Abstract
Purpose
Positional (supine dependent) obstructive sleep apnea (POSA) affects about 55% of adults with obstructive sleep apnea (OSA). We aimed to study the prevalence and risk factors for POSA in children.
Methods
Cross-sectional analysis of data obtained in 171 children with moderate to severe OSA confirmed by polysomnography (PSG) performed over a 2-year period. POSA is defined by an obstructive apnea–hypopnea index (oAHI) in the supine position ≥ 2× oAHI in the non-supine position.
Results
The overall prevalence of POSA was 18.7%. Children with POSA were significantly older (p < 0.001), had a higher prevalence of obesity (p = 0.04), a lower tonsil score (p = 0.049), and less severe OSA (lower oAHI) (p = 0.02) compared to children without POSA, while age was the only significant independent predictor of POSA. The ratio AHI supine to AHI non-supine was not significantly higher during REM than during NREM sleep in children with POSA.
Conclusions
POSA is less common in children compared to adults and the prevalence of POSA increases with age. Although OSA worsens during REM sleep, this was not observed for POSA. Future studies should investigate the prevalence of POSA in specific subgroups and upper airway characteristics of POSA in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
OSA in children occurs among all ages with an estimated prevalence from 1.2 up to 5% [1, 2]. OSA is more common in children with obesity, Down syndrome (DS), and craniofacial abnormalities.
It is known that body position during sleep can affect the frequency and severity of the respiratory events. This phenomenon is referred to as positional obstructive sleep apnea (POSA) when a higher number of respiratory events occur during the supine sleeping position [3,4,5]. In adults, POSA is commonly defined by an AHI in the supine position ≥ 2× AHI in the non-supine position [3]. It has been demonstrated that about 55% of the adult patients with OSA have POSA [6]. In children, however, only few studies have been conducted investigating the effect of sleep position on OSA severity and the results are contradictory. Different studies [7,8,9] suggest that the effect of position on OSA severity might be influenced by different factors such as age, obesity, history of adenotonsillectomy (AT), and location of the upper airway obstruction. In children, it is known that sleep stage has an important effect on OSA severity as well and the apnea/hypopnea index is higher in REM sleep than during NREM sleep [10, 11].
The aims of the present study are threefold: (1) to investigate the prevalence of POSA in children with OSA; (2) to identify patient-related factors associated with POSA; (3) to investigate whether POSA is a REM-related phenomenon in children.
Methods
This is a cross-sectional, single-center study performed at the Pediatric Sleep Center of the Antwerp University Hospital, Belgium.
All children (aged between 1 and 18 years) undergoing a baseline polysomnography (PSG) between January 01, 2015, and December 31, 2016, with a diagnosis of moderate to severe OSA (obstructive apnea/hypopnea index (oAHI) > 5/h), were eligible for inclusion. We included children with obesity, Down syndrome, or a previous history of adenotonsillectomy (AT). The latter are classified as children with persistent OSA. Children with neuromuscular diseases, craniofacial malformations, or other syndromes leading to more complex upper airway anatomy were excluded. Sleep studies performed to evaluate the effect of anti-inflammatory treatment or upper airway surgery or for titration of continuous positive airway pressure (CPAP) or Bi-level positive airway pressure (Bipap) were also excluded.
Basic demographic data and polysomnographic parameters were collected from the hospitals’ electronical medical records and entered in a database. To investigate the influence of age on the occurrence of POSA, the study population was divided into three age groups: < 6 years, between 6 and 12 years, and above 12 years. Body weight and length as determined at the time of PSG were used to calculate the BMI z-score and BMI percentile score. Obesity was defined as a BMI z-score greater than or equal to 2 or a BMI greater than the 95th percentile for gender and age [12, 13]. Tonsils were graded from 0 to 4 according to Brodsky [14].
POSA is defined as oAHI supine ≥ 2× oAHI non-supine according to Cartwright et al. [3]. In addition, it was required that the patient spent at least 30 min in each sleeping position (supine and non-supine).
All children underwent nocturnal PSG for at least 6 h at the Pediatric Sleep Disorders Center of the Antwerp University Hospital, Belgium. The following variables were continuously measured and recorded by a computerized polysomnograph (Brain RT, OSG, Rumst, Belgium), electroencephalography (C4/A1, C3/A2, F3/A2 and F4/A1), electro-oculography, electromyography of the anterior tibialis and chin muscles, and electrocardiography. Respiratory effort was measured by respiratory inductance plethysmography and oxygen saturation by a finger probe connected to a pulse oximeter. Airflow was measured by means of nasal pressure cannula and thermistor, and snoring was detected by means of a microphone at the suprasternal notch. Children were also monitored on audio/videotape using an infrared camera. Polysomnography was manually scored by certified technicians according to international guidelines [15]. Sleep stage distribution is expressed as the amount of a given sleep stage relative to total sleep time (TST). The obstructive apnea–hypopnea index (oAHI) was defined as the number of obstructive apneas and hypopneas per hour of sleep. Obstructive sleep apnea was defined as an oAHI ≥ 2/h [16]. OSA was classified as mild (oAHI between 2 and 5/h), moderate (oAHI ≥ 5 and up to 10/h), or severe (oAHI ≥ 10/h) [17]. For the assessment of body position, the Schwarzer Polygraphy Adaptor, with a built-in position sensor, was used. This device was fixed on the patient’s chest. The children are observed through an infrared video camera and these data are included in the PSG recording.
The total sleep time and time in the supine position and non-supine positions were recorded. Using these values, the oAHI in the different sleeping positions was calculated, accounting for the number of obstructive apneas and hypopneas per hour of total sleep time in supine and in non-supine sleep, respectively [18].
Statistical analysis was performed using SPSS version 24.0 (SPSS Inc., Chicago, IL).
Normally distributed data are expressed as a mean and standard deviation and non-normally distributed data are expressed as a median (25th–75th percentile).
In this study, the presence or absence of positional OSA is the main variable of interest. This variable was coded as a binary variable.
Patient characteristics between positional and non-positional OSA patients were compared by Student’s t test or Mann–Whitney U test according to data distribution. Chi-square test was performed for analyzing categorical variables. When sample sizes were too small, Fisher’s exact test was used for comparing categorical variables. The Wilcoxon signed-rank test was used when comparing related samples.
The OSA severity in the supine (oAHI supine) and the non-supine (oAHI non-supine) positions was correlated with age, BMI z-score, and tonsil score, using the Spearman rank correlation test.
A binary logistic regression was performed to identify independent factors associated with positional OSA. The independent variables included were age, BMI z-score, history of AT, and tonsil score. The significance level was set to p < 0.05.
Results
Data from 171 children were available for analysis. Patient characteristics are displayed in Fig. 1.
The majority of study patients (n = 102; 60%) were non-obese and non-syndromal children without a history of previous upper airway surgery.
Children were 5.8 ± 4.9 years old, mostly male (60.4%) with a mean BMI z-score of 0.35 ± 1.81 and severe obstructive sleep apnea with a median oAHI of 11.7/h (7.0–19.3). Most children were younger than 6 years (n = 110).
OSA severity (oAHI) was not significantly different in children with persistent OSA 9.4/h (5.7–18.0) compared to those without prior surgery 12.4/h (7.6–19.3) (p = 0.17). Children with persistent OSA were older than those without previous AT: 10.0 ± 5.2 vs 5.1 ± 4.4 years, respectively (p < 0.001).
Children slept significantly longer in the non-supine position (306 min, (177.2–426.4)) than in the supine position (215.0 min (87.3–316.3)) (p < 0.001). The oAHI supine was not significantly higher than the oAHI non-supine for the entire study group: 11.1/h (6.8–18.8) vs 9.8/h (5.6–17.6), respectively. In general, oAHI supine was not correlated with age, BMI z-score, and tonsil score. The oAHI non-supine in contrast did correlate negatively with age (r = − 0.26; p < 0.01) and BMI z-score (r = − 0.16; p = 0.03), and positively with tonsil score (r = 0.22; p < 0.01).
Of the 171 children included in this study, 19% (n = 32) fulfilled the criteria for POSA. The prevalence of POSA was 22.2% in children with Down syndrome and this is not significantly different from the 18.1% prevalence in non-syndromal children. The prevalence of POSA in the different subgroups of non-syndromal children is displayed in Fig. 2. POSA was significantly more prevalent in non-syndromal children with obesity (p = 0.02) or non-syndromal children with a history of prior adenotonsillectomy (p = 0.03) compared with the group of non-syndromal, non-obese children without prior upper airway surgery.
The prevalence of POSA in the different age categories is presented in Fig. 3.
The prevalence of POSA increased with age, and children with POSA were significantly older than those without POSA: 9.4 ± 5.4 years vs 5.0 ± 4.4 years (p < 0.01). In the youngest age category (< 6 years), there is no significant difference between oAHI supine and oAHI non-supine. However, in the middle and oldest age categories, a significant difference was found between oAHI supine and oAHI non-supine (Table 1).
Patient characteristics and polysomnographic data for POSA and non-POSA children among the entire study population are presented in Tables 2 and 3, respectively.
There is no difference in gender or prevalence of DS between the POSA and the non-POSA groups. When analyzing the entire study population, there is no difference in the number of children with a history of adenotonsillectomy between POSA and non-POSA. The BMI z-score did not differ significantly between children with or without POSA, but the percentage of children with obesity was higher in the POSA group (34.4% vs 18.0%; p = 0.04). The tonsil scores were lower in the POSA group compared to the non-POSA group (p = 0.049). In children with a history of tonsillectomy, oAHI supine of 12.3/h (7.4–20.8) was significantly higher than oAHI non-supine of 5.9/h (2.7–12.2) (p ≤ 0.006).
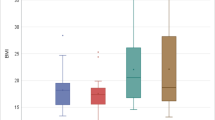
Figure 4 illustrates that children with POSA have less severe OSA based upon oAHI compared to children without POSA.
Children with POSA had a lower total sleep time (TST) than children without POSA, and for this reason, the other time variables are shown as percentage of TST. Children with POSA spent more time in the supine position (p = 0.03). They also had a lower proportion of REM sleep, but when corrected for age these differences disappeared.
To investigate which variables could predict the presence of POSA, we performed a binary logistic regression analysis with the following variables: age, BMI z-score, history of AT, and tonsil score. Age was the only significant predictor of POSA (p < 0.01), with an odds ratio of 1.2. (95% CI, 1.128–1.403).
In four subjects, REM sleep was not recorded properly during PSG; their data were excluded from REM-related analysis. In the remaining 167 patients, the effect of REM sleep and the interaction with sleep position were studied. oAHI during REM sleep (18.9/h; 12.2–32.0) was significantly higher than the oAHI in NREM sleep (7.5/h; 4.5–15.4) (p < 0.001).
The oAHI was higher in the supine (19.6/h; 8.8–33.8) than in the non-supine position (14.5/h; 7.1–26.6) during REM sleep (p = 0.006). This difference was not observed for NREM sleep: oAHI 6.9/h (2.8–14.4) supine vs 6.8/h (2.8–12.7) non-supine (p = 0.4). Although oAHI during supine REM sleep was higher in children with POSA (20.4/h; 12.0–33.9) compared with non-POSA (18.5/h; 8.2–33.8), this difference was not significant (p = 0.6).
To evaluate the effect of REM and NREM sleep on positional differences of oAHI, the ratio oAHI supine to oAHI non-supine was calculated in REM sleep and in NREM sleep. This ratio was not significantly higher in REM sleep than in NREM sleep in POSA patients.
Discussion
This cross-sectional study aimed to investigate the prevalence of POSA in children, to identify risk factors, and to investigate the effect of sleep stage. The prevalence of POSA was 19% in a cohort of children with moderate to severe OSA. In addition, the prevalence of POSA was not significantly higher in children with DS compared to non-syndromal children. Age was a significant predictor of POSA with an increasing risk as children grow older. Based on the results of this study, the positional difference in OSA severity seems not to be influenced by sleep stage (REM vs non-REM). Finally, as in adults, children with OSA predominantly occuring in the supine position, on average, have milder and less severe OSA (Fig. 4).
In our study, 19% of patients fulfilled the diagnostic criterion of POSA defined as oAHI supine ≥ 2× oAHI non-supine [3]. This is different from previous reports where 9 to 31% of the study patients fulfilled this criterion [8, 19]. These differences may be related to sample size or study population. We included children over a wide age span, with obesity, or a prior history of upper airway surgery, and only excluded children with craniofacial malformation or syndromic conditions other than Down syndrome. The study population therefore represents a large clinical sample of children diagnosed with moderate to severe OSA in a tertiary referral center during a 2-year period.
In adults, POSA is often defined as a supine to non-supine AHI ratio of ≥ 2 but this definition is not universally accepted [20]. Since the publication by Cartwright et al. in 1991, various definitions for POSA in adults have emerged in literature and more recently different subtypes were defined with different clinical characteristics. The Amsterdam Positional OSA classification (APOC) differentiates OSA patients into true positional, non-positional, and multifactorial (OSA severity partly influenced by position) [6]. However, at present, the clinical role of subclassifying POSA in adults is unclear [20]. Also in children, there is no consensus on the definition of POSA, and the use of different criteria is likely to affect POSA prevalence.
When defining “positional patients” as children in whom AHI supine is greater than AHI non-supine, the prevalence of positional patients increases to 54% in the study by Nisbet et al. [19], 37% in the study by Cuhadaroglu et al. [8], and 56% in our study population. At least, questions remain about the definition of POSA in children and whether adult criteria are applicable to children.
We identified age as an important factor influencing the severity and prevalence of POSA. In our study population, the risk of getting POSA increased with 12% for each year and the POSA group was significantly older than the group of non-POSA children. This is in line with data published by Zhang et al. [9] who concluded that the oAHI supine was significantly higher in children aged 5–13 years.
The prevalence of POSA was investigated in different subgroups of children with OSA. Although the number of patients in these subgroups is small, some trends could be observed.
The prevalence of POSA in non-syndromal children with persistent OSA following previous AT was 31%, which is higher than the overall 18% of POSA in our study population. OSA severity was not significantly different in children with persistent OSA compared to those without previous AT but 13 out of 22 were obese. Since the prevalence of POSA in the obese group was also much higher (30.3%), the higher prevalence in the AT group could be at least partially related to the high prevalence of obesity. Nevertheless, after correction for these factors through regression analysis, a history of AT and obesity were not significant predictors of POSA in non-syndromal children.
The relationship between degree of tonsillar hypertrophy and POSA is unclear. In children with tonsil score 0, we observed an oAHI supine that was significantly higher than oAHI non-supine. This difference was not observed in children where tonsils were still present (regardless of tonsillar size).
Only a few studies looked at the effect of tonsillar hypertrophy on AHI in different sleep positions with conflicting results.
Cuhadaroglu et al. [8] used video endoscopy to evaluate the upper airway and concluded that OSA in children with adenotonsillar hypertrophy is worse in the supine position. Kim et al. [21] evaluated the influence of sleeping position on the respiratory parameters in 19 children with OSA vs 31 children classified as habitual snorers. The AHI was significantly higher in the supine position compared to the non-supine position in the subpopulation of children with OSA. In the control group, the difference in AHI between the supine and non-supine positions was not significant. The authors identified enlarged tonsils as contributing factors to the positional difference in AHI. The findings in these two small studies are different from those reported by Dayyat et al. [22]. In a large study including 430 OSA children and 185 controls, Dayyat et al. concluded that the presence of obesity increases the effect of body position on respiratory disturbance while tonsil size appeared to have no influence on positional differences in breathing during sleep. Our data suggest that absence of tonsils (after tonsillectomy) in non-syndromic children is associated with POSA but this effect may be partially mediated by age and obesity. Part of the difference between these studies might also be related to the larger percentage (47.4%) of obese children in the study by Dayyat et al. compared with 26.3% in the study by Kim et al. and 22.9% in our population of non-syndromic children.
In line with previous studies [19, 23], the prevalence of POSA in children with DS did not differ significantly from the prevalence in non-syndromal children. Despite the high prevalence of OSA in children with Down syndrome, they do not have a higher prevalence of POSA.
The association between obesity and POSA in children remains controversial [7, 8, 21]. The prevalence of obesity was higher in children with POSA. When looking at OSA severity in the obese subgroup, it was found that the oAHI in the supine position was significantly higher than non-supine and there was a negative correlation between BMI z-score and oAHI non-supine. In that perspective, our data are in line with those published by Dayyat et al. [7] who found that the AHI supine was twice as high than the AHI in the prone position in obese children. Obese children may be more susceptible to positional differences and the effect of gravity on fat depositions surrounding the upper airway.
Overall, children spent a larger part of their sleep time in the non-supine position, and only the children with POSA spent more time in the supine. Thus, children with POSA spent more time in the supine position which is their most unfavorable sleep position. This could result in more sleep fragmentation and makes them more symptomatic. Our finding is in contrast with recent data reported by Walter et al. [24]. These authors studied children with OSA and non-snoring controls within a small age range (3–5 years) and found that all children spent significantly more time supine than in any other sleep position, a difference that might be explained by the broader age range in the present study.
Based on oAHI and RDI, it appears that children with POSA have less severe OSA. This is consistent with the findings in adults, where patients with POSA have milder disease [25, 26].
In children, OSA worsens during REM sleep, and pediatric OSA is mainly a REM-related phenomenon [10, 11]. Our data show an interaction between sleep state (REM vs non-REM) and sleep position (supine vs non-supine).
oAHI in REM supine was significantly higher than oAHI in REM non-supine. A difference that was not found during NREM sleep suggesting that the effect of sleep position is most pronounced during REM sleep. Children with POSA had less REM sleep, a finding that might be related to the fact that children with POSA were older than non-positional patients and the overall decrease in REM sleep with age. On the other hand, Cartwright et al. observed that a large proportion of adult patients had no REM sleep while sleeping supine and the authors concluded that the supine sleeping position is not well tolerated by positional patients during REM sleep [27]. Our data also show that children with POSA experience more severe apneas during REM sleep. In children with POSA, the ratio of AHI supine to AHI non-supine was not significantly different during REM as compared to NREM sleep. These data indicate that the positional effect is present during all sleep stages and that POSA is not worsening during REM sleep. This is also in accordance with the data published in adults were it has been observed that supine AHI is elevated equally in both sleep stages compared to non-supine [27].
Data obtained during drug-induced sedation endoscopy in adult patients showed that nearly all positional patients had a partial improvement in upper airway collapse while in the lateral position and that most reduction occurred at the level of the tongue base and epiglottis [28]. Tongue base obstruction has been identified as an important cause of persistent OSA in children following AT [29]. In the future, it would be interesting to evaluate upper airway characteristics in children with POSA through drug-induced sedation endoscopy or imaging studies to further delineate the role of tonsillar hypertrophy and tongue base obstruction in the pathogenesis of POSA in children. This could provide more insight into upper airway characteristics of these patients and guide therapeutic decision-making. In adults with POSA, positional modification techniques aiming to prevent patients from sleeping supine are a valuable treatment option [30,31,32]. Positional therapy has occasionally been used in children as well, but currently there are no data available on the efficacy of this treatment in children [33]. Positional therapy is a non-invasive treatment option and its role in the management of POSA in children should be evaluated in future studies.
There are some limitations to this study that should be mentioned. Children with craniofacial malformations and neuromuscular diseases were excluded. These diseases are a risk factor for developing OSA and consequently could also be a risk factor for POSA. Although we prospectively enrolled all patients diagnosed with moderate to severe OSA in a 2-year period, we cannot exclude referral bias because we serve as a tertiary referral center and it might be not fully representative to extrapolate our data to the general pediatric population. Third, sleep position was measured by means of a position sensor fixed to the chest and confirmed by video analysis obtained through infrared camera. In a recent paper by Walter et al., sleep position was determined manually for each 30-s epoch, and the authors did not rely on a position sensor [24]. These authors argued that this helped them to identify a “true supine” position vs a “semi-supine” position where the head and neck are in a lateral position relative to the trunk. Based upon this measurement technique for sleep position, Walter et al. concluded that 3–5-year-old children (non-snoring controls and those with OSA) predominantly sleep in the supine position, a finding that is in contrast with our present data. Further studies should investigate the most accurate technique for measuring sleep position in children.
We choose to include only children with moderate to severe OSA because this is clinically most relevant. In the pediatric age group, an oAHI > 5/h with or without associated morbidity has been used as an indication for treatment [16]. We cannot exclude that our findings might have been different if we had also included children with mild disease. In adults, POSA is more common in patients with mild–moderate OSA but definitions of mild and moderate disease are different from those used in children.
Conclusions
Supine-dependent OSA is less prevalent in children compared to adults. Although OSA worsens during REM sleep in children, this was not the case for POSA. Age is a significant and independent predictor of POSA with an increasing risk as children grow older. Children with POSA have milder OSA as compared to non-POSA children. Future studies should reach a consensus about the definition of POSA in children, assess POSA prevalence in specific subgroups of children, and investigate upper airway characteristics in children with POSA.
References
Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, Schechter MS, Ward SD, Sheldon SH, Shiffman RN, Lehmann C, Spruyt K (2012) Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 130(3):714–755
Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Vela-Bueno A, Fedok F, Vlasic V, Graff G (2009) Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep 32(6):731–736
Cartwright RD (1984) Effect of sleep position on sleep apnea severity. Sleep 7(2):110–114
Joosten SA, O'Driscoll DM, Berger PJ, Hamilton GS (2014) Supine position related obstructive sleep apnea in adults: pathogenesis and treatment. Sleep Med Rev 18(1):7–17
Richard W, Kox D, den Herder C, Laman M, van Tinteren H, de Vries N (2006) The role of sleep position in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol 263(10):946–950
Frank MH, Ravesloot MJ, van Maanen JP, Verhagen E, de Lange J, de Vries N (2015) Positional OSA part 1: towards a clinical classification system for position-dependent obstructive sleep apnoea. Sleep Breath 19(2):473–480
Dayyat E, Maarafeya MM, Capdevila OS, Kheirandish-Gozal L, Montgomery-Downs HE, Gozal D (2007) Nocturnal body position in sleeping children with and without obstructive sleep apnea. Pediatr Pulmonol 42(4):374–379
Cuhadaroglu C, Keles N, Erdamar B, Aydemir N, Yucel E, Oguz F, Deger K (2003) Body position and obstructive sleep apnea syndrome. Pediatr Pulmonol 36(4):335–338
Zhang XW, Li Y, Zhou F, Guo CK, Huang ZT (2007) Association of body position with sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Acta Otolaryngol 127(12):1321–1326
Goh DY, Galster P, Marcus CL (2000) Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med 162(2 Pt 1):682–686
El-Kersh K, Cavallazzi R, Patel PM, Senthilvel E (2016) Effect of sleep state and position on obstructive respiratory events distribution in adolescent children. J Clin Sleep Med 12(4):513–517
Must A, Anderson SE (2006) Body mass index in children and adolescents: considerations for population-based applications. Int J Obes 30(4):590–594. https://doi.org/10.1038/sj.ijo.0803300
Flegal KM, Ogden CL (2011) Childhood obesity: are we all speaking the same language? Adv Nutr 2(2):159–166
Brodsky L (1989) Modern assessment of tonsils and adenoids. Pediatr Clin N Am 36(6):1551–1569
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM, American Academy of Sleep M (2012) Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 8(5):597–619
Kaditis AG, Alonso Alvarez ML, Boudewyns A, Alexopoulos EI, Ersu R, Joosten K, Larramona H, Miano S, Narang I, Trang H, Tsaoussoglou M, Vandenbussche N, Villa MP, Van Waardenburg D, Weber S, Verhulst S (2016) Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management. Eur Respir J 47(1):69–94
Marcus CL, Moore RH, Rosen CL, Giordani B, Garetz SL, Taylor HG, Mitchell RB, Amin R, Katz ES, Arens R, Paruthi S, Muzumdar H, Gozal D, Thomas NH, Ware J, Beebe D, Snyder K, Elden L, Sprecher RC, Willging P, Jones D, Bent JP, Hoban T, Chervin RD, Ellenberg SS, Redline S, Childhood Adenotonsillectomy T (2013) A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 368(25):2366–2376
Fernandes do Prado LB, Li X, Thompson R, Marcus CL (2002) Body position and obstructive sleep apnea in children. Sleep 25(1):66–71
Nisbet LC, Phillips NN, Hoban TF, O'Brien LM (2014) Effect of body position and sleep state on obstructive sleep apnea severity in children with Down syndrome. J Clin Sleep Med J 10(1):81–88
Omobomi O, Quan SF (2017) Positional therapy in the management of positional obstructive sleep apnea-a review of the current literature. Sleep Breath 22:297–304. https://doi.org/10.1007/s11325-017-1561-y
Kim HY, Dhong HJ, Lee JK, Chung SK, Jung SC (2011) Sleep quality and effects of position on sleep apnea in East Asian children. Auris Nasus Larynx 38(2):228–232
Dayyat E, Kheirandish-Gozal L, Sans Capdevila O, Maarafeya MM, Gozal D (2009) Obstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophy. Chest 136(1):137–144
Senthilvel E, Krishna J (2011) Body position and obstructive sleep apnea in children with Down syndrome. J Clin Sleep Med 7(2):158–162
Walter LM, Dassanayake DUN, Weichard AJ, Davey MJ, Nixon GM, Horne RSC (2017) Back to sleep or not: the effect of the supine position on pediatric OSA: sleeping position in children with OSA. Sleep Med 37:151–159
Lee SA, Paek JH, Chung YS, Kim WS (2017) Clinical features in patients with positional obstructive sleep apnea according to its subtypes. Sleep Breath 21(1):109–117
Mador MJ, Choi Y, Bhat A, Dmochowski J, Braun M, Gottumukkala VA, Grant BJ (2010) Are the adverse effects of body position in patients with obstructive sleep apnea dependent on sleep stage? Sleep Breath 14(1):13–17
Cartwright RD, Diaz F, Lloyd S (1991) The effects of sleep posture and sleep stage on apnea frequency. Sleep 14(4):351–353
Victores AJ, Hamblin J, Gilbert J, Switzer C, Takashima M (2014) Usefulness of sleep endoscopy in predicting positional obstructive sleep apnea. Otolaryngol Head Neck Surg 150(3):487–493
Boudewyns A, Abel F, Alexopoulos E, Evangelisti M, Kaditis A, Miano S, Villa MP, Verhulst SL (2017) Adenotonsillectomy to treat obstructive sleep apnea: is it enough? Pediatr Pulmonol 52(5):699–709
Barnes H, Edwards BA, Joosten SA, Naughton MT, Hamilton GS, Dabscheck E (2017) Positional modification techniques for supine obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev 36:107–115
Ravesloot MJL, White D, Heinzer R, Oksenberg A, Pepin JL (2017) Efficacy of the new generation of devices for positional therapy for patients with positional obstructive sleep apnea: a systematic review of the literature and meta-analysis. J Clin Sleep Med 13(6):813–824
Beyers J, Dieltjens M, Kastoer C, Opdebeeck L, Boudewyns AN, De Volder I, Van Gastel A, Verbraecken JA, De Backer WA, Braem MJ, Van de Heyning PH, Vanderveken OM (2018) Evaluation of a trial period with a sleep position trainer in patients with positional sleep apnea. J Clin Sleep Med 14(4):575–583
Tapia IE, Marcus CL (2013) Newer treatment modalities for pediatric obstructive sleep apnea. Paediatr Respir Rev 14(3):199–203
Acknowledgments
The authors are grateful to Ir. Marc Willemen (sleep technician) and Mrs. Kristien Wouters (statistician) for their advice with the revised manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the parents or legal caregivers for all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper was presented as poster at the European Society for Pediatric Otorhinolaryngology (ESPO) meeting in Stockholm, June 2–5, 2018.
Rights and permissions
About this article
Cite this article
Verhelst, E., Clinck, I., Deboutte, I. et al. Positional obstructive sleep apnea in children: prevalence and risk factors. Sleep Breath 23, 1323–1330 (2019). https://doi.org/10.1007/s11325-019-01853-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-019-01853-z