AHIpost > 20 or ESSpost > 10; only 2 had an AHIpost of 20 events/h, one had AHI reduction < 50%, three had ESS reduction < 50%, and two patients had reduction of both parameters by < 50%. This can also be explained by the evidence of non-anatomical features as the pathophysiologic cause of OSAS, such as loop gain or low arousal threshold, which would probably not benefit from surgical interventions alone. In this regard, in the future, a consensus should be obtained to define criteria to better choose the most appropriate therapeutic option [30].
Our results applying non-resective pharyngoplasty techniques obtaining an 89% rate of success is at least comparable with that obtained by Montevecchi et al. with a 73% of success applying the same criteria and performing similar surgical procedures in terms of BRP alone or as a part of multilevel surgery [21]. Comparing the results of Pang et al. [11], that performed 73 ESS pharyngoplasty in a unilevel or multilevel setting, judging the outcome similarly to our criteria but not considering the ESS questionnaire, they got a success rate ranging from 67 to 90% depending on the severity of the OSAHS grade, again comparable with our rate of success, confirming that such techniques are reproducible with comparable results.
Despite the good results obtained in our study and the interesting findings regarding the association of wider ∆A-P measure and a successful outcome, this should be validated in further prospective studies, homogeneous for surgical treatments, to verify the reproducibility of pharyngeal measures assessment, that could subsequently be tested as objective targets to be achieved at the end of the surgical procedures, besides the self-judgment of the surgeon.
Anyway, in selected non-obese patients affected by OSAS with palatal or lateral pharyngeal wall collapse, who refuse or who cannot tolerate PAP therapy, non-resective pharyngoplasty, in a unilevel or multilevel setting, is a surgical technique with a high success rate; variables such as AHIpre or AP variation seems to be useful parameters associated with the surgical outcomes that should be further studied to be validated as predictors of success.
References
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM (2013) Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 177:1006–1014. https://doi.org/10.1093/aje/kws342
Frey WC, Pilcher J (2003) Obstructive sleep-related breathing disorders in patients evaluated for bariatric surgery. Obes Surg 13:676–683. https://doi.org/10.1381/096089203322509228
Kohler M, Stradling JR (2010) Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol 7:677–685. https://doi.org/10.1038/nrcardio.2010.145
Flemons WW (2002) Clinical practice. Obstructive sleep apnea. N Engl J Med 347:498–504. https://doi.org/10.1056/NEJMcp012849
Epstein LJ, Kristo D, Strollo PJ Jr et al (2009) Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 5:263–276
Vroegop AV, Vanderveken OM, Boudewyns AN et al (2014) Drug-induced sleep endoscopy in sleep-disordered breathing: report on 1,249 cases. Laryngoscope, In
(1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22:667–689
Tang JA, Salapatas AM, Bonzelaar LB, Friedman M (2017) Long-term incidence of velopharyngeal insufficiency and other sequelae following uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg 156:606–610. https://doi.org/10.1177/0194599816688646
Cahali MB (2003) Lateral pharyngoplasty: a new treatment for obstructive sleep apnea hypopnea syndrome. Laryngoscope 113:1961–1968
Salamanca F, Costantini F, Mantovani M, Bianchi A, Amaina T, Colombo E, Zibordi F (2014) Barbed anterior pharyngoplasty: an evolution of anterior palatoplasty. Acta Otorhinolaryngol Ital 34:434–438
Pang KP, Piccin O, Pang EB, Pang KA, Chan YH, Rotenberg BW (2016) Combined expansion pharyngoplasty and anterior palatoplasty for the treatment of OSA. Indian J Otolaryngol Head Neck Surg 68:528–533. https://doi.org/10.1007/s12070-016-1020-2
Pang KP, Woodson BT (2007) Expansion sphincter pharyngoplasty: a new technique for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg 137:110–114. https://doi.org/10.1016/j.otohns.2007.03.014
Mantovani M, Minetti A, Torretta S, Pincherle A, Tassone G, Pignataro L (2012) The velo-uvulo-pharyngeal lift or “roman blinds” technique for treatment of snoring: a preliminary report. Acta Otorhinolaryngol Ital 32:48–53
Vicini C, Hendawy E, Campanini A, Eesa M, Bahgat A, AlGhamdi S, Meccariello G, DeVito A, Montevecchi F, Mantovani M (2015) Barbed reposition pharyngoplasty (BRP) for OSAHS: a feasibility, safety, efficacy and teachability pilot study. “We are on the giant’s shoulders”. Eur Arch Otorhinolaryngol 272:3065–3070. https://doi.org/10.1007/s00405-015-3628-3
Barbieri M, Missale F, Incandela F, Fragale M, Barbieri A, Roustan V, Canevari FR, Peretti G (2019) Barbed suspension pharyngoplasty for treatment of lateral pharyngeal wall and palatal collapse in patients affected by OSAHS. Eur Arch Otorhinolaryngol 276:1829–1835. https://doi.org/10.1007/s00405-019-05426-4
Rashwan MS, Montevecchi F, Cammaroto G et al (2017) Evolution of soft palate surgery techniques for obstructive sleep apnea patients: a comparative study for single-level palatal surgeries. Clin Otolaryngol. https://doi.org/10.1111/coa.13027
Sorrenti G, Piccin O (2013) Functional expansion pharyngoplasty in the treatment of obstructive sleep apnea. Laryngoscope 123:2905–2908. https://doi.org/10.1002/lary.23911
Sher AE, Schechtman KB, Piccirillo JF (1996) The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep 19:156–177
Sher AE (2002) Upper airway surgery for obstructive sleep apnea. Sleep Med Rev 6:195–212
Miljeteig H, Mateika S, Haight JS, Cole P, Hoffstein V (1994) Subjective and objective assessment of uvulopalatopharyngoplasty for treatment of snoring and obstructive sleep apnea. Am J Respir Crit Care Med 150:1286–1290. https://doi.org/10.1164/ajrccm.150.5.7952554
Montevecchi F, Meccariello G, Firinu E et al (2017) Prospective multicentre study on barbed reposition pharyngoplasty standing alone or as a part of multilevel surgery for sleep apnoea. Clin Otolaryngol. https://doi.org/10.1111/coa.13001
Vicini C, Montevecchi F, Pang K et al (2014) Combined transoral robotic tongue base surgery and palate surgery in obstructive sleep apnea-hypopnea syndrome: expansion sphincter pharyngoplasty versus uvulopalatopharyngoplasty. Head Neck 36:77–83. https://doi.org/10.1002/hed.23271
Vicini C, De Vito A, Benazzo M et al (2012) The nose oropharynx hypopharynx and larynx (NOHL) classification: a new system of diagnostic standardized examination for OSAHS patients. Eur Arch Otorhinolaryngol 269:1297–1300. https://doi.org/10.1007/s00405-012-1965-z
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14:540–545
Vignatelli L, Plazzi G, Barbato A, Ferini-Strambi L, Manni R, Pompei F, D’Alessandro R, GINSEN (Gruppo Italiano Narcolessia Studio Epidemiologico Nazionale (2003) Italian version of the Epworth sleepiness scale: external validity. Neurol Sci 23:295–300. https://doi.org/10.1007/s100720300004
De Vito A, Carrasco Llatas M, Vanni A et al (2014) European position paper on drug-induced sedation endoscopy (DISE). Sleep Breath 18:453–465. https://doi.org/10.1007/s11325-014-0989-6
Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S, Denkert C (2012) Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One 7:e51862. https://doi.org/10.1371/journal.pone.0051862
Huang Y, Malhotra A, White DP (2005) Computational simulation of human upper airway collapse using a pressure-/state-dependent model of genioglossal muscle contraction under laminar flow conditions. J Appl Physiol 99:1138–1148. https://doi.org/10.1152/japplphysiol.00668.2004
Millman RP, Carlisle CC, Rosenberg C, Kahn D, McRae R, Kramer NR (2000) Simple predictors of uvulopalatopharyngoplasty outcome in the treatment of obstructive sleep apnea. Chest 118:1025–1030
Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A (2013) Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 188:996–1004. https://doi.org/10.1164/rccm.201303-0448OC
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Surgical procedures were performed by Barbieri Marco and Barbieri Andrea. Material preparation and data collection were performed by Fragale Marco, Incandela Fabiola, and Roustan Valeria. Formal analysis and picture drawing were performed by Missale Francesco. The first draft of the manuscript was written by Missale Francesco, Fragale Marco, Incandela Fabiola, and Arceri Carlotta. Peretti Giorgio, Canevari Frank Rikki, and Barbieri Marco reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors certify that they have no affiliation with or involvement in any organization or entity with any financial interest.
Ethical approval
The research did not involve any animal models; the research involved human participants in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards; and informed consent was obtained from all individual participants included in the study. Ethical review and approval was not required for this study in accordance with the national and institutional requirements (Ethics committee IRCCS Ospedale Policlinico San Martino, Genoa). However, all patients preoperatively signed a consent form for disclosure of privacy in managing personal data for scientific purposes.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Table 1
Summary of statistics for quantitative variables. (DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Missale, F., Fragale, M., Incandela, F. et al. Outcome predictors for non-resective pharyngoplasty alone or as a part of multilevel surgery, in obstructive sleep apnea-hypopnea syndrome. Sleep Breath 24, 1397–1406 (2020). https://doi.org/10.1007/s11325-019-01985-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-019-01985-2